ABSTRACT
PM2.5 sampling was conducted at a curbside location in Delhi city for summer and winter seasons, to evaluate the effect of PM2.5 and its chemical components on the visibility impairment. The PM2.5 concentrations were observed to be higher than the National Ambient Air Quality Standards (NAAQS), indicating poor air quality. The chemical constituents of PM2.5 (the water-soluble ionic species SO42-, NO3−, Cl−, and NH4+, and carbonaceous species: organic carbon, elemental carbon) were analyzed to study their impact on visibility impairment by reconstructing the light extinction coefficient, bext. The visibility was found to be negatively correlated with PM2.5 and its components. The reconstructed bext showed that organic matter was the largest contributor to bext in both the seasons which may be attributed to combustion sources. In summer season, it was followed by elemental carbon and ammonium sulfate; however, in winter, major contributions were from ammonium nitrate and elemental carbon. Higher elemental carbon in both seasons may be attributed to traffic sources, while lower concentrations of nitrate during summer, may be attributed to volatility because of higher atmospheric temperatures.
Implications: The chemical constituents of PM2.5 that majorly effect the visibility impairment are organic matter and elemental carbon, both of which are products of combustion processes. Secondary formations that lead to ammonium sulfate and ammonium nitrate production also impair the visibility.
Introduction
Visibility impairment has become a highly prevalent phenomenon in developing countries including India, due to exponential growth and development in all sectors. It has resulted from an increase in atmospheric pollutant concentrations that frequently violates the National Ambient Air Quality Standards (NAAQS), particularly the ambient PM2.5. Visibility impairment is primarily caused due to extinction of light by scattering and/or absorption by fine particles. Visibility impairment due to urban aerosol has been the subject of numerous air pollution studies around the world over the past several decades. Previous studies revealed that the size, chemical composition, and mass concentration of airborne particles substantially affect visibility (Kim et al. Citation2006; Wang et al. Citation2006; Yuan et al. Citation2006). Although the extinction of visible light from gaseous species can also impair visibility, such species have a much weaker influence (Sloane et al. Citation1991).
Delhi, the capital city of India, has a population of more than 14 million. The city is surrounded by other major growth centers of adjoining states such as Haryana and Uttar Pradesh, known as the National Capital Region (NCR) (Gulia et al. Citation2015). Delhi is reported to have 29 planned industrial areas, which lead to intercity goods distribution by diesel-fueled heavy duty vehicles (HDVs). Approximately 44,000 freight vehicles enter Delhi city every day, of which 40% are HDVs (United Nations Environment Programme [UNEP] Citation2015). All these sources cause high emissions of PM2.5, which exceed the prescribed National Ambient Air Quality Standards (NAAQS) and lead to visibility degradation. Very limited literature is available regarding the effect of PM2.5 composition on visibility degradation in New Delhi (Khanna et al. Citation2014; Khare and Khanna Citation2016; Tiwari et al. Citation2014; Citation2013).
The major objectives of this study were twofold: one, to assess the relationship between PM2.5 and visual range; and two, to assess the causes of visibility impairment by relating light extinction coefficients (bext) to PM2.5 chemical composition.
Materials and methods
Site description
The PM2.5 sampling has been conducted at a curbside location on national highway NH-2 (). The sampling site is in the proximity of one of the busiest intersections of Delhi, Ashram Chowk. The site has been selected based on the high traffic activity on NH-2 during the day and movement of HDVs for interstate goods transport during the night, as this junction acts as a connecting link to other two states, Uttar Pradesh and Haryana (Khanna, Khare, and Gargava Citation2015). Approximately 10% of the freight vehicles enter into Delhi city through the NH-2 entry point (United Nations Environment Programme [UNEP] 2015). The traffic composition varies during the day, with dominance of cars and two-wheelers during the morning and evening peak hours. The prominent wind directions in summer and winter are from the southwest and west, respectively. The average temperature was 12.94 ± 1.86ºC during winter and 33.36 ± 3.24ºC during summer sampling.
Sampling details
PM2.5 sampling has been conducted during December 2013–January 2014 and May–June 2014 for winter and summer seasons, respectively. Samples have been collected in a sampling cycle of 12–12 hours (i.e., morning sampling 9 a.m. to 9 p.m. and night 9 p.m. to 9 a.m.) every day in alternate weeks using a collocated PM2.5 sampler (Ecotech Instrument AAS-127), which operates at a constant flow rate of 16.67 L/min. The samples have been collected on 47-mm polytetrafluoroethylene (PTFE) Teflon filters (Whatman PTFE filters, with pore size 2 µm and PP ring supported) and quartz filters (Pall Life Sciences, Tissuquartz 2500QAT-UP) for inorganic and carbonaceous analysis, respectively. The samples have been analyzed for metallic and ionic species and carbon fractions. Forty-one metals have been analysed using energy-dispersive x-ray fluorescence (ED-XRF; PANalytical Epsilon 5) from Si to Pb, at the Central Pollution Control Board (CPCB), New Delhi. The ionic species have been analyzed using ion chromatography (IC; Dionex ICS-1000) for cationic (sodium, ammonium, potassium) and anionic (sulfate, nitrate, chloride) species. The carbon fractions have been analyzed using the IMPROVE_A Thermal Optical/reflectance protocol (DRI model 2001 thermal/optical carbon analyzer) for four fractions of organic carbon (OC1, OC2, OC3, OC4; temperature: ambient to 580ºC) and three fractions of elemental carbon (EC1, EC2, EC3; temperature >580ºC, ≤840ºC) at the National Environmental Engineering Research Institute (NEERI), New Delhi. The pyrolyzed carbon has been corrected by subtracting from OC4 and adding to the EC1 component.
The daily visual range (VR) data have been collected from the Delhi International airport. The VR is measured by using a transmissometer, Drishti, developed by CSIR-National Aerospace Laboratories. It uses a novel light intensity modulation technique with synchronous demodulation detection, which provides hourly VR data. Daily optical light extinction coefficients (bext) are estimated from the well-known Koschmieder equation as shown in eq 1 (Koschmieder Citation1924):
Results and discussion
Chemical composition of PM2.5
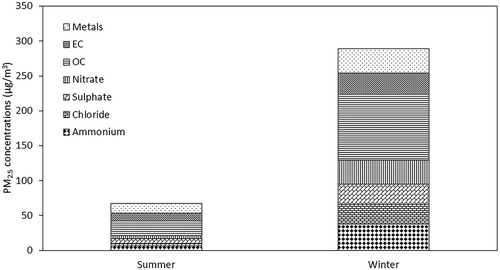
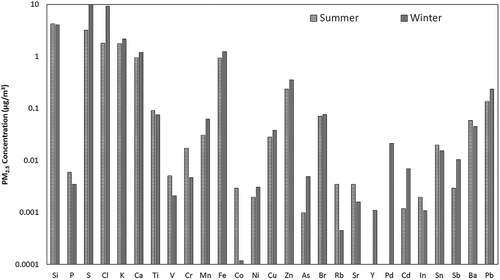
The daily mean PM2.5 12-hr concentrations at the sampling site have been observed to be 60.5 ± 21.4 μg m−3 and 293.1 ± 36.7 μg m−3 in summer and winter seasons, respectively. These concentrations have been observed to be higher than the NAAQS of 60 μg m−3 as prescribed by the NAAQS (CPCB Citation2009). OC has attributed highest percentage of PM2.5 in both summer (31%) and winter (33%), as shown in . OC concentrations seem to be significantly higher in winter than summer, due to the presence of higher emissions from combustion sources during the former. This was followed by metals and EC, with a higher percentage of EC in summer than in winter. OC and EC have shown a high correlation in both seasons, indicating common sources such as vehicular emissions. However, their regression analysis indicates the presence of other sources of OC, as well such as combustion sources. During the winter season, biomass, wood, and waste combustion is often used for heating across the city under uncontrolled conditions in the open areas (Fu et al. Citation2010; Pant et al. Citation2015; Yadav, Tandon, and Attri Citation2013). Among the metal species, sulfur was the most abundant in both seasons, followed by crustal elements like Si, K, Ca, Fe, and Zn as shown in . Metallic analysis shows higher emissions from industrial and vehicular emissions during summer, and from coal and biomass burning during winter. A high correlation has been obtained for S with As and Ni and moderate correlation with Pb and Br, which may indicate coal combustion, as Ni, V, K, Cd, Se, Cl, and As are the tracers for coal combustion and oil combustion (Yu et al. Citation2013). Pb along with Mn and Ba are emitted from fuel additives, and K from biomass burning, which has been higher during winter than during summer (Begum, Biswas, and Hopke Citation2007; Satsangi et al. Citation2013). Zn, Cu, and Ni are used as tracers for metallurgical industries; however, Zn and Cl are also emitted from incineration (Harrison et al. Citation1997; Moreno et al. Citation2013; Srimuruganandam and Nagendra Citation2012).
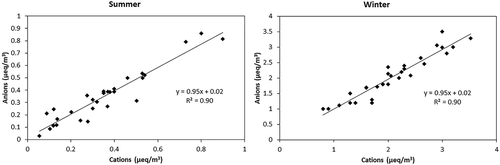
Among the ions, sulfate has been found to be the maximum in summer, followed by ammonium and nitrate; however, in winter, ammonium has been found to be the maximum, followed by nitrate and sulfate. This is in accordance with observations from previous studies with sulfate being higher in summer and nitrate in winter, as ammonium nitrate is volatile in nature and thus has been lost in summer due to volatilization (Chatterjee et al. Citation2010; Chow et al. Citation2005; Kim, Choi, and Ghim Citation2015). In summer, the concentration of ions has been higher in the day than at night as the ionic formation is favored by high temperature and strong solar radiation (Zhang et al. Citation2006). Ion equivalency has been estimated using anions (sulfate, nitrate, and chloride) and cations (ammonium) for both seasons to understand the neutralization of the ions in the atmosphere, and the anions have been found to be neutralized by ammonium in both seasons ().
Correlation of visual range with PM2.5
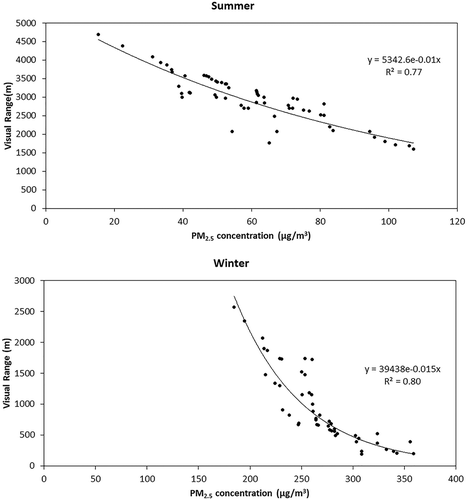
shows the VR as a function of PM2.5 mass concentrations for summer and winter seasons. In both the seasons, VR shows a significant correlation with PM2.5, although they are negatively correlated. VR showed an exponential fit correlation with PM2.5 concentrations; with sulfate and nitrate concentrations, it showed a power profile. VR showed a negative correlation with all of the PM2.5 constituents, that is, sulfate, nitrate, ammonium, OC, and EC ().
Table 1. Correlation between visual range and PM2.5 chemical constituents.
Effect of chemical components on light extinction coefficients
The degradation of visibility occurs as a result of the scattering and absorption of light by particles and gases in the atmosphere. The light extinction (bext), which is the sum of light scattering by particles (bsp), absorption by particles (bap), scattering by gas (bsg), and absorption by gas (bag), is reconstructed according to the revised IMPROVE algorithm (Pitchford et al. Citation2007). The revised algorithm reduces bias for high and low light extinction extremes. It reduces the underprediction of high haze periods and the overprediction of low haze periods compared with the performance of the original algorithm (Pitchford et al. Citation2007). This revised algorithm has been applied by a large number of studies with concentrations comparable to New Delhi concentrations, particularly in China (Chen et al. Citation2016; Cheng et al. Citation2017; Fu et al. Citation2016; Jung et al. Citation2009; Wang et al. Citation2015). The algorithm is shown in eq 2:
where fS(RH) and fL(RH) are the water growth factors for small- and large-sized distribution of sulfate and nitrate, respectively, and fSS(RH) is the water growth factor for sea salt. These water growth factors vary as a function of relative humidity (RH). The water growth factors have been used from Pitchford et al. (Citation2007) based on the hourly relative humidity data. These have been averaged out for the 12-hr period. The constant numbers in the preceding equation are extinction efficiencies for each chemical species under dry conditions. Sulfate and nitrate refer to ammonium sulfate and ammonium nitrate complexes, which are estimated as 1.38 times the mass of sulfate and 1.29 times the mass of nitrate, assuming that sulfate and nitrate are fully neutralized by ammonium. Organic mass is estimated as 1.8 times the OC concentrations to account for unmeasured fractions. Small and large modes of sulfate, nitrate, and organics indicate formation through dry and aqueous mechanisms and are determined by the mass of each of these components, where the large mode is estimated by dividing the total concentration of the component by 20 μg m−3 and the small mode is estimated by subtracting the large mode concentrations from the total concentration. If the total concentration of a component exceeds 20 μg m−3, all of it is assumed to be in the large mode.
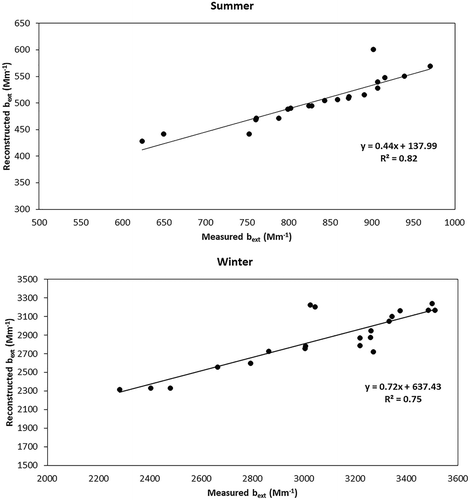
The estimated bext using the IMPROVE equation was 484.39 ± 28.8 Mm−1 and 2875.8 ± 321.6 Mm−1 in summer and winter seasons, respectively. The bext was significantly correlated with PM2.5 concentrations (r2 = 0.94, p < 0.001), demonstrating the strong influence of PM2.5 particles on visibility degradation. The reconstructed light extinction coefficient was compared with that derived from the visual range in eq 1 using the Koschmieder equation. The estimated bext using the equation was 832 ± 56.1 Mm−1 and 3139 ± 632.9 Mm−1 in summer and winter seasons, respectively. There is significant difference in the values estimated using the two methods; however, the reconstructed chemical bext correlated strongly with the optical bext estimated from the Koschmieder equation (r2 = 0.78, p < 0.001), as shown in . Lowenthal and Kumar (Citation2016) recommended using a factor of 2.1 instead of 1.8 for calculating organic matter (OM) from OC, which allows for accounting of water-soluble OM. Using this factor significantly increases the bext to 550.58 ± 30.4 Mm−1 and 3110.04 ± 283.1 Mm−1 in summer and winter, respectively reducing the variation from the estimated bext using Koschmeider equation (summer 832 ± 56.1 Mm−1 and winter 3139 ± 632.9 Mm−1).
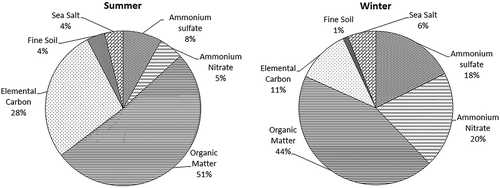
On average, OM is the largest contributor to the light extinction coefficient, accounting for 40% of chemical bext, as shown in . The fractional contribution of OM to bext is the highest in summer (44%), followed by winter (37%). The next most important contributors to bext are elemental carbon (31%) and ammonium sulfate [(NH4)2SO4], with minor contributions from ammonium nitrate (NH4NO3) and fine soil (~7%). However, in winter, major contributions are from ammonium nitrate (22%), ammonium sulfate (20%), and elemental carbon (13%). Higher elemental carbon in both seasons may be attributed to traffic sources, while lower concentrations of nitrate during summer may be attributed to volatility because of higher atmospheric temperatures. The values obtained in this study are in accordance with previous studies conducted in India (Ram et al. Citation2012; Singh et al. Citation2008; Tiwari et al. Citation2014).
Conclusion
The PM2.5 mass concentrations were observed to be threefold higher than the NAAQS for the winter season. PM2.5 and its components were strongly correlated with the visual range, although the correlation was negative in nature. The reconstructed bext was 484.39 ± 28.8 Mm−1 and 2875.8 ± 321.6 Mm−1 in summer and winter seasons, respectively, which indicates poor air quality for visibility in Delhi city. Based on a method established by the IMPROVE program, organic matter (OM) is found to be the largest contributor to visibility impairment overall, accounting for 40% of bext, followed by ammonium sulfate and nitrate, elemental carbon, and fine dust. The particle scattering coefficient, bsp, and particle absorption coefficient, bap, contributed the majority of the light extinction coefficient, and thus, particle emissions must be regulated for better air quality and visibility.
Acknowledgment
The authors thank CPCB, Delhi, and NEERI, Delhi Zonal office, for providing necessary laboratory support.
Additional information
Funding
Notes on contributors
Isha Khanna
Isha Khanna is a research associate at Department of Civil Engineering, Indian Institute of Technology Delhi, Hauz Khas, New Delhi, India.
Mukesh Khare
Mukesh Khare is a professor at Department of Civil Engineering, Indian Institute of Technology Delhi, Hauz Khas, New Delhi, India.
Prashant Gargava
Prashant Gargava is an additional director and head of Air Quality Management Division at Central Pollution Control Board, Delhi, India.
Anwar Ali Khan
Anwar Ali Khan is an additional director at the Environment Department, Uttar Pradesh, India.
References
- Begum, B. A., S. K. Biswas, and P. K. Hopke. 2007. Source apportionment of air particulate matter by chemical mass balance (CMB) and comparison with positive matrix factorization (PMF) model. Aerosol Air Qual. Res. 7:446–468. doi:10.4209/aaqr.2006.10.0021.
- Chatterjee, A., A. Adak, A. K. Singh, M. K. Srivastava, S. K. Ghosh, S. Tiwari, P. C. S. Devara, and S. Raha. 2010. Aerosol chemistry over a high altitude station at northeastern Himalayas, India PLOS ONE 5:e11122. doi:10.1371/journal.pone.0011122.
- Chen, X., S. Lai, Y. Gao, Y. Zhang, Y. Zhao, D. Chen, J. Zheng, L. Zhong, S.-C. Lee, and B. Chen. 2016. Reconstructed light extinction coefficients of fine particulate matter in rural Guangzhou, Southern China. Aerosol Air Qual. Res. 16:1981–1990. doi:10.4209/aaqr.2016.02.0064.
- Cheng, Z., X. Ma, Y. He, J. Jiang, X. Wang, Y. Wang, L. Sheng, J. Hu, and N. Yan. 2017. Mass extinction efficiency and extinction hygroscopicity of ambient PM 2.5 in urban China. Environ. Res. 156:239–246.
- Chow, J. C., J. G. Watson, D. H. Lowenthal, and K. L. Magliano. 2005. Loss of PM2.5 nitrate from filter samples in central California. J. Air Waste Manage. Assoc. 55:1158–1168. doi: 10.1080/10473289.2005.10464704.
- CPCB, 2009. Central Pollution Control Board: National Ambient Air Quality Standards. http://www.moef.nic.in/sites/default/files/notification/Recved%20national.pdf. (accessed February 14, 2018)
- Fu, P. Q., K. Kawamura, C. M. Pavuluri, T. Swaminathan, and J. Chen. 2010. Molecular characterization of urban organic aerosol in tropical India: Contributions of primary emissions and secondary photooxidation. Atmos. Chem. Phys. 10:2663–2689. doi:10.5194/acp-10-2663-2010.
- Fu, X., X. Wang, Q. Hu, G. Li, X. Ding, Y. Zhang, Q. He, T. Liu, Z. Zhang, Q. Yu, R. Shen, and X. Bi. 2016. Changes in visibility with PM 2.5 composition and relative humidity at a background site in the Pearl River Delta region. J. Environ. Sci. (China) 40:10–19. doi:10.1016/j.jes.2015.12.001.
- Gulia, S., S. M. S. Nagendra, M. Khare, and I. Khanna. 2015. Urban air quality management—A review. Atmos. Pollut. Res. 6:286–304. doi:10.5094/APR.2015.033.
- Harrison, R. M., D. J. T. Smith, C. A. Pio, and L. M. Castro. 1997. Comparative receptor modelling study of airborne particulate pollutants in Birmingham (United Kingdom), Coimbra (Portugal) and Lahore (Pakistan). Atmos. Environ. 31:3309–3321. doi:10.1016/S1352-2310(97)00152-0.
- Jung, J., H. Lee, Y. J. Kim, X. Liu, Y. Zhang, J. Gu, and S. Fan. 2009. Aerosol chemistry and the effect of aerosol water content on visibility impairment and radiative forcing in Guangzhou during the 2006 Pearl River Delta campaign. J. Environ. Manage. 90:3231–3244. doi:10.1016/j.jenvman.2009.04.021.
- Khanna, I., M. Khare, and P. Gargava. 2015. Health risks associated with heavy metals in fine particulate matter : A case study in Delhi City, India. J. Geosci. Environ. Protect. 3:72–77. doi:10.4236/gep.2015.32012.
- Khanna, I., M. Khare, P. Gargava, B. K. Jakhmola, and N. Mishra. 2014. Chemical characterization of fine particulate matter at a kerbside of national highway in Delhi. Paper presented at Better Air Quality Conference, Sri Lanka, November 19–21.
- Khare, M., and I. Khanna. 2016. Case studies of source apportionment from the Indian Sub-continent. In: Airborne particulate matter: Sources, atmospheric processes and health, ed. R. E. Hester, R. M. Harrison, and X. Querol, 315–343. Cambridge, UK: Royal Society of Chemistry.
- Kim, C. H., Y. Choi, and Y. S. Ghim. 2015. Characterization of volatilization of filter-sampled PM2.5 semi-volatile inorganic ions using a backup filter and denuders. Aerosol Air Qual. Res. 15:814–820.
- Kim, Y. J., K. W. Kim, S. D. Kim, B. K. Lee, and J. S. Han. 2006. Fine particulate matter characteristics and its impact on visibility impairment at two urban sites in Korea: Seoul and Incheon. Atmos. Environ. 40:593–605. doi:10.1016/j.atmosenv.2005.11.076.
- Koschmieder, H. 1924. Theorie der horizontalen Sichtweite. Beitr. Phys. Freien Atmos. 12:33–53.
- Lowenthal, D. H., and N. Kumar. 2016. Evaluation of the IMPROVE equation for estimating aerosol light extinction. J. Air Waste Manage. Assoc. 667:1096–2247.
- Moreno, T., A. Karanasiou, F. Amato, F. Lucarelli, S. Nava, G. Calzolai, M. Chiari, E. Coz, B. Artíñano, J. Lumbreras, et al. 2013. Daily and hourly sourcing of metallic and mineral dust in urban air contaminated by traffic and coal-burning emissions. Atmos. Environ. 68:33–44. doi:10.1016/j.atmosenv.2012.11.037.
- Pant, P., S. J. Baker, A. Shukla, C. Maikawa, K. J. Godri Pollitt, and R. M. Harrison. 2015. The PM10 fraction of road dust in the UK and India: Characterization, source profiles and oxidative potential. Sci. Total Environ. 530–31:445–452. doi:10.1016/j.scitotenv.2015.05.084.
- Pitchford, M., W. Malm, B. Schichtel, N. Kumar, D. Lowenthal, and J. Hand. 2007. Revised algorithm for estimating light extinction from IMPROVE particle speciation data revised algorithm for estimating light extinction from IMPROVE particle speciation data. J. Air Waste Manage. Assoc. 5711:1326–1336.
- Ram, K., M. M. Sarin, A. K. Sudheer, and R. Rengarajan. 2012. Carbonaceous and secondary inorganic aerosols during wintertime fog and haze over urban sites in the Indo-Gangetic plain. Aerosol Air Qual. Res. 12:355–366.
- Satsangi, A., T. Pachauri, V. Singla, A. Lakhani, and K. Maharaj Kumari. 2013. Water soluble ionic species in atmospheric aerosols: Concentrations and sources at Agra in the Indo-Gangetic Plain (IGP). Aerosol Air Qual. Res. 13:1877–1889.
- Singh, T., P. S. Khillare, V. Shridhar, and T. Agarwal. 2008. Visibility impairing aerosols in the urban atmosphere of Delhi. Environ. Monit. Assess. 141:67–77. doi:10.1007/s10661-007-9879-8.
- Sloane, C. S., J. Watson, J. Chow, L. Pritchett, and L. W. Richards. 1991. Size-segregated fine particle measurements by chemical species and their impact on visibility impairment in Denver. Atmos. Environ. 256:1013–1024. doi:10.1016/0960-1686(91)90143-U.
- Srimuruganandam, B., and S. M. S. Nagendra. 2012. Source characterization of PM10 and PM2.5 mass using a chemical mass balance model at urban roadside. Sci. Total Environ. 433:8–19. doi:10.1016/j.scitotenv.2012.05.082.
- Tiwari, S., D. S. Bisht, A. K. Srivastava, A. S. Pipal, A. Taneja, M. K. Srivastava, and S. D. Attri. 2014. Variability in atmospheric particulates and meteorological effects on their mass concentrations over Delhi, India. Atmos. Res. 145:45–56. doi:10.1016/j.atmosres.2014.03.027.
- Tiwari, S., A. K. Srivastava, D. S. Bisht, P. Parmita, M. K. Srivastava, and S. D. Attri. 2013. Diurnal and seasonal variations of black carbon and PM2.5 over New Delhi, India: Influence of meteorology. Atmos. Res. 125–26:50–62. doi:10.1016/j.atmosres.2013.01.011.
- United Nations Environment Programme. 2015. Promoting low carbon transport in India: Assessment of heavy duty vehicle characteristics in Delhi.
- Wang, H., M. Tian, X. Li, Q. Chang, J. Cao, F. Yang, Y. Ma, and K. He. 2015. Chemical composition and light extinction contribution of PM 2.5 in urban Beijing for a 1-year period. Aerosol Air Qual. Res. 15:2200–2211.
- Wang, J.-L., Y.-H. Zhang, M. Shao, and X.-L. Liu. 2006. The quantitative relationship between visibility and mass concentration of PM2.5 in Beijing. WIT Trans. Ecol. Environ. 86:595–610.
- Yadav, S., A. Tandon, and A. K. Attri. 2013. Characterization of aerosol associated non-polar organic compounds using TD-GC-MS: A four year study from Delhi, India. J. Hazard. Mater. 252–253:29–44. doi:10.1016/j.jhazmat.2013.02.024.
- Yu, L., G. Wang, R. Zhang, L. Zhang, Y. Song, B. Wu, X. Li, K. An, and J. Chu. 2013. Characterization and source apportionment of PM 2.5 in an urban environment in Beijing. Aerosol Air Qual. Res. 13:574–583.
- Yuan, C.-S., C.-G. Lee, S.-H. Liu, J.-C. Chang, C. Yuan, and H.-Y. Yang. 2006. Correlation of atmospheric visibility with chemical composition of Kaohsiung aerosols. Atmos. Res. 82:663–679. doi:10.1016/j.atmosres.2006.02.027.
- Zhang, W.-J., Y.-L. Sun, G.-S. Zhuang, and D.-Q. Xu. 2006. Characteristics and seasonal variations of PM2.5, PM10, and TSP aerosol in Beijing. Biomed. Environ. Sci. 19:461–468.