ABSTRACT
A novel nanosize metallic calcium/iron dispersed reagent was synthesized and tested as coagulant/catalyst in a hybrid zero valent iron (ZVI)/H2O2 oxidation process to treat leachate. Two different types of leachates, one from municipal solid waste (MSW) tipping hall (MSWIL) and second from an MSW landfill site (MSWLL), were collected and characterized. The morphology, elemental composition, and mineral phases of the nano-Ca/CaO and nano-Fe/Ca/CaO were characterized by scanning electron microscopy–electron dispersive spectroscopy (SEM-EDS) and x-ray powder diffraction (XRD) analysis. The coagulation process with 2.5 g L−1 nano-Ca/CaO attained 64.0, 56.0, and 20.7% removal of color, chemical oxygen demand (COD), and total suspended solids (TSS) in MSWLL. With only 1.0 g L−1 of nano-Fe/Ca/CaO, relatively high color, COD and TSS removal was achieved in MSWLL at 67.5, 60.2, and 37.7%, respectively. The heavy metal removal efficiency reached 91–99% after treatment with nano-Fe/Ca/CaO in both leachate samples. The coupling process, using 1.0 g L−1 of nano-Fe/Ca/CaO and 20 mM H2O2 doses, achieved enhancement removal of color, COD, and TSS, up to 95%, 96%, and 66%, respectively, without initial pH control. After this treatment, the color, COD, TSS, and heavy metals were significantly decreased, fitting the Korean discharge regulation limit. A hybrid coupled zero valent iron (ZVI)/H2O2 oxidation process with novel nanosized metallic calcium/iron dispersed reagent proved to be a suitable treatment for dealing with leachate samples.
Implications: Conventional treatments (biological or physicochemical) are not sufficient anymore to reach the level of purification needed to fully reduce the negative impact of landfill leachates on the environment. This implies that new treatment alternatives species must be proposed. A coupled zero valent iron (ZVI)/H2O2 oxidation process proved to be a suitable treatment for dealing with leachate samples. Coagulation with nFe/Ca/CaO allows 91–99% of heavy metals removal. The coupled coagulation–oxidation process by nFe/Ca/CaO reveals excellent ability to treat leachate. After coupled treatment the color, COD, and TSS were also much lower than the discharge regulation limit.
Introduction
Sanitary landfill for municipal solid waste (MSW) disposal continues to be widely accepted and used in several countries. This method generally offers lower operation and maintenance costs compared to others (Wang et al., Citation2002; Tatsi et al., Citation2003). MSW incineration is another waste disposal choice, but only when simpler and less expensive choices are unavailable. However, entering the incineration facility, waste trucks make their way to the tipping hall and the waste is offloaded. Water microspray is employed to suppress dust generation. This process results in the generation of fresh leachate/wastewater (U.S. Environmental Protection Agency (EPA), Citation1995; Ehring Citation1982). However, both leachates from MSW landfill sites/tipping halls are often defined as hazardous and heavily polluted wastewaters (Ehring, Citation1982; EPA, Citation1995; Wang et al., Citation2002; Tatsi et al., Citation2003). Landfill leachate may contain large amounts of organic contaminants, which can be measured in terms of chemical oxygen demand (COD), ammonia, halogenated hydrocarbons, suspended solids, heavy metals, and inorganic salts (Amokrane et al., Citation1997; Wang et al., Citation2002; Tatsi et al., Citation2003). The specific composition of the leachate determines its relative treatability (Mott et al., Citation1987; Diamadopoulos, Citation1994; Kang et al., Citation2002a; Aguilar et al., Citation2005). With the continuous hardening of the discharge standards in most countries and the aging of landfill sites with more and more stabilized leachates, conventional treatments (biological or physicochemical) are not sufficient to reach the level of purification needed to fully reduce the negative impact of landfill leachates on the environment (Lema et al., Citation1988; Petruzzelli et al., Citation2000; Trebouet et al., Citation2001; Marttinen et al., Citation2002). Therefore, new treatment alternatives must be proposed (Renou et al., Citation2008; Nwabanne et al., Citation2009; Dabhade et al., Citation2009; Uemura, Citation2010).
Advanced oxidation processes (AOPs) involving the production of highly reactive hydroxyl radicals (•OH) have shown great potential for organic pollutant treatment in wastewater. On the other hand, the catalytic decomposition of hydrogen peroxide by ferrous ions (i.e., Fenton’s reagent) was first described by Fenton and is one of the most commonly used AOPs for wastewater treatment (Huang et al., Citation1993; Trujillo et al., Citation2006; Hermosilla et al., Citation2009). Recently there have been numerous reports about landfill leachate treatment by the classical Fenton process (H2O2/ferrous salt) (Trujillo et al., Citation2006; Deng et al., Citation2007; Zhang et al., Citation2005; Zhang et al., Citation2009). However, its major disadvantage is that when the chloride or sulfate salt of iron (i.e., FeCl3 and FeSO4) is used, the production of Fe(OH)3 sludge requires further separation and proper disposal, with a consequent increase in operation costs (Benatti et al., Citation2006). In addition to the classical Fenton reaction, recent studies have focused on the Fenton process with alternative iron sources (Barbusinski and Majewski, Citation2003) such as elemental iron (Fe°), which is one of the most widely used zero-valent metals (such as Fe°, Zn°, Sn°, and Al°) because it is readily available, inexpensive, and nontoxic (Joo and Cheng, Citation2006). These results have opened promising perspectives since their conception, as fast and economical alternative iron sources for Fenton reactions. Some authors document information on zero-valent iron (ZVI) effectiveness in Fenton oxidation in a variety of contaminants, such as treatment of methyl tert-butyl ether (MTBE) (Bergendahla and Thies, Citation2004), discoloration of azo dye compounds (Barbusinski and Majewski, Citation2003), dichlorodiphenyltrichloroethane (DDT) degradation (Boussahel et al., Citation2007), phenolic compound removal (Barbusinski and Majewski, Citation2003; Kallel et al., Citation2009a), and COD reduction (Barreto et al., Citation2008; Kallel et al., Citation2009b). On the other hand, not many studies consider heavy metal treatment in landfill leachates (Jung et al., Citation2009).
Recently, utilization of nanorange (1–200 nm) catalysts in water purification has become more popular (Kallel et al., Citation2009a; Kallel et al., Citation2009b). In terms of catalysts or reagents for water purification, nanoparticles offer a high specific surface area (SSA), and in some cases (e.g., for nano-zero-valent iron [nZVI]) different properties in comparison to their bulk phase might positively influence the reaction efficiency (Barreto et al., Citation2008). This can be an advantage for the treatment of large contaminant molecules such as dyes, organic loads, and heavy metals, which are considerably limited in diffusion through microporous catalysts.
On the other hand, we have previously reported that treatment of waste containing multipollutants (i.e., persistent organic pollutants [POPs], heavy metals, and radioactive cesium) with a nanometallic calcium/iron dispersion mixture is most effective (Mallampati et al., Citation2012; Mitoma et al., Citation2013; Mallampati et al., Citation2014a; Mallampati et al., Citation2014b; Mallampati et al., Citation2015). Nanometallic calcium/iron has been never used as a catalyst for the purpose of multicontaminant treatment in landfill leachate. The nanometallic iron/calcium is a promising catalyst for oxidative processes using H2O2. Its catalytic properties are often attributed to its Fe° content, which is considered to be the key parameter in Fenton-like reactions (Hanna et al., Citation2008; Klara et al., Citation2012; Xue et al., Citation2009). Although for larger particles the formation of a passivating shell can prevent complete oxidation of iron, nanosized iron may be more sensitive in this process. Furthermore, the magnetic properties of nanosized iron allow easy and complete separation from the treated medium by means of magnetic separation (Sun et al., Citation1998).
The aim of this investigation is to synthesize and characterize a novel nanosize metallic calcium/iron dispersed reagent as a coagulant/catalyst in the hybrid zero valent iron (ZVI)/H2O2 oxidation process to treat two different type leachates, one from an MSW tipping hall (MSWIL) and the second from an MSW landfill site (MSWLL). Contrary to other studies in which H2O2 is added to the raw leachate together with the coagulant (Hanna et al., Citation2008; Klara et al., Citation2012; Xue et al., Citation2009), the idea developed here was to use in a first step nano-Fe/Ca/CaO as coagulant in order then to use the nonprecipitated Fe remaining in dissolution as a catalyst in the oxidation process. By the nanocoagulation process, the colloidal particles’ electrostatic surface potential is neutralized. The resulting destabilized particles stick together into larger floes. These floes are able to aggregate with suspended polluting matter. Therefore, nanocoagulantion initiates organic compounds encapsulation through the formation of immobile salts, for example, by reacting with water, yielding a Ca-associated (CaCO3/Ca(OH)2) precipitate that encloses/binds organic particle surfaces (Mallampati et al., Citation2012; Mallampati et al., Citation2014b) (eq 1). On the other hand, with nFe/Ca/CaO, the iron particle addition in the aqueous phase dissolves ferrous ions and electrons transfer to react with the organic molecules on the iron’s surface. This has proved to be a fast and successful process to reduce a variety of organic compounds (eq 2).
In the zero valent iron (ZVI)/H2O2 oxidation process, ferrous ion reacts with hydrogen peroxide, producing a hydroxyl radical. (OH). The oxidation reaction is characterized by the catalytic decomposition of H2O2 as described in the following (eqs 3–5). Hydroxyl radicals created by the process can be scavenged by excess Fe2+ (eq 4). The major reactions for the generation of oxidizing radicals may be represented in the following (eq 5). The nano-iron comes from the excess of coagulant (nFe/Ca/CaO), rendering unnecessary further additions. Furthermore, treated compounds that do not precipitate in the coagulation process may be removed in the subsequent zero valent iron (ZVI)/H2O2 oxidation process. Therefore, this hybrid coupled coagulation–Fenton process with nFe/Ca/CaO and H2O2 appears to be a suitable treatment of leachate.
The advantages of this coupled process are threefold: (i) Coagulation considerably reduces leachate COD and color, thus leading to a lower requirement for H2O2, (ii) nanosize iron (Fe°) comes from the excess of coagulant (nano-Fe/Ca/CaO), rendering unnecessary further Fe addition, and (iii) unprecipitated/untreated compounds that do not precipitate/are not treated in the coagulation process may be removed in the subsequent Fenton process. By this treatment method the production of residual sludge can be minimized/eliminated. The coagulation with nano-Fe/Ca/CaO was optimized to achieve the best starting conditions for the Fenton process by generating a low COD and high Fe concentration. The morphology, elemental composition, and mineral phases of the nano-Fe/Ca/CaO were characterized. The Fenton process was also optimized in terms of the amount of nano-Fe/Ca/CaO and H2O2 doses.
Material and methods
Leachate sample collection and characterization
Two leachate samples were taken by polyethylene bottles from the municipal sanitary landfill, located in Ulsan, South Korea, in July 2015. First, fresh leachate was collected from the municipal solid waste reception area/tipping hall and named MSWIL. Next, raw leachate was collected from the influent end of the detention pond near the entrance of the landfill and named MSWLL. Both leachate samples were stored in a cold room in the laboratory at 4ºC to minimize biological and chemical reactions (American Public Health Association [APHA], American Water Works Association [AWWA], and Water Pollution Control Federation [WPCF], Citation1995). The leachate quality main parameters (pH, total suspended solids [TSS] color, COD, and heavy metals) were measured according to standard methods (APHA, Citation1992; APHA, AWWA, and WPCF, Citation1992, Citation1995). The pH measurement was performed using a pH meter (HACH sension 4) and a pH probe (HACH platinum series pH electrode, model 51910, HACH Co., USA). Color measurements were reported as true color (filtered using 0.45-µm filter paper) assayed at 400 nm using a DR 2800 HACH spectrophotometer adapted from the Standard Methods for the Examination of Water and Wastewater (APHA, AWWA, and WPCF, Citation1992, Citation1995). The effects of filtration on color removal were corrected by control sample (pure water) comparison. COD was estimated by the standard dichromate method ASTM D1252 (ASTM, Citation2012). Heavy metal concentrations were measured using inductively coupled plasma–optical emission spectrometry (ICP-OES; Varian, 720-ES). Removal efficiency of color, COD, TSS and heavy metals was obtained using the following equation:
where Ci and Cf are the initial and final concentrations of leachate before and after treatment, respectively.
Nanometallic calcium/iron dispersed reagent synthesis
Solvent-free production of the coagulant/catalyst reagent, a dispersed mixture of nanometallic Ca/CaO (nCa/CaO) and nano-Fe/Ca/CaO (nFe/Ca/CaO), was through a planetary ball-milling process. Unlike conventional methods such as chemical synthesis and vapor-phase condensation, which typically involve toxic chemicals, sophisticated equipment, and extensive labor, the precision ball-milling method relies solely on mechanical impact forces generated by stainless-steel beads in a high-speed rotary chamber to break down the (nCa/CaO and nFe/Ca/CaO) particles (Mallampati et al., Citation2012; Mallampati et al., Citation2014a; Mallampati et al., Citation2014b; Mallampati et al., Citation2015). The system uses no toxic solvents and is scalable to large-scale manufacturing.
Granular particles of metallic calcium (Ca) (99%, 2–2.5 mm particle size distribution; 0.43–0.48 m2 g−1 surface area) and iron (Fe) (0.15 mm diameter) were purchased from Kishida Chemical Co. Ltd. The nCa/CaO and nFe/Ca/CaO (dry system) systems were prepared by combining metallic Ca and CaO, and Fe, Ca, and CaO, respectively, through a mechanochemical process. At room temperature under Ar, dry CaO (preheated at 825°C for 2 hr), and dry metallic Ca/iron (Fe) (Ca/CaO = 2/5; Fe/Ca/CaO = 2/2/5 ratios) were introduced into a planetary ball mill (20 pieces SUS, 32 g/ball). Milling was conducted for 1 hr at 600 rpm to achieve a rotation-to-revolution ratio of 1 to –2. The conditions for the dispersed nanosystems preparation were established after performing several experiments to ascertain optimal conditions (Mallampati et al., Citation2012; Mallampati et al., Citation2014a; Mallampati et al., Citation2014b; Mallampati et al., Citation2015). After preparation, the nCa/CaO and nFe/Ca/CaO systems were sampled for additional leachate treatment studies.
Nanometallic calcium/iron dispersed reagent characterization
To characterize the nCa/CaO and nFe/Ca/CaO composites, we employed scanning electron microscopy combined with electron dispersive spectroscopy (SEM-EDS), microanalysis, and elemental map and semiquantitative analyses (JSM6510A equipped with a Si(Li) probe with 138 eV resolution; JEOL). The SEM images were obtained at a magnification of 1,000× and 10,000×. Transmission electron microscopy (TEM) analysis was also carried out to characterize single particle of nFe/Ca/CaO composites (H-8100, Hitachi).
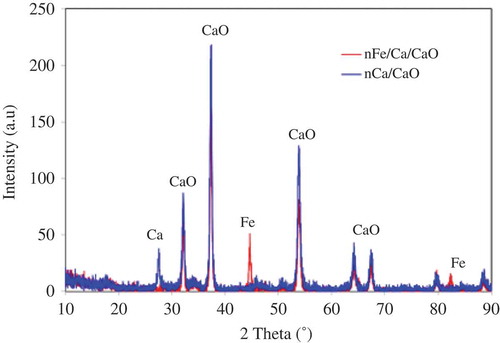
X-ray powder diffraction (XRD) analyses were performed to identify crystalline phases on the surface of nCa/CaO and nFe/Ca/CaO composites, and were conducted using XRD (RINT, Rigaku) using Cu Kα Ni-filtered radiation (λ = 15.418 Å). XRD data were collected for values of 2θ from 10 to 90º. In the cases of freshly prepared samples, a drying step under inert atmosphere was performed and the samples were subjected directly to analysis. Particle size distributions of nCa/CaO and nFe/Ca/CaO were performed by a ZETA-SIZER, nano-series, Malvern (nano-ZS) in ethanol.
Leachate treatment with nanometallic calcium/iron dispersed reagents
Prior to the experiments, the leachate-containing bottles were sufficiently mixed in order to make homogeneous conditions for all samples. A preliminary set of blank assays was performed in order to characterize the evolution of pH, COD, TSS, color, and heavy metal removal without addition of any coagulants. Next, the two types of coagulants used were nCa/CaO and/or nFe/Ca/CaO. Different doses, such as adding 0.5, 1.0, and 2.5 g L−1 of coagulant (nCa/CaO or nFe/Ca/CaO), were slowly added to a 1000-mL leachate sample in Erlenmeyer flasks kept in cool water. The coagulant and the leachate were dynamically mixed for 10 min at 500 rpm, followed by slow mixing for 30 min at 60 rpm by a magnetic stir bar and allowed to settle for 10 min. To study the effects of coagulant (nCa/CaO or nFe/Ca/CaO) doses on the coagulation process, supernatant was withdrawn using a pipette from a point located ~2 cm below the liquid level for pH, COD, TSS, color, and heavy metal measurement. For the purpose of coagulation, the initial pH of leachate was not adjusted, directly treated. The laboratory procedures met all quality assurance/quality control (QA/QC) requirements, including the use of triplicate experimental replication and matrix spikes.
The zero valent iron (ZVI)/H2O2 oxidation treatment
In order to improve the nFe/Ca/CaO efficiency on color, COD, and TSS removal we explored hydrogen peroxide (H2O2) addition, creating the Fenton treatment. In the zero valent iron (ZVI)/H2O2 treatment experiments, a fixed dose of H2O2 (20 mM) was added to the coagulation process. Leachate samples were treated in precisely the same manner described in the preceding, and supernatant was sampled to determine COD, color, and TSS so that H2O2 addition effects on Fenton coagulation (nFe/Ca/CaO) could be studied. The treatment efficiency was measured with respect to nFe/Ca/CaO dose and addition of H2O2 solution, respectively. For COD analysis, all tests were made after the total removal of residual H2O2 from all samples, because the residual H2O2 increases the COD value since it acts as a reductant, especially in chromate-based analysis of COD (Barbusinski and Filipek, Citation2001). Deng’s method was used to eliminate the remaining H2O2 and stop the oxidation reaction (Deng, Citation2007).
Results and discussion
Leachate characterization
The main characteristics of the leachate are presented in . All values presented in the table are the average values of three replications. suggests that the both leachates have moderate concentrations of color, organic matter, and heavy metals. However, the heavy metals concentrations are high in MSWIL. The high levels of pH and heavy metals in MSWIL may be due to the presence of some type of heavy-metal-contaminated waste or chemical agent (cleaning, deodorizing), or due to some very alkaline waste having recently been freshly tipped. Further, the values of color, COD, and heavy metals exceed the standard discharge limits of the Korean Water Quality Standards (Bae et al., Citation2012) (). The presence of high concentrations of color and COD was supplemented by the presence of dissolved organics. These organic compounds may present in the form of recalcitrant humic acid and carbohydrate materials (Artiola and Fuller, Citation1982; Langlais et al., Citation1991). This implies that there is a need to treat the leachate before it can be discharged.
Table 1. Properties of the two different leachates used for this study.
Nanometallic calcium/iron dispersed reagent characterization
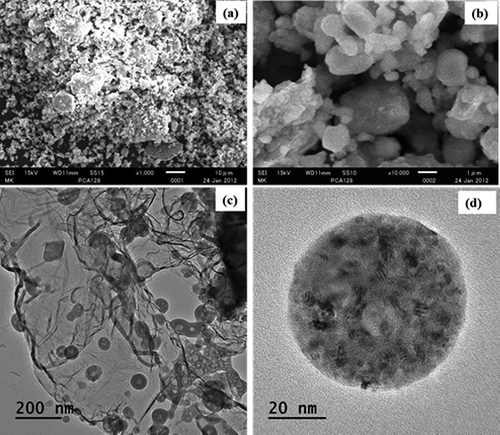
SEM images of the nCa/CaO and nFe/Ca/CaO composite were taken at two different magnifications, 1,000× and 10,000×. Microstructure images are presented in . The nCa/CaO and nFe/Ca/CaO particles exhibit porous surfaces, as shown in and . We also observe that the nCa/CaO and nFe/Ca/CaO microstructures include solid spherical and irregularly shaped amorphous particles that formed chain-like aggregates, which are apparent at higher magnifications ( and ). The maximum particle size distribution observed was 263 and 206 nm for nCa/CaO and nFe/Ca/CaO, respectively. Through XRD analysis, the broad peaks at 2θ of 45º and 85º confirmed the presence of elemental iron (Fe) in the nFe/Ca/CaO sample. A similar result was obtained by Giasuddin (Citation2007). This peak is referred to as the XRD spectrum, as shown in . However, the surface compositions of the nCa/CaO and nFe/Ca/CaO particles were slightly different chemically.
Figure 1. SEM and TEM microstructure of nano-Fe/Ca/CaO at different magnifications: (a) nFe/Ca/CaO at 1,000× magnification and (b) nFe/Ca/CaO at 10,000× magnification, (c) TEM analysis of single particle at 200nm, and (d) TEM analysis of single particle at 20 nm.
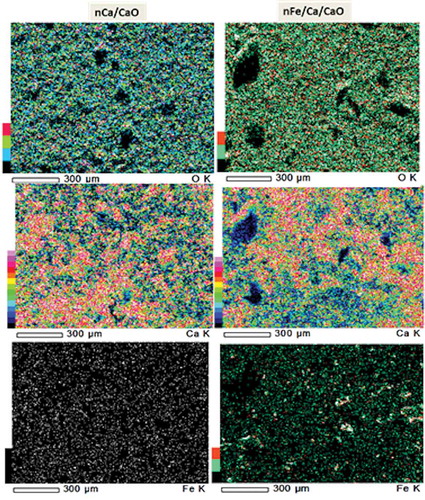
The surface composition was analyzed using an EDS attached to the SEM. illustrates the EDS elemental maps of the nCa/CaO and nFe/Ca/CaO. shows the percentage of elements present. All values presented in the table are the average values of three experimental replications. The EDS elemental mapping image of nCa/CaO shows that the surface is primarily comprised of calcium (Ca) and oxygen (O2). The nFe/Ca/CaO surface is primarily comprised of calcium (Ca), oxygen (O2), and iron (Fe) (). As presented in , Ca, at 65.8 mass percent, was the most abundant element present on the surface of nCa/CaO, followed by O at 34.2 mass percent. In nFe/Ca/CaO, Fe at 6.2 mass percent was observed, in addition to Ca at 62.4 and O at 31.4 mass percent, respectively.
Table 2. Surface chemical compositions of nCa/CaO and nFe/Ca/CaO.
Leachate treatments with nanometallic calcium/iron dispersed reagents
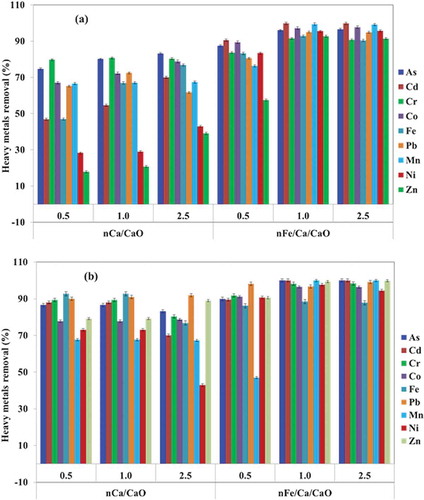
A preliminary set of blank assays was performed in order to compare the efficiency of removal of color, COD and TSS with different doses of nCa/CaO and/or nFe/Ca/CaO coagulation in both MSWIL and MSWLL samples. shows the averages values of three experimental replications. As presented in , nCa/CaO treatment revealed low color, COD, and TSS removal capacity, although by adding 2.5 g L−1, it attained 29.2, 12.7, and 5.7% of color, COD, and TSS, respectively in MSWIL. Noticeably, nCa/CaO showed better removal efficiency, that is, 64.0, 56.0, and 20.7% for color, COD, and TSS, respectively, in MSWLL. On the other hand, only with 1.0 g L−1 of nFe/Ca/CaO dose there was relative enhancement removal of color of about 45.8 and 67.5% from 240 to 130 nTu and 222 to 72 nTu achieved in both MSWIL and MSWLL samples, compared with nCa/CaO treatment (). Similarly, the COD concentration was reduced (33.9 and 60.2%) from 472 to 312 mg L−1 and 352 to 130 mg L−1 in both MSWIL and MSWLL leachate samples, respectively, with 1.0 g L−1 nFe/Ca/CaO. We observed more evidence of this removal trend in TSS, with 1.0 g L−1 nFe/Ca/CaO (17.0 and 37.7%) in MSWIL and MSWLL, respectively. Color removal efficiency, COD, and TSS were higher than when other conventional coagulation methods are applied (Amokrane et al., Citation1997; Petruzzelli et al., Citation2000; Trebouet et al., Citation2001; Tatsi et al., Citation2003).
Table 3. Evolution of landfill leachate characteristics during varying coagulation concentrations (0.5, 1.0, and 2.5 g L−1; nCa/CaO and nFe/Ca/CaO) and treatment processes.
On the other hand, we do not observe further color, COD, and TSS reduction by coagulant dose increase to 2.5 g L−1 in leachate samples with nFe/Ca/CaO (). This behavior may be explained by the charge neutralization theory (Melia, Citation1972; Sincero and Sincero, Citation2003; Wang et al., Citation2002). When the coagulant nCa/CaO was added to the leachate, cations and hydrolyzed products interacted with negative colloids to neutralize their charges, which promoted colloid destabilization. In the appropriate dosage, colloids can absorb cations and become positively charged, and hence may stablize again as a result of electrical repulsion. This process diminishes the capacity for organic matter removal (Melia, Citation1972; Sincero and Sincero, Citation2003; Wang et al., Citation2002).
However, the highest removal of color and COD was achieved at a dose of 1.0 g L−1 of nFe/Ca/CaO in the MSWLL sample compared with the MSWIL (). This further may be because, at pH 7, nFe/Ca/CaO can effectively react with organic substances in the MSWLL leachate. In general, organic colloids are hydrophilic while inorganic colloids are hydrophobic. The primary charges on hydrophilic colloids are due to polar groups such as carboxylic (-COOH) and amine (-NH2) groups (Sincero and Sincero, Citation2003). Under acidic conditions the H+ counterions neutralize the primary charges, reducing the zeta potential and the force of repulsion between particles (Melia, Citation1972). At pH 7, the highest removal of COD, TSS, and color occurred, resulting from agglomeration and settling of the colloids. Wang et al. (Citation2002) observed that within the pH range 3–8, coagulation–flocculation was most effective in the lower pH range for landfill leachate. On the other hand, under the alkaline condition of pH 12.3, MSWIL nFe/Ca/CaO reactivity with organic substance is less, because of the large amount of nonbiodegradable organic matter. In contrast, in both leachate samples, TSS removal was comparatively decreased in terms of color and COD removal (). This may be due to the precipitation of presented metallic Ca and CaO to CaCO3/Ca(OH)2 (Mallampati et al., Citation2014a; Mallampati et al., Citation2015).
Heavy metal removal efficiency reached about 91–99% after treatment with 1.0 g L−1 of nFe/Ca/CaO ( and ). Of note, after treatment with nFe/Ca/CaO, the heavy metal concentrations were lower than the discharge limit of the Korean Water Quality Standards (Bae et al., Citation2012). Furthermore, the effects of nCa/CaO and nFe/Ca/CaO dosage on heavy metal removal in MSWIL and MSWLL were studied and the results are presented in . With 1.0 g L−1 of nFe/Ca/CaO, dose enhancement of heavy metal removal was achieved in comparison with nCa/CaO in both leachate MSWIL and MSWLL samples.
The zero valent iron (ZVI)/H2O2 oxidation treatment
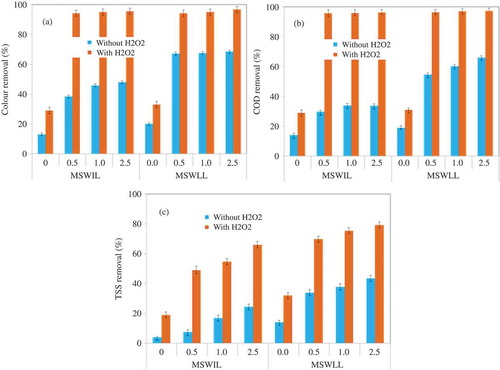
The highest amounts of removal of color, COD, TSS, and heavy metals were achieved at a dosage of 1.0–2.5 g L−1 of nFe/Ca/CaO in the coagulation process for both MSWIL and MSWLL samples. However, the color, COD, and TSS concentrations in both MSWIL and MSWLL samples still exceeded the standard discharge limits of the Korean Water Quality Standards (Bae et al., Citation2012). Generally, the coagulation stage is essential to reduce total suspended solids, organic content, and color in order to improve the later treatment’s efficiency. Leachate’s brown color is mainly due to the presence of high concentrations of humic substances, which represent the majority of organic compounds in the leachate (Petruzzelli et al., Citation2000; Renou et al., Citation2008). Additionally, these substances are considered hydroxyl radical scavengers. Lower turbidity values favor oxidation performance. Further, in order to improve the nFe/Ca/CaO effiency of color, COD and TSS removal, we studied the influence of hydrogen peroxide (H2O2) (coagulation–oxidation process) on leachate treatment with the same manner as for the nFe/Ca/Cao process. Blank assays were also done in order to compare the efficiency of these systems and the classical Fenton process. The removal effect on color, COD, and TSS with and without H2O2 addition on different dosages of nFe/Ca/CaO, during the coagulation–oxidation process, in both MSWIL and MSWLL samples is as shown in . Color, COD, and TSS removal increased with nFe/Ca/CaO dosage in the 0.5–2.5 g L−1 range. Addition of H2O2 enhanced color, COD, and TSS removal, at 95%, 96%, and 66%, respectively, with the 1.0 g L−1 nFe/Ca/CaO dose, compared to no H2O2 addition, in both MSWIL and MSWLL samples (–). As described in the preceding, the increase of nFe/Ca/CaO dosage to 2.5 g L−1 did not improve removals significantly.
However, Fenton oxidation was hindered at pH values above 2 because the absence of H+ can inhibit H2O2 decomposition and reduce the production of .OH radicals. Therefore, the oxidation potential of .OH decreased as pH increased (Zhang et al., Citation2005; Zhang et al., Citation2009). pH and the H2O2/Fe molar ratio are the most important variables in Fenton process. Several authors have demonstrated that optimal H2O2/Fe molar ratio is independent of initial COD and ferrous iron dosage (Zhang et al., Citation2005; Deng Citation2007). Furthermore, Zhang et al. (Citation2005, Citation2009), report that the optimal H2O2/Fe molar ratio is related to initial pH. However, in the present work, we achieved perfect COD and color removal without pH control (i.e., we directly used our nFe/Ca/CaO composite).
Generally, during the Fenton oxidation process with the chloride or sulfate salt of iron (i.e., FeCl3 and FeSO4) and, mainly during the pH adjustment to 8, a large amount of flocs of various sizes in the wastewater/leachate were observed. According to Walling and Kato (Citation1971), the small flocs were ferric hydroxo complexes formed by complex chain reactions of ferrous and hydroxide ions. Therefore, the major disadvantage is the production of Fe(OH)3 sludge that requires further separation and proper disposal, with a consequent increase in operation costs (Benatti et al., Citation2006). However in the present study using nFe/Ca/CaO, the generation volume of the residual chemical/catalytic sludge was very low compared to other reported studies with chloride or sulfate salt of the iron (i.e., chemical/catalytic sludge generation was about 40 mL) (Benatti and Tavares, Citation2012; Miled et al., Citation2015). However, in the present study, the volume is very low, only about 8–10 mL. This may be due to the fact that the nanosize iron (Fe°) comes from the excess of coagulant (nano-Fe/Ca/CaO), rendering unnecessary further Fe addition, and unprecipitated/untreated compounds that do not precipitate/are not treated in the coagulation process may be removed in the subsequent Fenton process. These results indicate that the coupled coagulation–Fenton process with nFe/Ca/CaO and H2O2 appears to be a suitable treatment of the leachate without any initial pH control.
The mechanism of the Zero valent iron (ZVI)/H2O2 process with a nanometallic calcium/iron dispersed reagent
portrays a schematic diagram of a possible pathway for the enhanced coupled H2O2 oxidation process for the treatment of landfill leachate by the novel nanosized metallic calcium/iron dispersal reagent. Colloidal particles generally carry a negative electrical charge. They are surrounded by an electrical double layer (due to attachment of positively charged ions from the ambient solution) and thus inhibit the particles getting too close. They remain finely divided and don’t agglomerate. Due to their low specific gravity, they do not settle out (). When the double layer is compressed by the nanocoagulant (nFe/Ca/CaO) (generally positively charged in this case, Ca2+/Fe2+), the particles’ electrostatic surface potential is neutralized. The resulting destabilized particles stick together sufficiently when contact is made. Subsequent gentle and prolonged (several minutes) mixing cements the microscopic coagulated particles into larger floes. These floes are able to aggregate with suspended polluting matter. When increased sufficiently in size and weight, the particles settle to the bottom (). Consequently, by the nanocoagulation process, the particle surface area increases and the heavy metal mobility decreases because of the higher adsorption rate (Mallampati et al., Citation2012; Mallampati et al., Citation2014a). Therefore, nanocoagulantion initiates heavy metals/organic compounds encapsulation through the formation of immobile salts, for example, by reacting with water, yielding a Ca-associated (CaCO3/Ca(OH)2) precipitate that encloses/binds heavy metal/organic particle surfaces () (eq 1) (Mallampati et al., Citation2012; Mallampati et al., Citation2014b).
Figure 6. A possible pathway of coupled zero valent iron (ZVI)/H2O2 oxidation process for the treatment of landfill leachate: (a) nFe/Ca/CaO acts as coagulant, (b) heavy metals and organic compound encapsulation through the formation of immobile salts, and (c) nonprecipitated Fe remaining in dissolution was used as catalyst in the further Fenton process.
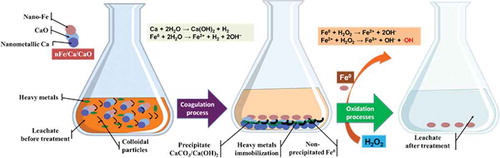
On the other hand, with nFe/Ca/CaO, the iron particle addition in the aqueous phase dissolves ferrous ions and electrons transfer to react with the organic molecules on the iron’s surface (eq 2). This has proved to be a fast and successful process to reduce a variety of organic compounds. It is intended that toxic contaminants (e.g., heavy metals and organic contaminants) would be able to degrade (Kang et al., Citation2002b; Vedrenne et al., Citation2012; Taha and Ibrahim, Citation2014). Nanosized iron particles with high specific surface area can decrease the color/COD concentration. Removal efficiencies of color/COD incremented promptly during the treatment (). Furthermore, the control mechanism is a contaminant metal function of the standard redox potential in the presence of nano-iron (Fe).
Heavy metals and organic compounds are adsorbed directly to the nano-Fe surface, where they are rendered immobile—for example, Zn2+, Cd2+, Cr6+, Cu2+, Pb2+, As3+, and more (Kanel et al., Citation2005; Uzum et al., Citation2009). The standard redox potential of zerovalent iron is −0.41 V, which is similar to that of Cd2+ (−0.40 V), Cr3+ (−0.42 V), Pb2+ (−0.13 V), and As3+ (−0.23 V). Therefore, the removal of As, Cd, Cr, and Pb ions by nano-Fe results from sorption (Li and Zhang, Citation2007). Consequently, the treatment of leachate with nano-Fe/Ca/CaO can reduce heavy metal leachability while minimizing their solubility by the reduction of heavy metals, fixing by adsorption, or heavy metal precipitation ().
The amount of organic compounds and heavy metals exposed to leaching is reduced. In order to achieve the best conditions for Fenton oxidation, the coagulation process was intended to reduce COD as much as possible to avoid interference from other organic compounds and to reduce the dose of H2O2 required. This was accomplished by maintaining a high concentration of dissolved iron (Perdigon-Melon et al., Citation2010; Carlos et al., Citation2015). In the zero valent iron (ZVI)/H2O2 oxidation process, ferrous ion reacts with hydrogen peroxide, producing a hydroxyl radical. (OH). The oxidation reaction is characterized by the catalytic decomposition of H2O2 as described earlier (eqs 3 to 5) . Hydroxyl radicals created by the process can be scavenged by excess Fe2+ (eq 4). The major reactions for the generation of oxidizing radicals may be represented by eq). The nano-iron comes from the excess of coagulant (nFe/Ca/CaO), rendering unnecessary further additions. Furthermore, treated compounds that do not precipitate in the coagulation process may be removed in the subsequent zero valent iron (ZVI)/H2O2 oxidation process (). Therefore, this hybrid coupled coagulation–Fenton process with nFe/Ca/CaO and H2O2 appears to be a suitable treatment of leachate.
Conclusion
A coupled zero valent iron (ZVI)/H2O2 oxidation process with novel nanosize metallic calcium/iron dispersed reagent proved to be a suitable treatment for dealing with leachate samples. The coupling process, using a 1.0 g L−1 of nFe/Ca/CaO and 20 mM of H2O2, achieved enhancement of color, COD, and TSS removal, at 95%, 96%, and 66%, respectively, without initial pH control. The heavy metal removal efficiency also reached about 91–99% after treatment nFe/Ca/CaO in both leachate samples. Further, the color, COD, TSS, and heavy metals concentrations were lower than the discharge limits of the Korean Water Quality Standards. These results encourage the idea that the hybrid coagulation–Fenton process with nFe/Ca/CaO appears to be a suitable treatment of the leachate.
Funding
This work was supported by the 2017 Research Fund of University of Ulsan.
Additional information
Funding
Notes on contributors
Son Dong Lee
Son Dong Lee is a Ph.D. student in the Department of Civil and Environmental Engineering, University of Ulsan, Ulsan, Republic of Korea.
Srinivasa Reddy Mallampati
Srinivasa Reddy Mallampati is an assistant professor in the Department of Civil and Environmental Engineering, University of Ulsan, Ulsan, Republic of Korea.
Byoung Ho Lee
Byoung Ho Lee is a professor in the Department of Civil and Environmental Engineering, University of Ulsan, Ulsan, Republic of Korea.
References
- Aguilar, M.I., J. Saez, M. Llorens, A. Soler, J.F. Ortuno, V. Meseguer, and A. Fuentes. 2005. Improvement of coagulation-flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere 58:47–56. doi:10.1016/j.chemosphere.2004.09.008
- American Public Health Association (APHA), American Water Works Association (AWWA), and Water Pollution Control Federation (WPCF). 1992. Standard Methods for the Examination of Water and Wastewater, 18th ed. Washington, DC: APHA, AWWA, and WPCF.
- American Public Health Association (APHA), American Water Works Association (AWWA), and Water Pollution Control Federation (WPCF). 1995. Standard Methods for the Examination of Water and Wastewater, 19th ed. Washington, DC: APHA, AWWA, and WPCF.
- Amokrane, A., C. Comel, and J. Veron. 1997. Landfill leachates pre-treatment by coagulation–flocculation. Water Res. 31:2775–82. doi:10.1016/S0043-1354(97)00147-4
- Artiola, F.J., and H.W. Fuller. 1982. Humic substances in landfill leachates: I. Humic acid extraction and identification. J. Environ. Qual. 11:663–69.
- ASTM International. 2012. Standard Test Methods for Chemical Oxygen Demand of Water. ASTM D1252-06. West Conshocken, PA: ASTM International.
- Bae, M.J., J.S. Kim, and Y.S. Park. 2012. Evaluation of changes in effluent quality from industrial complexes on the Korean nationwide scale using a self-organizing map. Int. J. Environ. Res. Public Health. 9:1182–1200. doi:10.3390/ijerph9041182
- Barbusinski, K., and K. Filipek. 2001. Use of Fenton’s reagent for removal of pesticides from industrial wastewater. Pol. J. Environ. Stud. 10:207–12.
- Barbusinski, K., and J. Majewski. 2003. Discoloration of azo dye acid red 18 by Fenton reagent in the presence of iron powder. Pol. J. Environ. Stud. 12:151–55.
- Barreto, M.R., F.T. Silva, and T.C.B. Paiva. 2008. Combined zerovalent iron and Fenton processes for the treatment of Brazilian TNT industry wastewater. J. Hazard. Mater. 165:1224–28.
- Benatti, C.T., C.R.G. Tavares, and T.A. Guedes. 2006. Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology. J. Environ. Manage. 80:66–74. doi:10.1016/j.jenvman.2005.08.014
- Benatti, C.T., and C.R.G. Tavares. 2012. Fenton’s process for the treatment of mixed waste chemicals.Faculdade Ingá–UNINGÁ, Universidade Estadual de Maringá–UEM, Brazil. Unpublished.
- Bergendahla, J.A., and T.P. Thies. 2004. Fenton’s oxidation of MTBE with zero-valent iron. Water Res. 38:327–34.
- Boussahel, R., D. Harik, M. Mammar, and S. Lamara-Mohamed. 2007. Degradation of obsolete DDT by Fenton oxidation with zero-valent iron. Desalination 206:369–72. doi:10.1016/j.desal.2006.04.059
- Carlos, A., E.D. Torres-Socías, J.A. Peres, M.I. Maldonado, I. Oller, S. Malato, and M.S. Lucas. 2015. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 286:261–68.
- Dabhade, M.A., M.B. Saidutta, and D.V.R. Murthy. 2009. Adsorption of phenol on granular activated carbon from nutrient medium: Equilibrium and kinetic study. Int. J. Environ. Res. 3:557–68.
- Deng, Y. 2007. Physical and oxidative removal of organics during Fenton treatment of mature municipal landfill leachate. J. Hazard. Mater. 146:334–40. doi:10.1016/j.jhazmat.2006.12.026
- Diamadopoulos, E. 1994. Characterization and treatment of recirculation-stabilized leachate. Water Res. 28:2439–45. doi:10.1016/0043-1354(94)90062-0
- Ehrig, H.J. 1982. Quality and quantity of sanitary landfill leachate. Waste Manage. Res. 1:53–68. doi:10.1016/0734-242X(83)90024-1
- Giasuddin, A.B.M., S.R. Kanel, and H. Choi. 2007. Adsorption of humic acid onto nanoscale zero-valent iron and its effect on arsenic removal. Environ. Sci. Technol. 41:2022–27. doi:10.1021/es0616534
- Hanna, K., T. Kone, G. Medjahdi. 2008. Synthesis of the mixed oxides of iron and quartz and their catalytic activities for the Fenton-like oxidation. Catal. Commun. 9:955–59. doi:10.1016/j.catcom.2007.09.035
- Hermosilla, D., M. Cortijo, and C.P. Huang. 2009. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 407:3473–81. doi:10.1016/j.scitotenv.2009.02.009
- Huang, C.P., C. Dong, and Z. Tang. 1993. Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manage. 13:361–77. doi:10.1016/0956-053X(93)90070-D
- Joo, S.H., and I.F. Cheng. 2006. Nanotechnology for Environmental Remediation. New York NY: Springer.
- Jung, D., Z. Yongsheng, Z. Weihong, and H. Mei. 2009. Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater. J. Hazard. Mater. 161:224–30.
- Kallel, M., C. Belaida, T. Mechichib, M. Ksibia, and B. Elleucha. 2009a. Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem. Eng. J. 150:391–95. doi:10.1016/j.cej.2009.01.017
- Kallel, M., C. Belaida, R. Boussahel, M. Ksibi, A. Montiel, and B. Elleuch. 2009b. Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J. Hazard. Mater. 163:550–554.
- Kanel, S.R., B. Manning, L. Charlet, and H. Choi. 2005. Removal of arsenic(III) from groundwater by nanoscale zero valent iron. Environ. Sci. Technol. 39:1291–98. doi:10.1021/es048991u
- Kang, K.H., H.S. Shin, and H. Park. 2002a. Characterization of humic substances present in landfill leachates with different landfill ages and its implications. Water Res. 36:4023– 32. doi:10.1016/S0043-1354(02)00114-8
- Kang, S.F., C.H. Liao, and M.C. Chen. 2002b. Pre-oxidation and coagulation of textile wastewater by the Fenton process. Chemosphere 46:923–28. doi:10.1016/S0045-6535(01)00159-X
- Klara, R., K.D. Frank, and G. Anett. 2012. Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions—Influence of Fe(II)/Fe(III) ratio on catalytic performance. J. Hazard. Mater. 241:433–40.
- Langlais, B., D.A. Reckhow, and D.R. Brink, eds. 1991. Ozone in Water Treatment: Allocation and Engineering. Chelsea MI: Lewis.
- Lema, J.M., R. Mendez, and R. Blazquez. 1988. Characteristics of landfill leachates and alternatives for their treatment: a review. Water Air Soil Pollut. 40:223–50.
- Li, X.Q., and W.X. Zhang. 2007. Sequestration of metal cations with zerovalent iron nanoparticles—A study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J. Phys. Chem. C 111:6939–46. doi:10.1021/jp0702189
- Mallampati, S.R., Y. Mitoma, T. Okuda, S. Sakita, and C. Simion. 2014a. Preferential removaland immobilization of stable and radioactivecesium in contaminated fly ash with nanometallic Ca/CaO methanolsuspension. J. Hazard. Mater. 279:52–59.
- Mallampati, S.R., Y. Mitoma, T. Okuda, S. Sakita, and C. Simion. 2014b. Simultaneous decontamination of cross-polluted soils with heavy metals and PCBs using a nano-metallic Ca/CaO dispersion mixture. Environ. Sci. Pollut. Res. 21:9270–77. doi:10.1007/s11356-014-2830-y
- Mallampati, S.R., Y. Mitomab, T. Okuda, C. Simiond, and B.K. Lee. 2015. Solvent-free synthesis and application of nano-Fe/Ca/CaO/[PO4] composite for dual separation and immobilization of stable andradioactive cesium in contaminated soils. J. Hazard. Mater. 297:74–82. doi:10.1016/j.jhazmat.2015.04.071
- Mallampati, S.R., T. Okuda, Y. Mitoma, S. Sakita, and M. Kakeda. 2012. Enhanced heavy metal immobilization in soil by grinding with addition of nanometallicCa/CaO dispersion mixture. Chemosphere 89:717–23. doi:10.1016/j.chemosphere.2012.06.030
- Marttinen, S.K., R.H. Kettunen, K.M. Sormunen, R.M. Soimasuo, and J.A. Rintala. 2002. Screening of physical–chemical methods for removal of organic material, nitrogen and toxicity from low strength landfill leachates. Chemosphere 46:851–58. doi:10.1016/S0045-6535(01)00150-3
- Miled, W., S. Soula, and N. Ladhari. 2015. Treatment of water soluble dyes in real textile wastewater by Fenton’s process. Int. J. Sci. Res. Eng. Technol. 3:102–6.
- Mitoma, Y., S.R. Mallampati, H. Miyata, and M. Kakeda. 2013. Decomposition of polychlorinated biphenyls in soil with a dispersion mixture of metallic calcium and calcium oxide. Arch. Environ. Contam. Toxicol. 64:180–86. doi:10.1007/s00244-012-9829-5
- Mott, H.V., K.E. Hartz, and D.R. Yonge. 1987. Landfill leachates. J. Environ. Eng. 113:476–85. doi:10.1061/(ASCE)0733-9372(1987)113:3(476)
- Nwabanne, J.T., O.D. Onukwuli, and C.M. Ifeakandu. 2009. Biokinetics of anaerobic digestion of municipal waste. Int. J. Environ. Res. 3:511–16.
- O’Melia, C.R. 1972. Coagulation and flocculation. In Physicochemical Processes for Water Quality Control, ed. W.J. Weber, Jr., chap. 2. New York, NY: Wiley-Interscience.
- Perdigón-Melón, J.A., J.B. Carbajo, A.L. Petre, R. Rosal, and E. García-Calvo. 2010. Coagulation–Fenton coupled treatment for ecotoxicity reduction in highly polluted industrial wastewater. J. Hazard. Mater. 181:127–32. doi:10.1016/j.jhazmat.2010.04.104
- Petruzzelli, D., A. Volpe, N. Limoni, and R. Passino. 2000. Coagulants removal and recovery from water clarifier sludge. Water Res. 34:2177–82.doi:10.1016/S0043-1354(99)00357-7
- Renou, S., J.G. Givaudan, S. Poulain, F. Dirassouyan, and P. Moulin. 2008. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 150:468–93.doi:10.1016/j.jhazmat.2007.09.077
- Sincero, A.P., and G.A. Sincero. 2003. Physical-Chemical Treatment of Water and Wastewater. Boca Raton FL: CRC Press.
- Sun, Z.X., F.W. Su, W. Forsling, and P.O. Samskog. 1998. Surface characteristics of magnetite in aqueous suspension. J. Colloid Interface Sci. 197:151–59. doi:10.1006/jcis.1997.5239
- Taha, M.R., and A.H. Ibrahim. 2014. Characterization of nano zero-valent iron (nZVI) and its application in sono-Fenton process to remove COD in palm oil mill effluent. J. Environ. Chem. Eng. 2:1–8. doi:10.1016/j.jece.2013.11.021
- Tatsi, A.A., A.I. Zouboulis, K.A. Matis, and P. Samaras. 2003. Coagulation–flocculation pretreatment of sanitary landfill leachates. Chemosphere 53:737–44. doi:10.1016/S0045-6535(03)00513-7
- Trebouet, D., J.P. Schlumpf, P. Jaouen, F. Quemeneur. 2001. Stabilized landfill leachate treatment by combined physicochemical- nanofiltration processes. Water Res. 35:2935–42. doi:10.1016/S0043-1354(01)00005-7
- Trujillo, D., X. Font, A. Sanchez. 2006. Use of Fenton reaction for the treatment of leachate from composting of different wastes. J. Hazard. Mater. 138:201–204. doi:10.1016/j.jhazmat.2006.05.053
- Uemura, S.H. 2010. Mineral requirements for mesophilic and thermophilic anaerobic digestion of organic solid waste. Int. J. Environ. Res. 4:33–40.
- U.S. Environmental Protection Agency. 1995. Decision Maker’s Guide to Solid Waste Management, Vol. II. http://www.epa.gov/garbage/dmg2.htm.
- Uzum, C., T. Shahwan, A.E. Eroglu, K.R. Hallam, T.B. Scott, and I. Lieberwirth. 2009. Synthesis and characterization of kaolinite-supported zerovalent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl. Clay Sci. 43172–81. doi:10.1016/j.clay.2008.07.030
- Vedrenne, M., R. Vasquez-Medrano, D. Prato-Garcia, B.A. Frontana-Uribe, and J.G. Ibanez. 2012. Characterization and detoxification of a mature landfill leachate using a combined coagulation-flocculation/photo Fenton treatment. J. Hazard. Mater. 205:208–215. doi:10.1016/j.jhazmat.2011.12.060
- Walling, C., and S. Kato. 1971. The oxidation of alcohols by Fenton’s reagent: The effect of copper ion. J. Am. Chem. Soc. 93:4275–81. doi:10.1021/ja00746a031
- Wang, Z.P., Z. Zhang, Y.J. Lin, N.S. Deng, T. Tao, and K. Zhuo. 2002. Landfill leachate treatment by acoagulation–photooxidation process. J. Hazard. Mater. 95:153–159. doi:10.1016/S0304-3894(02)00116-4
- Xue, X.F., K. Hanna, N.S. Deng. 2009. Fenton-like oxidation of rhodamine B in the presence of two types of iron(II, III) oxide. J. Hazard. Mater. 166:407–414. doi:10.1016/j.jhazmat.2008.11.089
- Zhang, H., H.J. Choi, P. Canazo, C.P. Huang. 2009. Multivariate approach to the Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 161:1306–1312. doi:10.1016/j.jhazmat.2008.04.126
- Zhang, H., H.J. Choi, and C. Huang. 2005. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 125:166–174. doi:10.1016/j.jhazmat.2005.05.025