 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Biogas containing H2S has limited use in electricity and heat production as H2S can be corrosive to metal equipment. Bio-filtration has proved to be a suitable technology for biogas desulfurization because of economical and environmental benefits over physicochemical techniques. In the present study, a response surface methodology using 32 full factorial design was employed to determine the effects of two operating parameters, namely empty bed retention time (EBRT: 100–180 sec) and liquid recirculation velocity (LRV: 2.4–7.1 m3 m−2 h−1) on H2S removal efficiency (%) in single-stage and triple-stage bio-trickling filters (SBTF and TBTF) treating an H2S-rich biogas. Quadratic model was found to be the best predictive model for H2S removal efficiency. The results indicated that H2S removal efficiency was significantly influenced by the synergistic effect of linear terms of EBRT and LRV with a greater effect associated with EBRT. However, the quadratic term of LRV had an antagonistic effect. The quadratic term of EBRT and cross-product term between EBRT and LRV did not exhibit a significant effect on H2S removal efficiency. The predicted values from the established models showed a close agreement with the experimental data with the coefficient of determination (R2) of 0.99 for H2S removal efficiency in both SBTF and TBTF. Response analysis demonstrated that the performance of TBTF was superior compared to SBTF.
Implications: Bio-trickling filter technology has gained a lot of attention for biogas desulfurization because it is economically and environmentally superior over chemical methods. Empty bed retention time (EBRT) and liquid recirculation velocity (LRV) are crucial variables influencing the performance of bio-trickling filters. In this work, the authors established a model that can properly predict H2S removal efficiency in a single/triple bio-trickling filter (SBTF and TBTF) treating H2S-rich biogas with regard to the individual and interaction effects between EBRT and LRV. Analysis with the help of response surface methodology indicated that TBTF was more efficient compared to SBTF for H2S removal.
Introduction
Hydrogen sulfide (H2S) is a toxic and foul-smelling compound. It has a low odor threshold between 0.0001 and 0.03 parts per million (ppm) (Rava Citation2008). H2S has been reported to cause a wide range of adverse impacts on human health including headaches and eye irritation (1–10 ppm) (Cheng et al. Citation2018), serious eye damage (50 ppm) (Ko, Xu, and Jang Citation2015) and instantaneous death at concentrations over 1000 ppm (Charnnok et al. Citation2013). H2S not only causes life-threatening circumstances but it can also accelerate the corrosion of metal equipment where H2S, oxygen, and moisture coexist. In addition, flaring biogas containing H2S releases sulfur dioxide (SO2) into the air which is one of the most pollutants contributing to acid deposition, more commonly known as acid rain, because it can react with water vapor and oxygen in the air to form sulfuric acid which is deposited to the earth when there is rain.
H2S is released, as a by-product, from various industrial sectors including natural gas production sites, oil refineries (Vikrant et al. Citation2018), Kraft paper mills (Rava Citation2008) and geothermal power plants (Bassani et al. Citation2018; Gunnarsson et al. Citation2013). Landfill gas is also recognized as a major source of H2S emission (Ko, Xu, and Jang Citation2015; Rasi Citation2009). The off-gas from either anaerobic digestion processes (Almenglo et al. Citation2016; Ziemiński and Kopycki Citation2016) or intensive livestock operations (Su et al. Citation2013) contain a considerable amount of H2S. The biogas from an upflow anaerobic sludge blanket (UASB) reactor treating a concentrated rubber latex processing wastewater (Songkhla, Thailand) could contain H2S at a concentration as high as 26000 ppm (Saelee et al. Citation2009).
To avoid the above-mentioned problems, H2S must be removed from biogas prior to further utilization. Biotrickling filter (BTF) has become a widely accepted technology for biogas desulfurization and is a cost-efficient and environmentally friendly alternative to physico-chemical methods (Khoshnevisan et al. Citation2017). In BTFs treating a contaminated gas, the gas and recirculation liquid streams are passed through a packed bed on which a microbial consortium is immobilized. The contaminants are transported from the gas stream into the bio-film where they are biodegraded (Mudliar et al. Citation2010).
Desulfurizing BTFs are often operated under aerobic conditions. The final products can be either elemental sulfur (S0) or sulfuric acid depending on the availability of oxygen for the microorganisms in the BTF (Khoshnevisan et al. Citation2017; López et al. Citation2016). Qiu and Deshusses (Citation2017) studied the effect of H2S-to-O2 molar ratio on the performance of a monolith desulfurizing BTF with an inlet H2S concentration of around 1000 ppm corresponding to an H2S-S loading rate of 127 g-S m−3 h−1. The results indicated that H2S removal efficiency at H2S-to-O2 molar ratio of 2:1 was 50%. Analysis of sulfur species in the liquid phase indicated that S0 accounted for about 40% of the total sulfur species recovered in the liquid. Tomàs et al. (Citation2009) determined S0 in the biofilm formed on the surface of packing material of a full-scale BTF treating 80 m3 h−1 biogas with an average H2S concentration of 3000 ppm. They observed that 68–99% of total solids in the biofilm was S0 due to low oxygen availability. Since S0 formation in desulfurizing BTFs leads to bed clogging and a subsequent increase in pressure drop (Vikrant et al. Citation2018), oxygen appropriate load should be supplied to enhance H2S oxidation to sulfuric acid (Tayar et al. Citation2019). Although sulfuric acid formation acidifies the biofilm, the successful application of acidic desulfurizing BTFs has been reported (Chaiprapat et al. Citation2015; Montebello et al. Citation2014; Tu et al. Citation2017).
Liquid recirculation velocity (LRV) and empty bed retention time (EBRT) are two important operating factors that influence the efficiency of desulfurizing BTFs (Chaiprapat et al. Citation2015; Charnnok et al. Citation2013). The use of recirculating liquid in BTFs allows supplying oxygen and regulating pH inside the reactor, as well as provides the nutrients required for microorganisms to grow and degrade the biogas contaminant. Good care should be taken to control LRV because high values of LRV could increase the thickness of the liquid layer on the biofilm surface that may reduce the contaminant mass transfer from the gas phase to the biofilm (López et al. Citation2016; Mirmohammadi, Sotoudeheian, and Bayat Citation2017). It is worth mentioning that a long travel path of recirculating liquid through BTF bed causes liquid channeling leading to partial wetting of the bed which negatively influences the BTF performance. Bed division with multiple-point recirculating liquid injection could help deliver liquid more evenly to the bed and avoid the flow channeling problems (Chaiprapat et al. Citation2015). EBRT, empty bed volume-to-influent biogas flow rate ratio, represents the time that an element of the biogas needs to travel from the empty bed inlet to the outlet. A number of studies have indicated that the longer EBRT of the desulfurizing BTF, the higher H2S removal efficiency (Guerrero and Bevilaqua Citation2015; Vikromvarasiri et al. Citation2017). Although desulfurizing BTFs at longer EBRT could improve the treatment efficiency, excessively large BTF volume results in higher fabrication and material costs (Khoshnevisan et al. Citation2017). Most desulfurizing BTFs have been operated at an EBRT shorter than 7 min, for instance, 0.15–0.95 min (Solcia et al. Citation2014), 0.67–1.67 min (Zhuo et al. Citation2019), 1.67–3.0 min (Chaiprapat et al. Citation2015) and 5.5–6.3 min (Zhou et al. Citation2015).
The aim of this study was to develop a model for H2S removal efficiency in a single/triple-stage BTF (SBTF and TBTF) in terms of LRV and EBRT using a 32 full factorial design (FFD) combined with response surface methodology (RSM), and to evaluate the adequacy and predictive capability of the model based on statistical criteria.
Materials and methods
Data acquisition
Data were taken from the experiments carried out by Chaiprapat et al. (Citation2015). Two lab-scale BTFs including SBTF and TBTF were operated under acidic condition (pH < 4.0) and a temperature of 31 ± 2.3°C, for desulfurization of H2S-rich biogas containing an average H2S concentration of 5522 ppm. Coconut husk used as bed media was submerged in the full-scale anaerobic digester effluent containing a sulfide concentration of about 31 mg/L (Songkhla, Thailand). Aeration was continuously applied and fresh effluent from the anaerobic digester was replaced every 2 days. After a 2-week inoculation, the media were packed into the BTFs and aerated anaerobic digester effluent was used as the recirculating liquid. Two variables, namely EBRT and LRV ranging from 100 to 180 sec, and 2.4 to 7.1 m3 m−2 h−1, respectively, were tested to evaluate H2S removal efficiency (Chaiprapat et al. Citation2015).
Choice of statistical model
The RSM coupled with a 32 FFD was used to establish a functional relationship between EBRT and LRV (termed independent variables) and responses (H2S removal efficiency in SBTF and TBTF). The 32 FFD matrix containing the independent variables in terms of actual and coded values (−1, 0 and +1 for low, medium and high level, respectively), and the actual values of the responses are outlined in in which each row describes an experiment. The relationship between actual and coded values of independent variables is given by Equation (1).
Table 1. 32 FFD matrix containing tested levels of the independent variables along with the values of responses used in this study (adapted from Chaiprapat et al. 2015).
where Xi and xi represent the coded and actual values of independent variable “i”, xmin and xmax are the actual values of the independent variable “i” at the minimum and maximum points, respectively (Singh et al. Citation2018).
The 32 FFD experimental data () were fitted to linear, two-factor interaction (2FI) and quadratic models in order to obtain an adequate functional relationship between independent variables and responses. Since two independent variables (X1 and X2) were tested, the linear, 2FI and quadratic models are expressed as Equations (2), (3) and (4), respectively.
where Y represents the predicted response; b0, b1-b2, b12, and b11-b22 are the model intercept, linear coefficients, interaction coefficient, and quadratic coefficients, respectively.
In this study, firstly, the best predictive model (for each response) was selected based on the sequential model sum of squares analysis and model summary statistics. Secondly, analysis of variance (ANOVA) was used to calculate the model coefficients, and to evaluate the significance of the model and the effect of each model term on the response. Thirdly, diagnostic plots were generated to assess the models' adequacy and clarify whether there is any violation against the ANOVA assumptions. Lastly, the selected models were graphically represented as three-dimension (3D) response surface and two-dimension (2D) contour plots to provide better insight about the individual and simultaneous influence of the independent variables on the responses, and then the optimal value of responses was determined.
Statistical and graphical analysis
Design-Expert® software 10.0.7.0 (trial version, Stat-Ease Inc., Minneapolis, USA) was used for statistical analysis and graphical representation of the experimental data. ANOVA was performed with respect to a 95% confidence level. The overall model and the model terms were considered to be statistically significant when their corresponding “p-value” was less than 0.05.
Results and Discussion
The effect of two operational parameters (EBRT: 100–180 sec and LRV: 2.4–7.1 m3 m−2 h−1) on the performance of a desulfurizing SBTF and TBTF was investigated using a 32 FFD of RSM. The dependent variable (response) was H2S removal efficiency (%).
Model fitting
and represent the results of the sequential model sum of squares and model summary statistics for H2S removal efficiency in SBTF and TBTF, respectively. As seen in , both linear and quadratic models were significant (p-value < 0.05). However, the quadratic model showed the higher adjusted coefficient of determination (R2adj.) and predicted R2 (R2pred.) values, and lower residual error sum of squares (PRESS) (). Therefore, the quadratic model was chosen for further analysis.
Table 2. Sequential model sum of squares for H2S removal efficiency in SBTF and TBTF.
Table 3. Model summary statistics for H2S removal efficiency in SBTF and TBTF.
The ANOVA for the quadratic model was performed to develop an equation relating H2S removal efficiency and the two aforementioned variables (LRV and EBRT) and to check the significance of the effect of each term of the model on the removal efficiency (). The larger the t-value and smaller the p-value, the more significant is the corresponding coefficient term.
Table 4. ANOVA analysis for the quadratic model of H2S removal efficiency in SBTF and TBTF.
According to , the linear terms of EBRT (X1) and LRV (X2), as well as the quadratic term of X2 (X22), showed a significant effect (p-value < 0.05) on H2S removal efficiency whereas the quadratic term of X1 (X12), and the cross-product term between X1 and X2 (X1 X2) were insignificant (p-value > 0.05). Among the significant model terms, X1 and X2 positively influenced H2S removal efficiency, with a greater effect associated with X1, whereas X22 exhibited a negative effect.
The final equation for the responses in terms of the coded and actual values of the independent variables is given by Equations (5)–(6) and Equations (7)–(8), respectively.
where Y1 and Y2 represent H2S removal efficiency (%) in SBTF and TBTF, respectively; (X1, X2) and (x1, x2) are the coded and actual values of the independent variables EBRT and LRV, respectively.
Model adequacy
The adequacy of the selected models was checked using the parity plot of the predicted values against the actual values for the responses (). The actual values of responses were obtained from the experimental design matrix (), and the corresponding predicted values were obtained by calculation from Equations (5)–(8).
Figure 1. Comparison between predicted and experimental (actual) values for the responses (a) H2S removal efficiency in SBTF (Y1) and (b) H2S removal efficiency in TBTF (Y2).
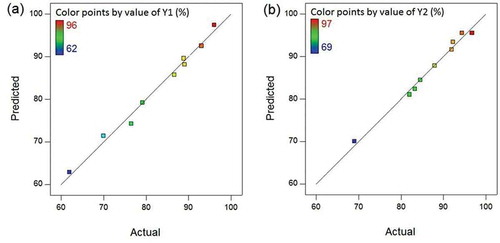
It is evident from , that the data points on the plot were dispersed close to the 45-degree line (100% correlation line) with R2 value of 0.988 and 0.987 for H2S removal efficiency in SBTF and TBTF, respectively. This implies that the models could satisfactorily explain the variability in the responses. The models did not explain only about 1.3% (= 100 × (1-R2)) of the total variability. In addition, diagnostic plots including the normal probability plot (NPP) () and the plot of studentized residuals versus the predicted values for the responses () were constructed to clarify any problem in the experimental data. A studentized residual is the residual divided by its standard deviation where the residual is the difference between an actual value for the response and its corresponding predicted value.
Figure 2. Normal probability plot of residuals for the responses (a) H2S removal efficiency in SBTF (Y1) and (b) H2S removal efficiency in TBTF (Y2).
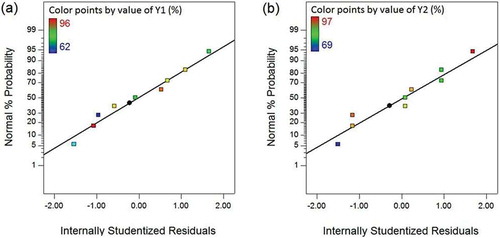
Figure 3. Internally studentized residuals versus predicted values of responses (Y1: H2S removal efficiency in SBTF; Y2: H2S removal efficiency in TBTF).
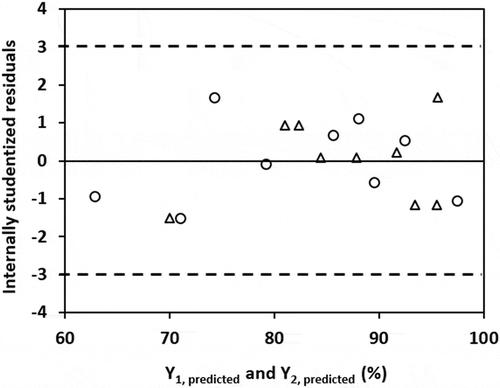
As displayed in , it is clear that the NPPs of residuals showed a straight-line pattern signifying that there was no violation of normality assumption. From , the data points for residuals randomly dispersed within the recommended limits −3 and +3 (Bustillo-Lecompte, Mehrvar, and Quiñones-Bolaños Citation2016; Safari et al. Citation2015), suggesting that the selected models are adequate and do not show any violation of the constant variance assumption.
Response surface analysis
Since residual analysis exhibited that the selected models had no statistical problems, the graphical representation of the responses such as 3D response surface and 2D contour plots were generated to provide better insight into the relationship between the independent variables and the responses. It was performed by substituting the values of independent variables within the experimental range (see ) in the models using Design-Expert software. and illustrate the interaction effect of EBRT and LRV on H2S removal efficiency in SBTF and TBTF expressed as 3D response surface and corresponding 2D contour plots.
Figure 4. (a) 3D response surface and (b) 2D contour plots illustrating the effect of EBRT and LRV on H2S removal efficiency in SBTF (Y1).
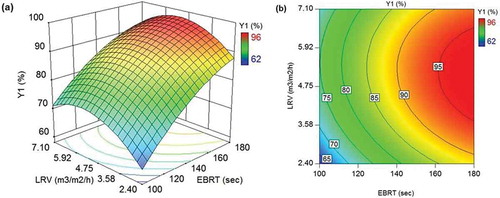
Figure 5. (a) 3D response surface and (b) 2D contour plots illustrating the effect of EBRT and LRV on H2S removal efficiency in TBTF (Y2).
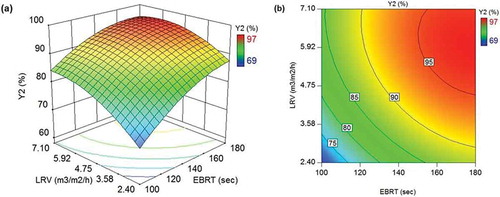
As depicted in and , H2S removal efficiency in both SBTF and TBTF changed nonlinearly with EBRT and LRV variables. Each variable showed a positive effect with the stronger effect belonged to EBRT. These results are in line with findings of Abdehagh et al. (Citation2014). The removal efficiency increased as LRV increased from 2.4 m3 m−2 h−1 (lowest in this study) up to a critical LRV (LRVC) value at which the removal efficiency reached an optimum value. Such enhancement in the efficiency of H2S removal could be associated with the higher amount of oxygen (through recirculating liquid) entered the BTFs. Chaiprapat et al. (Citation2015) reported that the LRVC in SBTF and TBTF at each EBRT tested was around 4.7 and 7.1 m3 m−2 h−1, respectively, whereas the models presented in this study demonstrated that the LRVC varied over a range of 5.1 to 5.5 m3 m−2 h−1 and 5.9 to 7.0 m3 m−2 h−1 in SBTF and TBTF, respectively ().
Table 5. Effect of EBRT and LRV on H2S removal efficiency in SBTF and TBTF (data from and ).
Further, increase beyond LRVC while EBRT held constant led to a negative influence on H2S removal efficiency that was obvious particularly in SBTF. This implies that, although oxygen is essential for H2S biofiltration, excessive amount of liquid in the bed could result in an increase in the liquid film thickness on the biofilm and, consequently, a reduction in H2S mass transfer from gas phase to the biofilm. The comparison between the performance of TBTF and SBTF at EBRT of 100 sec and LRV of 7.1 m3 m−2 h−1 clearly demonstrated that H2S removal efficiency in TBTF was 18.3% higher than that of SBTF. This is because the upper two stages in TBTF were not exposed to an excessive amount of liquid. Only the lower stage received entire LRV since one-third of LRV was fed to each stage. These findings suggest that TBTF could alleviate problems arising from excessive liquid in SBTF such as H2S mass transfer limitation.
shows that the optimal H2S removal efficiency in SBTF and TBTF increased from 74.9% to 97.6% and from 84.5% to 96.5%, respectively, as EBRT increased from 100 to 180 sec. These results are in line with findings of Montebello et al. (Citation2014) who observed an increase in H2S removal efficiency from (50%-60%) to (90%-100%) when EBRT increased from 33 to 130 sec in a BTF treating an inlet H2S concentration of 2000 ppm H2S. Comparable results were also obtained by Guerrero and Bevilaqua (Citation2015) who reported a rising trend in H2S removal efficiency from about (30%-50%) to (90%-98%) when EBRT increased from 96 to 174 sec in a BTF fed with biogas from a full-scale UASB reactor of the wastewater treatment plant of a brewery containing up 478 ppm H2S. Likewise, the findings of Vikromvarasiri et al. (Citation2017) towards biogas desulfurization using a BTF inoculated with Halothiobacillus neapolitanus NTV01 revealed that an increase in EBRT from 40 to 120 sec could enhance H2S removal efficiency from about 42% to 93% with an inlet H2S concentration of 1000 ppm H2S. A number of studies focused on BTF treating lower concentration of H2S (25–55 ppm H2S) indicated an improvement in efficiency of H2S removal (from 52% to 100%) when EBRT raised from 9 to 45 sec (Abdehagh et al. Citation2011; Jin, Veiga, and Kennes Citation2005; Namini et al. Citation2008). The longer EBRT could provide a longer time for H2S to transfer from gas phase to biofilm where it is oxidized by sulfide oxidizing bacteria.
At EBRT of 180 sec, the longest EBRT in this study, H2S removal efficiency in TBTF obtained 96.5%, which was almost similar to that achieved in SBTF (97.6%). This could be due to both SBTF and TBTF receiving the lowest H2S-S loading rate tested in this study (165 g-S m−3 h−1), and thus oxygen mass transfer was not hindered under this condition. However, when BTFs were operated at a shorter EBRT, the efficiency of H2S removal in TBTF was remarkably greater than that of SBTF. For instance, at EBRT of 100 sec (corresponding to an H2S-S loading rate of 304 g-S m−3 h−1), removal efficiency of 74.9% and 84.5% obtained in SBTF and TBTF, respectively. It can be concluded that dividing the BTF bed into multiple stages (each stage with its own recirculating liquid injection point) improves the treatment efficiency. The reason is that the bed staging allows more uniform distribution of the recirculating liquid through the BTF bed which can be translated into a more even oxygen distribution leading to a better bacterial growth and, consequently, an enhancement in H2S oxidation.
Conclusion
In this study, a modeling exercise was carried out on the available published work of Chaiprapat et al. (Citation2015). A statistical investigational technique (response surface methodology coupled with a 32 full factorial design) was applied to establish a predictive model in order to evaluate the individual and interaction effects of empty bed retention time (EBRT) and liquid recirculation velocity (LRV) on H2S removal efficiency in single-stage and triple-stage bio-trickling filters (SBTF and TBTF) treating a biogas stream containing H2S, and also to determine the optimal H2S removal efficiency. The quadratic polynomial model was the best model in fitting the experimental data because it showed higher R2adj. and R2pred., and lower PRESS compared to that calculated for the linear and 2FI models. The adequacy of the model was proven using robust statistical analysis. This study allowed drawing the following conclusions:
H2S removal efficiency in SBTF and TBTF was satisfactorily predicted using the quadratic model with coefficient of determination (R2) of 0.99.
Linear terms of each variable (EBRT and LRV) and the quadratic term of LRV significantly influenced H2S removal efficiency with the greater effect due to the linear term of EBRT.
Response analysis reiterated that both TBTF and SBTF showed almost similar H2S removal efficiency under long EBRT. However, at shorter EBRT, H2S removal efficiency in TBTF was superior compared to SBTF.
Additional information
Notes on contributors
Reza Salehi
Reza Salehi is currently an independent researcher who obtained his Ph.D. in Environmental Engineering from Polytechnique Montreal, Canada in November 2016. His research interest is mainly enhanced biological phosphorus removal (EBPR) and modelling of biological wastewater treatment systems.
Sumate Chaiprapat
Sumate Chaiprapat is an Associate Professor at Department of Civil Engineering, Prince of Songkla University, Thailand. He is also the director of Energy Systems Research Institute, Prince of Songkla University, Thailand. His major research interest is energy technology.
References
- Abdehagh, N., M. T. Namini, B. Bonakdarpour, and S. M. Heydarian. 2014. Effect of operating parameters on the performance of a thiobacillus thioparus-immobilized polyurethane foam biotrickling filter for hydrogen sulfide removal. CLEAN - Soil, Air, Water 42 (9):1311–17. doi:10.1002/clen.201300003.
- Abdehagh, N., M. T. Namini, S. M. Heydarian, B. Bonakdarpour, and D. Zare. 2011. Performance of a biotrickling filter employing thiobacillus thioparus immobilized on polyurethane foam for hydrogen sulfide removal. Iran. J. Environ. Health Sci. Eng. 8 (3):245–54.
- Almenglo, F., T. Bezerra, J. Lafuente, D. Gabriel, M. Ramírez, and D. Cantero. 2016. Effect of gas-liquid flow pattern and microbial diversity analysis of a pilot-scale biotrickling filter for anoxic biogas desulfurization. Chemosphere 157:215–23. doi:10.1016/j.chemosphere.2016.05.016.
- Bassani, A., D. Previtali, C. Pirola, G. Bozzano, I. S. Nadezhdin, A. G. Goryunov, and F. Manenti. 2018. H2S in geothermal power plants: From waste to additional resource for energy and environment. Chem. Eng. Trans. 70:127–32. doi:10.3303/CET1870022.
- Bustillo-Lecompte, C., M. Mehrvar, and E. Quiñones-Bolaños. 2016. Slaughterhouse wastewater characterization and treatment: An economic and public health necessity of the meat processing industry in Ontario, Canada. J. Geosci. Environ. Prot. 4 (4):175–86. doi:10.4236/gep.2016.44021.
- Chaiprapat, S., B. Charnnok, D. Kantachote, and S. Sung. 2015. Bio-desulfurization of biogas using acidic biotrickling filter with dissolved oxygen in step feed recirculation. Bioresour. Technol. 179:429–35. doi:10.1016/j.biortech.2014.12.068.
- Charnnok, B., T. Suksaroj, P. Boonswang, and S. Chaiprapat. 2013. Oxidation of hydrogen sulfide in biogas using dissolved oxygen in the extreme acidic biofiltration operation. Bioresour. Technol. 131:492–99. doi:10.1016/j.biortech.2012.12.114.
- Cheng, Y., T. Yuan, Y. Deng, C. Lin, J. Zhou, Z. Lei, K. Shimizu, and Z. Zhang. 2018. Use of sulfur-oxidizing bacteria enriched from sewage sludge to biologically remove H2S from biogas at an industrial-scale biogas plant. Bioresour. Technol. Rep. 3:43–50. doi:10.1016/j.biteb.2018.01.006.
- Guerrero, R. B. S., and D. Bevilaqua. 2015. Biotrickling filtration of biogas produced from the wastewater treatment plant of a brewery. J. Environ. Eng. 141 (8):04015010. doi:10.1061/(ASCE)EE.1943-7870.0000945.
- Gunnarsson, I., E. S. Aradóttir, B. Sigfússon, E. Gunnlaugsson, and B. M. Júlíusson. 2013. Geothermal gas emission from Hellisheidi and Nesjavellir power plants, Iceland. Geotherm. Resour. Counc. Trans. 37:785–89.
- Jin, Y., M. C. Veiga, and C. Kennes. 2005. Autotrophic deodorization of hydrogen sulfide in a biotrickling filter. J. Chem. Technol. Biotechnol. 80 (9):998–1004. doi:10.1002/jctb.1275.
- Khoshnevisan, B., P. Tsapekos, N. Alfaro, I. Díaz, M. Fdz-Polanco, S. Rafiee, and I. Angelidaki. 2017. A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res. J. 16:741–50. doi:10.18331/BRJ2016.4.4.6.
- Ko, J. H., Q. Xu, and Y. C. Jang. 2015. Emissions and control of hydrogen sulfide at landfills: A review. Crit. Rev. Environ. Sci. Technol. 45 (19):2043–83. doi:10.1080/10643389.2015.1010427.
- López, L. R., T. Bezerra, M. Mora, J. Lafuente, and D. Gabriel. 2016. Influence of trickling liquid velocity and flow pattern in the improvement of oxygen transport in aerobic biotrickling filters for biogas desulfurization. J. Chem. Technol. Biotechnol. 91 (4):1031–39. doi:10.1002/jctb.4676.
- Mirmohammadi, M., S. Sotoudeheian, and R. Bayat. 2017. Triethylamine removal using biotrickling filter (BTF): Effect of height and recirculation liquid rate on BTFs performance. Int. J. Environ. Sci. Technol. 14:1615–24. doi:10.1007/s13762-017-1273-7.
- Montebello, A. M., M. Mora, L. R. López, T. Bezerra, X. Gamisans, J. Lafuente, M. Baeza, and D. Gabriel. 2014. Aerobic desulfurization of biogas by acidic biotrickling filtration in a randomly packed reactor. J. Hazard Mater. 280:200–08. doi:10.1016/j.jhazmat.2014.07.075.
- Mudliar, S., B. Giri, K. Padoley, D. Satpute, R. Dixit, P. Bhatt, R. Pandey, A. Juwarkar, and A. Vaidya. 2010. Bioreactors for treatment of VOCs and odours - A review. J. Environ. Manag. 91 (5):1039–54. doi:10.1016/j.jenvman.2010.01.006.
- Namini, M. T., S. M. Heydarian, B. Bonakdarpour, and A. Farjah. 2008. Removal H2S from synthetic waste gas streams using a biotrickling filter. Iran. J. Chem. Eng. 5 (3):40–51.
- Qiu, X., and M. A. Deshusses. 2017. Performance of a monolith biotrickling filter treating high concentrations of H2S from mimic biogas and elemental sulfur plugging control using pigging. Chemosphere 186:790–97. doi:10.1016/j.chemosphere.2017.08.032.
- Rasi, S. 2009. Biogas composition and upgrading to biomethane. Ph.D. Thesis, Jyväskylä, Finland: University of Jyväskylä.
- Rava, E. M. E. 2008. Management of hydrogen sulphide generation at a Kraft paper mill. M.Sc. thesis, Pretoria, South Africa: University of Pretoria.
- Saelee, R., J. Chungsiriporn, J. Intamanee, and C. Bunyakan. 2009. Removal of H2S in biogas from concentrated latex industry with iron (III) chelate in packed column. Songklanakarin J. Sci. Technol. 31 (2):195–203.
- Safari, G. H., S. Nasseri, A. H. Mahvi, K. Yaghmaeian, R. Nabizadeh, and M. Alimohammadi. 2015. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 13:76–91. doi:10.1186/s40201-015-0234-7.
- Singh, Y., P. Singh, A. Sharma, P. Choudhary, A. Singla, and N. K. Singh. 2018. Optimization of wear and friction characteristics of Phyllanthus Emblica seed oil based lubricant using response surface methodology. Egypt. J. Pet. 27 (4):1145–55. doi:10.1016/j.ejpe.2018.04.001.
- Solcia, R. B., M. Ramírez, M. Fernández, D. Cantero, and D. Bevilaqua. 2014. Hydrogen sulphide removal from air by biotrickling filter using open-pore polyurethane foam as a carrier. Biochem. Eng. J. 84:1–8. doi:10.1016/j.bej.2013.12.019.
- Su, J. J., Y. C. Chang, Y. J. Chen, K. C. Chang, and S. Y. Lee. 2013. Hydrogen sulfide removal from livestock biogas by a farm-scale bio-filter desulfurization system. Water Sci. Technol. 67 (6):1288–93. doi:10.2166/wst.2013.696.
- Tayar, S. P., R. B. Solcia Guerrero, L. F. Hidalgo, and D. Bevilaqua. 2019. Evaluation of biogas biodesulfurization using different packing materials. ChemEngineering 3 (1):27. doi:10.3390/chemengineering3010027.
- Tomàs, M., M. Fortuny, C. Lao, D. Gabriel, J. Lafuente, and X. Gamisans. 2009. Technical and economical study of a full-scale biotrickling filter for H2S removal from biogas. Water Pract. Technol. 4 (2):wpt2009026. doi:10.2166/wpt.2009.026.
- Tu, X., J. Guo, Y. Yang, R. Feng, G. Sun, and J. Li. 2017. Biofilms formed within the acidic and the neutral biotrickling filters for treating H2S-containing waste gases. Royal Soc. Chem. Adv. 7 (41):25475–82. doi:10.1039/c7ra04053a.
- Vikrant, K., S. K. Kailasa, D. C. W. Tsang, S. S. Lee, P. Kumar, B. S. Giri, R. S. Singh, and K. H. Kim. 2018. Biofiltration of hydrogen sulfide: Trends and challenges. J. Cleaner Prod. 187:131–47. doi:10.1016/j.jclepro.2018.03.188.
- Vikromvarasiri, N., V. Champreda, S. Boonyawanich, and N. Pisutpaisal. 2017. Hydrogen sulfide removal from biogas by biotrickling filter inoculated with Halothiobacillus neapolitanus. Int. J. Hydrogen Energy 42 (29):18425–33. doi:10.1016/j.ijhydene.2017.05.020.
- Zhou, Q., H. Liang, S. Yang, and X. Jiang. 2015. The removal of hydrogen sulfide from biogas in a microaerobic biotrickling filter using polypropylene carrier as packing material. Appl. Biochem. Biotechnol. 175 (8):3763–77. doi:10.1007/s12010-015-1545-y.
- Zhuo, Y., Y. Han, Q. Qu, J. Li, C. Zhong, and D. Peng. 2019. Characteristics of low H2S concentration biogas desulfurization using a biotrickling filter: Performance and modeling analysis. Bioresour. Technol. 280:143–50. doi:10.1016/j.biortech.2019.02.007.
- Ziemiński, K., and W. J. Kopycki. 2016. Impact of different packing materials on hydrogen sulfide biooxidation in biofilters installed in the industrial environment. Energy Fuels 30 (11):9386–95. doi:10.1021/acs.energyfuels.6b01374.
