ABSTRACT
Reactive nitrogen species emission from the exhausts of gasoline and diesel vehicles, including nitrogen oxides (NOx) and nitrous acid (HONO), contributes as a significant source of photochemical oxidant precursors in the ambient air. Multiple laboratory and on-road exhaust measurements have been performed to estimate the NOx emission factors from various vehicles and their contribution to atmospheric pollution. Meanwhile, HONO emission from vehicle exhaust has been under-measured despite the fact that HONO can contribute up to 60% of the total hydroxyl budget during daytime and its formation pathway is not fully understood. A profound traffic-induced HONO to NOx ratio of 0.8%, established by Kurtenbach et al. since 2001, has been widely applied in various simulation studies and possibly linked to under-estimation of HONO mixing ratios and OH radical budget in the morning. The HONO/NOx ratios from direct traffic emission have become debatable when it lacks measurements for direct HONO emission from vehicles upon the fast-changing emission reduction technology. Several recent studies have reported updated values for this ratio. This study has reported the measurement of HONO and NOx emission as well as the estimation of exhaust-induced HONO/NOx ratios from gasoline and diesel vehicles using different chassis dynamometer tests under various real-world driving cycles. For the tested gasoline vehicle, which was equipped with three-way catalyst after-treatment device, HONO/NOx ratios ranged from 0 to 0.95 % with very low average HONO concentrations. For the tested diesel vehicle equipped with diesel particulate active reduction device, HONO/NOx ratios varied from 0.16 to 1.00 %. The HONO/NOx ratios in diesel exhaust were inversely proportional to the average speeds of the tested vehicles.
Implications: Photolysis of HONO is a dominant source of morning OH radicals. Conventional traffic-induced HONO/NOx ratio of 0.8% has possibly linked to underestimation of the total HONO budget and consequently underestimation of OH radical budget. The recently reported HONO/NOx ratio of ~1.6% was used to stimulate HONO emission, which resulted in increased HONO concentrations during morning peak hours and its impact of 14% OH increment in the morning. However, the results were still lower than the measured concentrations. More studies should be conducted to establish an updated traffic-induced HONO/NOx ratio.
Introduction
Reactive nitrogen species emission from the exhausts of gasoline and diesel vehicles, including nitrogen oxides (NOx) and nitrous acid (HONO), contributes as a significant source of photochemical oxidant precursors in the ambient air. Multiple laboratory and on-road exhaust measurements have been performed to estimate the NOx emission factors from various vehicles and their contribution to atmospheric pollution (Yang et al., Citation2015; Alvarez et al., Citation2008; Alves, Citation2015; Andersson et al., Citation2014; Huai et al., Citation2004; Suarez-Bertoa et al., Citation2015). Alvarez et al. (Citation2008) studied different vehicles certified under Euro 3 and Euro 4 categories. The authors reported NO2 to NOx mass ratios of 15–70%, with highest ratios of up to 70% observed in the diesel-particulate filter-equipped vehicles under urban driving conditions. A study by Alves (Citation2015) reported that gasoline and diesel vehicles with updated emission reduction technologies, which were under Euro 3–5 categories, showed an unclear impact on NOx and hydrocarbon emissions. Also, Yang et al. (Citation2015) tested 73 vehicles under the Euro 6 category with the worldwide harmonized light-duty test cycle (WLTC) and New European Driving Cycle (NEDC) from 2012 to 2015. The group reported that NOx emission from several vehicles exceeded 7–15 times the regulatory limit and predicted the average emission factor of 0.24 g/km, which was 3 times higher than the Euro 6 limit, based on the WLTC framework (Yang et al., Citation2015). This study reported on the emission of NOx from a three-way catalyst-installed gasoline car and a diesel-particle filter-installed car under constant speed and other real-world driving conditions (with hot and cold startup modes).
Meanwhile, HONO emission from vehicle exhaust has been undermeasured despite the fact that HONO can contribute up to 60% of the total hydroxyl budget during daytime and its formation pathway is not fully understood (Kleffmann et al., Citation2003; Kleffmann, Citation2007; Spataro et al., Citation2014). Kurtenbach et al. (Citation2001) performed a measurement of traffic-induced HONO emission in a tunnel study. The study established a profound HONO to NOx ratio of 0.8%, which has been widely applied in various simulation studies (Czader et al., Citation2012; Diao et al., Citation2016; Foley et al., Citation2010; Li et al., Citation2011). However, the application of this ratio has led to irreproducibility of HONO morning peaks attributed to traffic emission (Li et al., Citation2011; Czader et al., Citation2012). Recently, Rappengluck et al. (Citation2013) performed a continuous measurement at a highway junction in Houston, TX, and reported a HONO to NOx ratio of approximately 1.7%. Based on this result, a close HONO/NOx ratio of 1.6% was used to stimulate HONO emission for an urban site in Texas by Czader et al. (Citation2015). The authors reported increased HONO mixing ratios during morning peak hours and its impact of 14% OH increment in the morning at the measurement site and an overall 3% OH increment for the urban area. The HONO/NOx ratio from direct traffic emission has become debatable when there is also a lack of measurements for direct HONO emission from vehicles upon the rapidly changing technology for emission reduction. In this study, we performed the measurement of HONO and NOx emission and the estimation of HONO/NOx ratios from gasoline and diesel vehicles using different chassis dynamometer tests.
The Japanese automobile manufacturing industry has been introduced a number of new technologies to reduce exhaust emission from these types of vehicles (Japan Automobile Manufacturers Association, Citation2009). For gasoline vehicles, emission is reduced or eliminated in the combustion process through the use of electronically controlled fuel injection and combustion chamber improvements. In addition, three-way catalytic converters (TWC) and NOx storage-reduction (NSR) catalytic converters are installed in Japanese gasoline engines as after-treatment devices (Japan Automobile Manufacturers Association, Citation2009). For diesel vehicles, various measures have been applied in response to the 2005 stringent new long-term emission regulation. Those measures include combustion chamber improvements, exhaust gas recirculation (EGR), and advanced fuel-injection technology. Especially, NOx emission and particulate emission have been cut by using urea-selective catalytic reduction (urea-SCR) and diesel particulate filters as after-treatment devices (Japan Automobile Manufacturers Association, Citation2009). In compliance with the Japanese new long-term (JNL) emission regulation, the regulatory limits for medium-duty diesel and gasoline vehicles were, respectively, 0.63 and 2.55 g/km for CO, 0.024 and 0.05 g/km for nonmethane hydrocarbons (NMHC), 0.25 and 0.07 g/km for NOx (Ministry of Land, Infrastructure, Transport and Tourism, Citation2005). The limit for particulate matter (PM) was 0.015 g/km for medium-duty diesel vehicles and was not applicable to gasoline vehicles (Ministry of Land, Infrastructure, Transport and Tourism, Citation2005). In this study, two medium-duty gasoline and diesel vehicles, equipped with emission reduction devices, were tested in response to the JNL regulation using a chassis dynamometer and various real-world driving test cycles.
Materials and methods
Vehicles and fuels
Our study has investigated two medium-duty vehicles: a diesel-fueled RegiusAce Van (Manufacturer: Toyota, frame number: ADF-KDH201V); and a gasoline-fueled RegiusAce Van (Manufacturer: Toyota, frame number: CBF-TRH200V). The technical specifications of the vehicles are summarized in .
Table 1. Technical specification of the tested vehicles.
The diesel vehicle used was equipped with the diesel particulate active reduction system (DPR). The system consists of a catalyzed particulate filter, and a diesel oxidation catalyst (DOC). The characteristics and performance of the DPR device were reported elsewhere (Japan Automobile Manufacturers Association, Citation2009). Meanwhile, the gasoline vehicle used was equipped with a three-way catalytic converter (TWC) as the after-treatment device. The chassis dynamometer tests were conducted using commercial diesel and gasoline fuels. Properties of the tested diesel and gasoline fuels (Table S1) were analyzed by JX Nippon Oil & Energy Corporation. Sulfur content of the tested diesel and gasoline fuels were 0.0006 wt% and 0.0001 wt%, respectively.
Selected test cycles
The tested vehicles were investigated with four different driving cycles: JC08, LDV9, LDV26, and LDV43. JC08 is a real-world driving cycle for chassis dynamometer test defined by Japan Ministry of Land, Infrastructure and Transport. This test mode was firstly introduced to accurately evaluate the emission of light or medium-duty vehicles (gross weight of 3500 kg or less) in the real world. LDV9, LDV26, and LDV43 are real-world driving cycles defined by Japan Automobile Research Institute (JARI). The average speeds of JC08, LDV9, LDV26, and LDV43 were approximately 24, 9, 26, and 43 km/hr, respectively. Hot and cold startup modes were also investigated with each driving cycle. Figure S1 presents the time–speed profiles of the selected driving cycles in this work. In addition, a constant-speed driving mode of 30 km/hr was studied. Parameters of selected driving modes are listed in Table S1.
Instrumentation
Chassis dynamometer
The chassis dynamometer used for the experiments was optimized for light- to medium-duty vehicles. It was capable of simulating transient loads on such vehicles with the maximum speed of 160 km/hr. A direct current (DC) motor was installed to stimulate equivalent inertia weight, which ranged from 500 to 2475 kg with an adjustment pitch of 25 kg. A frame hydraulic floating mechanism was applied for dynamometer cradling. Therefore, mechanical loss of rotatory bearing was measured and compensated to ensure precise simulation. In addition, a vehicle cooling fan was operated and its wind speed (maximum wind speed: 110 km/hr) followed the vehicle speed during the test. Main specifications of this chassis dynamometer can be found in Table S3. During the experiments, the simulated inertia weights were maintained at 2490 kg and 2330 kg, while the rolling resistance values were controlled at 297.7 and 286.6 N, for diesel and gasoline vehicle tests, respectively.
Exhaust gas measurement devices
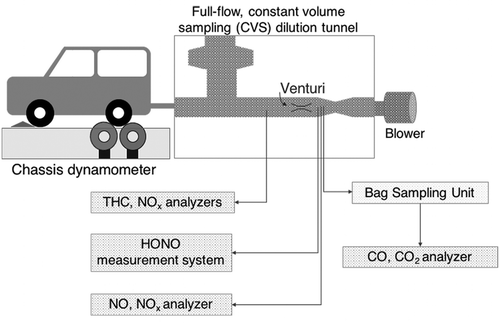
shows a schematic diagram of the exhaust gas measurement system. Exhaust emission from the tested vehicles was diluted using a full-flow, constant-volume-sampling (CVS) dilution tunnel. The exhaust was mixed with ambient air and cooled in the dilution tunnel. After that, samples (diluted exhaust) were introduced to the oven-housed Horiba MEXA-7200D measurement system to measure and continuously integrate CO, CO2, NOx, and THC concentrations. The bulk-stream gas sample was directed to the exhaust sampling unit (ESU), which recorded the CVS average flow and integrated mixing volume. The average flows of CVS were approximately 15 m3/min and 5 m3/min for diesel and gasoline vehicles tests, respectively. In front of the bulk-stream venturi, a gas sample for CO and CO2 measurement was extracted and introduced to sample bags in the bag sampling unit (BSU) for storage. Samples in the bags were then analyzed with the Horiba MEXA-7200D. Real-time NO and NOx concentrations were measured with the Horiba MEXA-1160 engine exhaust gas measurement device with sample gas extracted in front of the venturi tube.
In the same experiments, the sample for measurement of gaseous nitrous acid (HONO) was extracted in front of the bulk-stream venturi and continuously measured by an aqua-drag membrane denuder sampler (ADAMD) (Takenaka et al., Citation2004). Gaseous HONO was absorbed as nitrite ions on a thin water film created from a Milli-Q water flow, which was introduced at 70 µL/min from the lower end of ADAMD. At a sampling flow rate of 1.2 L/min, the system could attain a gas to solution volume ratio of 17,000. The HONO absorption efficiency of ADAMD was higher than 96% (Takenaka et al., Citation2004). Interference from NO2 in exhaust gas was identified by comparing the detected concentrations of the sample gas before and after passing through a Na2CO3-coated denuder. The NO2 interference was less than 0.1%. In the detection system, nitrite ions in the sampled solution reacted with the modified Griess–Saltzman reagent to form a purple azo dye, which was later detected by the Shimadzu SPD-20AV ultraviolet (UV) detector (λmax = 545 nm). The reagent contained 1 wt% sulfanilic acid, 0.01 wt% N-(1-napthyl) ethylenediamine dihydrochloride, and 5% sulfuric acid (Imanishi, Citation2012). The detection limit of this system was less than 0.1 ppb and the response time upon concentration change was less than 2 min.
Data acquisition and calculation
During the experiments, laboratory conditions (atmospheric pressure, temperature, relative humidity) were monitored by available devices of JARI. There were different time delays for various instruments because of the different sampling points and flows. Therefore, the recorded data of the instruments were then synchronized.
Ambient air, used as diluting gas in the CVS dilution tunnel, was cleaned and became NOx-free by passing through a high-efficiency particulate arresting (HEPA) filter and activated carbon. Before each test, a 20-min tunnel blank experiment was conducted twice in order to estimate the background constituents of the diluting gas (i.e., ambient air). During the experiments, the catalyzed particulate filter (DPF) was automatically (without external intervention) regenerated to reduce the soot loading on DPF. Our data excluded the emission during DPF regeneration period. Final gaseous concentrations were obtained after background removal and dilution factor (DF) multiplication.
Results and discussion
Emission of regulated gaseous compounds and CO2 from the tested vehicles under different driving cycles
The emission levels of regulated gaseous compounds and CO2 from the tested gasoline and diesel vehicles are summarized in .
Table 2. Emission characteristics of the tested vehicles under different driving cycles.
For the tested gasoline vehicle
Under the JC08 driving cycle (hot startup mode), the tested vehicle emissions were 0.02, 0.002, 0.003, and 286.6 g/km, respectively, for CO, THC, NOx, and CO2. It can be noted that the cold startup mode significantly elevated the emission levels of the tested vehicle, especially at low driving speed, as in the case of the LDV9 cycle test. These results were in agreement with previous studies. Alves (Citation2015) suggested that the vehicle’s emission control was ineffective in a short period during startup because of the excessive fuel–air ratio in the combustion chamber. The fuel–air ratio was enhanced, far above the effective stoichiometric ratio, to assist the ignition in the cold state. Therefore, the three-way catalytic converter (TWC) failed to perform complete oxidation and reduction to lower the levels of exhaust constituents. In addition, Weiss et al. (Citation2011) stated that the low catalyst temperatures resulted in ineffectiveness of the exhaust gas removal.
For the tested diesel vehicle
The emissions under hot and cold startup JC08 modes were, respectively, 0.01 and 0.11 g/km for CO, 0.001 and 0.01 g/km for NMHC, 0.38 and 0.51 g/km NOx, and 260.2 and 291.6 g/km for CO2. According to the JNL regulation, the regulatory limits for medium-duty diesel vehicles are 0.63, 0.024, and 0.25 g/km for CO, NMHC, and NOx emissions, respectively. The maximum allowable emissions are 0.84 g/km for CO, 0.032 g/km for NMHC, and 0.33 g/km for NOx (Ministry of Land, Infrastructure, Transport and Tourism, Citation2005). Results of the JC08 driving cycle test showed that the tested vehicle complied with the JNL regulation for CO and NMHC emission. In compliance with the regulation, the emission factor of NOx is calculated based on the following equation:
The obtained NOx emission of 0.4125 g/km from the tested diesel vehicle was 1.25 times higher than the regulatory limit of 0.33 g/km. In Japan, emission warranty mileage is 80,000 km for a passenger car or light- or medium-duty truck. By the time of the tests, the accumulated mileage of this tested car was over 80,000 km. High accumulative mileage has been reported to be one of the possible causes for the increase of NOx emission from diesel vehicles. Other reported causes include deterioration of the emission control system (e.g., exhaust gas circulation system), degradation of oxidation catalyst or particulate filter, and the test conditions (chassis dynamometer parameters, vehicle load) (Chen and Borken-Kleefeld, Citation2016; Carslaw et al., Citation2011; Yang et al., Citation2015; Kobayashi et al., Citation2008). Similar to the case of the tested gasoline vehicle, cold startup mode also elevated the levels of gaseous emissions from the tested diesel vehicle, which is in agreement with previous reports (Weiss et al., Citation2011; Alves, Citation2015; Windeatt et al., Citation2012).
Emission of reactive nitrogen species from the tested gasoline vehicle under different driving cycles
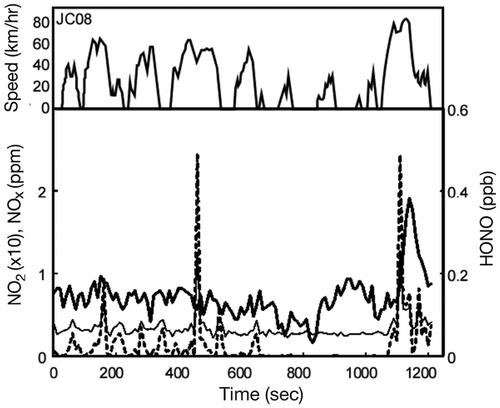
Reactive nitrogen species emission from the gasoline vehicle were measured under three cycles: JC08, JDV9, and JD26. shows the temporary profile of NO2, NOx, and HONO emission during the JC08 driving cycle test (hot startup mode). The plotted concentrations are the values before treating with the CVS dilution factor. The average HONO concentration over JC08 test mode was ~1.2 ppb (dilution factor = 8.4), with the highest concentration at the maximum speed of 81.6 km/hr. Calculated HONO/NOx ratios, which are listed in , varied from 0% to 0.95% with no specific trend. Overall, temporary concentrations of HONO and NO2 in exhaust gas were significantly low. Alvarez et al. (Citation2008) studied a light-duty gasoline vehicle at six driving cycles. The author reported rather low emissions of NOx (less than 0.057 g/km), with NO accounting for 97.8% of the total emission at both cold and hot startup modes.
Table 3. Summary of HONO/NOx ratios (unit: %).
Emission of reactive nitrogen species from the tested diesel vehicle under different driving cycles
Reactive nitrogen species emission from the diesel vehicle were measured under four real-driving cycles: JC08, JDV9, JD26, and one constant-speed running mode (30 km/hr). As in , the mass fraction of NO2 in total NOx emission increased from 21% to 31% as the average speed increased from 9 km/hr to 43 km/hr. The tested diesel vehicle was equipped with a diesel particulate active reduction system (DPR), which consists a diesel oxidation catalyst (DOC) placed in the upstream of a catalyzed particulate filter (DPF). The DOC facilitates the oxidation of NO to NO2 at low temperatures (e.g., below 250ºC). NO2 is then consumed in the oxidation of trapped soot on the DPF. However, the NO-to-NO2 conversion is limited by low-temperature kinetics, which might results in an imbalance between the amount of generated NO2 and the needed NO2 amount for soot oxidation (Russell and Epling, Citation2011). The smaller the amount of clogging soot in DPF, the smaller is the amount of consumed NO2 for oxidation. Excess NO2 is directed to the tailpipe without treatment, leading to an increase of observed NO2 emission.
Figure 3. Plot diagram of reaction nitrogen species emission from the tested diesel vehicle at different tested speeds. (a) Variability of NO2, NOx and HONO. (b) Variability of HONO/NO2 and HONO/NOx ratios.
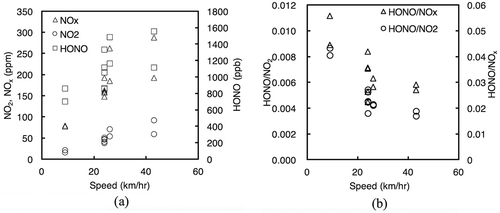
Also, shows the emission of nitrogen oxides increased as the average speeds increased, which is in great agreement with similar recent studies reported by Andersson et al. (Citation2014), Hayashizaki et al. (Citation2014), and Kwak et al. (Citation2014). The emission of HONO followed the same uphill trends. However, HONO/NO2 and HONO/NOx ratios behaved with the opposite trends. The lower the speeds, the higher were the observed ratios. shows the variability of HONO/NO2 and HONO/NOx ratios when the tested diesel vehicle changed its speed from 9 km/hr to 43 km/hr. A summary of HONO/NOx ratios over each driving cycle test can be found in . presents the temporal profile of reactive nitrogen species (NOx and HONO) emission from the tested diesel vehicle in hot start-up mode under various driving cycles. The plotted temporal concentrations were the values before being processed with CVS dilution factors. The average speed of each cycle is described in Table S2.
Figure 4. Temporal profile of reactive nitrogen emission from the tested diesel vehicle in hot startup mode under various driving cycles (HONO: thick line; NOx: dashed line). JC08 is a driving cycle for chassis dynamometer test defined by Japan Ministry of Land, Infrastructure, and Transport. LDV9, LDV26, and LDV43 are real driving cycles defined by Japan Automobile Research Institute.
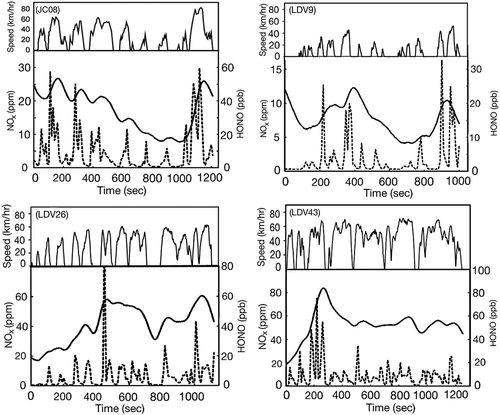
It is believed that HONO formation does not occur in the combustion chamber due to the high combustion temperature. Instead, HONO is likely formed in the vehicle tailpipe (Alicke, Citation2000; Spataro et al., Citation2014). Heterogeneous conversion of NO2 on particles in diesel exhaust is believed to significantly involve in the HONO formation (Arens et al., Citation2001; Kalberer et al., Citation1999; Salgado and Rossi, Citation2002). There has been a common understanding that diesel vehicles, regardless of the presence of the latest DPR after-treatment, emitted more particulate matter (PM) at lower average speed and less PM at higher speed (Alves, Citation2015; Andersson et al., Citation2014; S.H. Lee et al., Citation2015). Therefore, the decreasing trend of HONO to nitrogen oxides ratios with respect to the increasing speeds in our experiments was likely a consequence of associated NO2 increment and particulate matter decrement.
summarizes the comparison of traffic-induced and exhaust-induced HONO/NOx ratios, which were reported in previous studies and this study. The HONO/NOx ratio was 1.7% at a highway junction in Houston, TX, while such a ratio was 2.1 and 1.2% in Beijing and Hong Kong, China, respectively (Rappengluck et al., Citation2013; Yang et al., Citation2014; Xu et al., Citation2015). These ratios were much greater than the ratios of 0.29 and 0.8%, respectively, observed at two tunnels in the United States and Germany more than 15 years ago (Kurtenbach et al., Citation2001; Kirchstetter et al., Citation1996). The traffic composition studies suggested that the tunnels with higher heavy-duty vehicles flow were associated with higher HONO/NOx ratios. The results of our work suggested that HONO/NOx ratios in diesel-fueled vehicle exhaust were significantly higher than that in gasoline-fueled vehicle exhaust in the same real driving conditions, except for the cold startup LDV9 cycle (). However, our results were unfortunately not consistent with the single-vehicle study by Kurtenbach et al. (Citation2001), in which HONO/NOx ratios were comparable for a TWC-installed gasoline vehicle (0.66%) and an oxidation-catalyst-installed diesel vehicle (0.65%) under the same test conditions.
Table 4. Comparison of traffic-induced and exhaust-induced HONO/NOx ratios in various studies.
Summary
In summary, our study has conducted the chassis dynamometer measurement of reactive nitrogen species from the emission of two gasoline and diesel vehicles. HONO/NOx ratios of the tested vehicles under various real-world driving cycles were reported (see ). For the tested gasoline vehicles equipped with a TWC after-treatment device, HONO/NOx ratios ranged from 0% to 0.95% with very low average HONO concentrations. For the tested diesel vehicle equipped with a DPR device, HONO/NOx ratios varied from 0.16 to 1.00%. The results of the present work were compared with similar studies (see ). Measured HONO/NOx ratios were inversely proportional to the vehicle average speeds. This study has provided the profiles of direct HONO emission and HONO/NOx ratios from on-road vehicles under real-world driving conditions. Under the JC08 real driving cycles, the average HONO emission from the tested diesel vehicle (~868.8 ppb) was dramatically greater than that from the tested gasoline vehicle (1.2 ppb). Our study suggested that the unexpected exceedance of NO2 concentration for the soot oxidation process in the DPF was a significant factor that affected the production of HONO. The results also suggested a minor contribution of TWC-installed gasoline vehicle emission to the total HONO budget, while the contribution from the DPR-installed diesel emission is dramatically higher. More studies on single-vehicle HONO emissions should be conducted to establish a more precise traffic-induced HONO emission factor.
Supporting_Information_20160815.docx
Download MS Word (151.6 KB)Funding
This research was partially supported by the Japan Science and Technology Agency and Japan International Cooperation Agency, Science and Technology Research Partnership for Sustainable Development (SATREPS project entitled “Multi-beneficial measure for mitigation of climate change in Vietnam and Indochina countries by development of biomass energy”). This research was also partly supported by the Environment Research and Technology Development Fund (C-1001) of the Japan Ministry of Environment and by JSPS KAKENHI grant number 26740038.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
Notes on contributors
Ha T. Trinh
Ha T. Trinh is a Ph.D. student and Katsuma Imanishi is a former master’s student at the Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University.
Katsuma Imanishi
Ha T. Trinh is a Ph.D. student and Katsuma Imanishi is a former master’s student at the Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University.
Tazuko Morikawa
Tazuko Morikawa and Hiroyuki Hagino are researchers at the Japan Automobile Research Institute.
Hiroyuki Hagino
Tazuko Morikawa and Hiroyuki Hagino are researchers at the Japan Automobile Research Institute.
Norimichi Takenaka
Norimichi Takenaka is a professor at the Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University.
References
- Alicke, B. 2000. The Role of Nitrous Acid in the Boundary Layer. Ruperto Carola University, Heidelberg, Germany.
- Alvarez, R., M. Weilenmann, and J.-Y. Favez. 2008. Evidence of increased mass fraction of NO2 within real-world NO emissions of modern light vehicles—Derived from a Reliable online measuring method. Atmos. Environ. 42(19):4699–707. doi:10.1016/j.atmosenv.2008.01.046.
- Alves, C.A. 2015. Emissions from light-duty diesel and gasoline in-use vehicles measured on chassis dynamometer test cycles. Aerosol Air Qual. Res. 15(1):99–116. doi:10.4209/aaqr.2014.01.0006.
- Andersson, J., S. de Vries, M. Heaney, M. Keenan, J. Mansell, J. May, C. Favre, and D. Bosteels. 2014. On-road and chassis dynamometer evaluations of emissions from two Euro 6 diesel vehicles. SAE Int. J. Fuels Lubr. 7(3):919–34. doi:10.4271/2014-01-2826.
- Arens, F., L. Gutzwiller, U. Baltensperger, H.W. Gäggeler, and M. Ammann. 2001. Heterogeneous reaction of NO2 on diesel soot particles. Environ. Sci. Technol. 35:2191–99. doi:10.1021/es000207s.
- Bishop, G.A., and D.H. Stedman. 2015. Reactive nitrogen species emission trends in three light-/medium-duty United States fleets. Environ. Sci. Technol. 49(18):11234–40. doi:10.1021/acs.est.5b02392.
- Carslaw, D.C., S.D. Beevers, J.E. Tate, E.J. Westmoreland, and M.L. Williams. 2011. Recent evidence concerning higher NOx emissions from passenger cars and light duty vehicles. Atmos. Environ. 45 (39):7053–63. doi:10.1016/j.atmosenv.2011.09.063.
- Chen, Y., and J. Borken-Kleefeld. 2016. NOx emissions from diesel passenger cars worsen with age. Environ. Sci. Technol. 50(7):3327–32. doi:10.1021/acs.est.5b04704.
- Czader, B.H., Y. Choi, X. Li, S. Alvarez, and B. Lefer. 2015. Impact of updated traffic emissions on HONO mixing ratios simulated for urban site in Houston, Texas. Atmos. Chem. Phys. 15:1253–63. doi:10.5194/acp-15-1253-2015.
- Czader, B.H., B. Rappenglück, P. Percell, D.W. Byun, F. Ngan, and S. Kim. 2012. Modeling nitrous acid and its impact on ozone and hydroxyl radical during the Texas Air Quality Study 2006. Atmos. Chem. Phys. 12:6939–51. doi:10.5194/acp-12-6939-2012.
- Diao, L., A. Roy, B. Czader, S. Pan, W. Jeon, A.H. Souri, and Y. Choi. 2016. Modeling the effect of relative humidity on nitrous acid formation in the Houston Area. Atmos. Environ. 131(April):78–82. doi:10.1016/j.atmosenv.2016.01.053.
- Foley, K.M., S.J. Roselle, K.W. Appel, P.V Bhave, J.E. Pleim, T.L. Otte, R. Mathur, et al. 2010. Incremental Testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. Geosci. Model Dev. 3(1):205–26. doi:10.5194/gmd-3-205-2010.
- Hayashizaki, K., M. Hosoya, H. Urushibara, H. Hirabayashi, H. Honda, and Y. Nakamura. 2014. Emission characteristics from after-treatment system of medium and light duty engines. SAE Tech. Papers 1. doi:10.4271/2014-01-1501.
- Huai, T., T.D. Durbin, J. Wayne Miller, and J.M. Norbeck. 2004. Estimates of the emission rates of nitrous oxide from light-duty vehicles using different chassis dynamometer test cycles. Atmos. Environ. 38(38):6621–29. doi:10.1016/j.atmosenv.2004.07.007.
- Imanishi, K. 2012. Evaluation of Gaseous HONO Directly Emitted from Vehicles and Produced from NO2 on the Surface of Various Materials. Osaka Prefecture University, Osaka, Japan.
- Japan Automobile Manufacturers Association. 2009. Automotive Technologies in Japan: Solutions for Greater Safety, Improved Environmental Performance and Increased User Convenience. Japan Automobile Manufacturers Association. http://www.jama-english.jp/publications/autotech_2009.pdf.
- Kalberer, M., M. Ammann, F. Arens, H.W. Gäggeler, and U. Baltensperger. 1999. Heterogeneous formation of nitrous acid (HONO) on soot aerosol particles. J. Geophys. Res. 104:13825. doi:10.1029/1999jd900141.
- Kirchstetter, T.W., R.A. Harley, and D. Littlejohn. 1996. Measurement of nitrous acid in motor vehicle exhaust. Environ. Sci. Technol. 30(9):2843–49. doi:10.1021/es960135y.
- Kleffmann, J. 2007. Daytime sources of nitrous acid (HONO) in the atmospheric boundary layer. Chemphyschem 8:1137–44. doi:10.1002/cphc.200700016.
- Kleffmann, J., R. Kurtenbach, J. Lörzer, P. Wiesen, N. Kalthoff, B. Vogel, and H. Vogel. 2003. Measured and simulated vertical profiles of nitrous acid—Part I: Field measurements. Atmos. Environ. 37:2949–55. doi:10.1016/s1352-2310(03)00242-5.
- Kobayashi, S., S. Hasegawa, Y. Kondo, A. Fushimi, and K. Tanabe. 2008. Nitrogen dioxide emission from diesel vehicles equipped with exhaust aftertreatment systems. In Review of Automotive Engineering, 229–35. Tokyo, Japan: JSAE.
- Kurtenbach, R., K.H. Becker, J.A.G. Gomes, J. Kleffmann, J.C. Lörzer, M. Spittler, P. Wiesen, R. Ackermann, A. Geyer, and U. Platt. 2001. Investigations of emissions and heterogeneous formation of HONO in a road traffic tunnel. Atmos. Environ. 35:3385–94. doi:10.1016/s1352-2310(01)00138-8.
- Kwak, J.H., H.S. Kim, J.H. Lee, and S.H. Lee. 2014. On-road chasing measurement of exhaust particle emissions from diesel, CNG, LPG, and DME-fueled vehicles using a mobile emission laboratory. Int. J. Automot. Technol. 15:543–51. doi:10.1007/s12239-014-0057-z.
- Lee, B.H., G.W. Santoni, E.C. Wood, S.C. Herndon, R.C. Miake-Lye, M.S. Zahniser, S.C. Wofsy, and J.W. Munger. 2011. Measurements of nitrous acid in commercial aircraft exhaust at the alternative aviation fuel experiment. Environ. Sci. Technol. 45(18):7648–54. doi:10.1021/es200921t.
- Lee, S.H., J.H. Kwak, S.Y. Lee, and J.H. Lee. 2015. On-road chasing and laboratory measurements of exhaust particle emissions of diesel vehicles equipped with aftertreatment technologies (DPF, urea-SCR). Int. J. Automot. Technol. 16(4):551–59. doi:10.1007/s12239-015-0056-8.
- Li, Y., J. An, M. Min, W. Zhang, F. Wang, and P. Xie. 2011. Impacts of HONO sources on the air quality in Beijing, Tianjin and Hebei province of China. Atmos. Environ. 45(27):4735–44. doi:10.1016/j.atmosenv.2011.04.086.
- Ministry of Land, Infrastructure, Transport and Tourism. 2005. Japanese New Long-Term Emission. http://www.mlit.go.jp/jidosha/jidosha_tk10_000002.html (accessed May 30, 2015).
- Rappengluck, B., G. Lubertino, S. Alvarez, J. Golovko, B. Czader, and L. Ackermann. 2013. Radical precursors and related species from traffic as observed and modeled at an urban highway junction. J. Air Waste Manage. Assoc. 63:1270–86. doi:10.1080/10962247.2013.822438
- Russell, A., and W.S. Epling. 2011. Diesel oxidation catalysts. Catal. Rev. 53(4):337–423. doi:10.1080/01614940.2011.596429.
- Salgado, M.S., and M.J. Rossi. 2002. Flame soot generated under controlled combustion conditions: Heterogeneous reaction of NO2 on hexane soot. Int. J. Chem. Kinet. 34(11):620–31. doi:10.1002/kin.10091.
- Spataro, F., A. Ianniello, and A.I. Francesca Spataroa. 2014. Sources of atmospheric nitrous acid: State of the science, current research needs, and future prospects. J. Air Waste Manage. Assoc. 64(10):1232–50. doi:10.1080/10962247.2014.952846.
- Suarez-Bertoa, R., A.A. Zardini, V. Lilova, D. Meyer, S. Nakatani, F. Hibel, J. Ewers, M. Clairotte, L. Hill, and C. Astorga. 2015. Intercomparison of real-time tailpipe ammonia measurements from vehicles tested over the new world-harmonized light-duty vehicle test cycle (WLTC). Environ. Sci. Pollut. Res. 22(10):7450–60. doi:10.1007/s11356-015-4267-3.
- Takenaka, N., H. Terada, Y. Oro, M. Hiroi, H. Yoshikawa, K. Okitsu, and H. Bandow. 2004. A new method for the measurement of trace amounts of HONO in the atmosphere using an air-dragged aqua-membrane-type denuder and fluorescence detection. Analyst 129(11):1130. doi:10.1039/b407726a.
- Weiss, M., P. Bonnel, R. Hummel, A. Provenza, and U. Manfredi. 2011. On-road emissions of light-duty vehicles in Europe. Environ. Sci. Technol. 45(19): 8575–81. doi:10.1021/es2008424.
- Windeatt, J., G. Brady, P. Usher, H. Li, and A. Hadavi. 2012. Real world cold start emissions from a diesel vehicle. SAE Tech. Papers. University of Leeds, Leeds, UK. doi:10.4271/2012-01-1075.
- Xu, Z., T. Wang, J.Q. Wu, L.K. Xue, J. Chan, Q.Z. Zha, S.Z. Zhou, P.K.K. Louie, and C.W.Y. Luk. 2015. Nitrous acid (HONO) in a polluted subtropical atmosphere: Seasonal variability, direct vehicle emissions and heterogeneous production at ground surface. Atmos. Environ. 106:100–9. doi:10.1016/j.atmosenv.2015.01.061.
- Yang, L., V. Franco, P. Mock, R. Kolke, S. Zhang, Y. Wu, and J. German. 2015. Experimental assessment of NO emissions from 73 Euro 6 diesel passenger cars. Environ. Sci. Technol. 49(24):14409–15. doi:10.1021/acs.est.5b04242.
- Yang, Q., H. Su, X. Li, Y. Cheng, K. Lu, P. Cheng, J. Gu, S. Guo, M. Hu, L. Zeng, T. Zhu and Y. Zhang. 2014. Daytime HONO formation in the suburban area of the megacity Beijing, China. Sci. China Chem. 57:1032–42. doi:10.1007/s11426-013-5044-0.