ABSTRACT
Toxic gaseous elemental mercury (GEM) is emitted to the atmosphere through a variety of routes at rates estimated at over 5000 tonnes per annum, a large fraction of which is Anthropogenic. It is then widely disbursed atmospherically and eventually deposited, where it is subject to further biogeochemical cycling, including re-emission. Research into capture of point source mercury emissions revolves almost exclusively around the use of activated carbons, various catalytic oxidation substrates, or as a by-product of acidic treatments of flue gas during SOx and NOx reduction methods. GEM is very non-reactive in its native state, but capture rates are greatly enhanced if GEM is first oxidized, or at least where oxidation states play a role at the substrate GEM interface. Little research has been devoted to capture of GEM directly. However, presented here is a novel adaption of coir fibers for use as a substrate in capturing GEM emissions directly. Various coir modifications were investigated, with the most effective being fibers coated with CuI crystals dispersed in a non-crosslinked poly-siloxane matrix. Scanning electron microscopy was used to view surface morphologies, and sorption characteristics were measured using atomic absorption spectroscopy (AAS). These results indicate that coir fibers modified by CuI-[SiO2]n show great promise in their ability to efficiently sorb GEM, and could potentially be utilized in a variety of configurations and settings where GEM emissions need to be captured.
Implications
Highly toxic gaseous elemental mercury (GEM) has proved very difficult to capture, requiring complex catalytic oxidation or expensive gas scrubbing technologies. The modified coir fiber described in this work can effectively capture GEM without prior catalytic oxidation or any other physicochemical treatment of the gas. The solution provided here is made from renewable resources, is low cost, and the raw materials are readily available in bulk. Further, the mercury is bound in a stable and insoluble form that can be readily isolated from the substrate. This filtration device can be adapted to suit a variety of settings for GEM capture.
Introduction
Gaseous elemental mercury (GEM) capture is difficult due to the physical properties of this particular heavy metal. For example, mercury (Hg) has an extremely high surface tension of 485.5 mN m−1 at standard temperature and pressure, which greatly reduces solubility. The very high volatility of elemental mercury (Hg0(g)) is due to quantum effects caused by its unique e− subshell configuration (Norrby Citation1991). As Hg resides atmospherically mainly in gaseous form rather than as a particulate (Hgp), long-range transport is made possible by extended atmospheric times of >1 yr (Slemr, Schuster, and Seiler Citation1985), although more recent estimates have revised this residence time down to between 0.5 and 1 yr (Lindberg et al. Citation2007; Si and Ariya Citation2018). This toxic element is subsequently transformed into more reactive species upon photo-oxidation, and reactions with atmospheric halogens. This compares to other metals that predominate as particulates and have shortened atmospheric residence times in the range of hours to months (Galloway et al. Citation1982), although the picture is somewhat complicated by particulate matter size, season, and re-emission factors through volatilization (Amos et al. Citation2013; Prabhakar et al. Citation2014). The long-range transport of Hg causes widespread environmental toxicology impacts, enhanced by biogeochemical cycling.
The dispersion of GEM can be somewhat attenuated by point source emission reduction and capture (activated carbons and other scrubber technologies). Most current research into GEM capture centers around technologies developed for flue gas emissions from coal combustion. Although the Hg content of coal on average is low at about 0.5 mg kg−1 (Finkelman Citation1981), the sheer volume of coal combusted means large volumes of Hg is released atmospherically by this process. Treatment for SOx and NOx has incidentally reduced Hg emissions by about half since the 1970s (Muntean et al. Citation2014); however, total Hg current emissions from coal combustion are estimated at 38 Gg (Streets et al. Citation2018). The main areas of active research are in catalytic oxidation of Hg0 using fly ashes and solid-state metal catalysts, combined with particle scrubbers and membrane type technologies. Such technologies include acid and solid infusion-based technologies to either solubilize Hg, or to provide a binding surface on particulates, respectively, for subsequent capture in membranes and filters (Gao et al. Citation2013). Little research is aimed at direct capture of gaseous Hg0 without catalytic oxidation using sustainable technology, as is presented in the current study.
Besides current emissions, legacy pollution stemming from industrial uses of Hg dating back millennia remains an ongoing problem for environmental remediation specialists. Direct treatment of soils can be difficult and destructive. Treatments such as volatilizing Hg by thermal stimulation can be effective and relatively harmless to soils but the resultant GEM has to be captured. This can then be cooled to facilitate a return to metallic Hg but cannot be readily achieved for in situ treatments, and may require complete excavation of sites and transport to treatment facilities. However, GEM emissions can be increased via biological methods, such as the addition of Hg2+→Hg0 reducing bacteria, making this a practical route for remediation (Dash and Das Citation2012), but is again dependent on suitable capture methods. In mat form, the technology presented here could simply be rolled out over large areas as a Hg0 capture geotextile. Further, the technology can be manufactured to any desired shape meaning it has the potential for use in a variety of industrial settings, such as for coal combustion or cement manufacturing generated mercury emissions.
The use of renewable resources for environmental remediation of heavy metals makes sense from an economic and environmental perspective. Coir is derived from the mesocarp of germinated fruit of the coconut palm (Cocos nucifera), is a readily available renewable resource available in bulk at low cost, and has useful properties such as high tensile and flexural strength. It can readily be made into rope and matting by facile mechanical processes and is widely used as a geotextile. However, when employed as a geotextile, being cellulosic, coir is prone to chemical and biological degradation over time mediated by fungi, bacteria, and substrate characteristics (Balan Citation1995; Lekha Citation2004). This degradation can be reduced by various fiber surface modifications and other treatments (Nicholas Citation1982; Suni, Unnikrishnan, and Mathew Citation2016).
Surface modifications can also be beneficial for matrix adhesiveness if one desires fiber coatings, or for fiber use in polymer composites. For example, cellulosic fibers have surface waxes and oils, interfering with fiber matrix bonding, but these can be removed or reduced with various treatments such as alkali baths (Chandrasekar et al. Citation2017). Alkali treatment also increases fibrillation, which can increase surface area interlocking of matrix and fiber surfaces, and interfacial bonding is also improved by increased surface roughness (Liu et al. Citation2004; Punyamurthy et al. Citation2012). The challenge is to modify coir in such a way that it has GEM capture characteristics while reducing its tendency to degrade when applied in environmental or industrial settings.
The current work presents a facile and robust method of GEM capture using coir fiber coated with polymeric siloxane harboring CuI crystals that forms solid and insoluble copper(I) tetra-iodide mercurate when exposed to GEM without the need for oxidation or other catalytic treatments of the gas.
Materials and methods
Fiber pre-treatment
Coir fiber was obtained in pre-made mat form of 5 mm thickness from a local supermarket, and subsequently cut into appropriate size fractions or prized apart. Fibers were soaked in dH2O for 2 h, then rinsed under copious amounts of hot water to remove any grit and other particulates, followed by autoclaving at 121°C for 1 h, and thoroughly dried in a fume-hood for 48 h. All fibers not being immediately used were stored in sealed polypropylene bags.
Soxhlet extraction and alkaline treatment
Naturally occurring waxes and oils were removed by Soxhlet extraction prior to any other modifications. Fibers were added to a Soxhlet thimble and 100% C3H6O solvent was gently boiled for 24 h. Fibers were then washed under copious amounts of dH2O and allowed to dry in a fume-hood for 24 h. A second solvent of 1:2 solution of 70% ethyl acetate (EtOH)(Fisher Scientific [E12420]) and 100% C6H6, was used for 48 h, after which time the fiber was washed and dried as before. Dried fibers were totally submerged in each of 5% and 10% w/v solutions, respectively, of dissolved NaOH (Fisher Scientific as pellets [Lot 124,515]) at 25°C, and gently agitated on a VWR™ rocking platform for 1 h in sealed glass containers. Fibers were then washed under copious amounts of dH2O and allowed to dry for 24 hr in a fume-hood.
Coir fiber modifications
Polyisoprene
Pure latex liquid (cis-1,4 polyisoprene) (Shintani Laboratory, University of Nevada, Reno, Nevada) was added to 200 mL 100% tetrahydrofuran (THF) (Mallinckrodt Inc. (2858–06)) such that 1%, 5%, 10%, and 50% w/v solutions were made. These were placed in sealed glass jars and gently agitated on a VWR™ rocking platform overnight to fully dissolve polyisoprene. Untreated coir mat was cut into appropriate radius discs. The polyisoprene plus THF solutions were then poured over the coir discs seated on glass trays and placed in a fume-hood overnight to allow the THF to evaporate. A glass spreader was used to assist coating for the 50% solution due to high viscosity. The procedure was repeated with the addition of a mass equivalent to latex of either activated charcoal (Sigma-Aldrich [C6289]), or activated coconut pith (Fisher Scientific [5-690-A]) or CuI crystals (Strem Chemicals [93–2936]), with magnetic stirring during dissolution phase to keep particulates suspended.
Carboxymethylcellulose
Carboxymethylcellulose (CMC) sodium salt (Sigma-Aldrich [C-5103]) was slowly added to 200 mL dH2O in sufficient quantity to make 1%, 2%, 3%, and 4% w/v solutions, that were gently heated and stirred until CMC was fully dissolved. CMC was poured over the fibers seated in glass trays and placed in the fume-hood for 48 h to allow the water to evaporate. A glass spreader was needed for the 3% and 4% CMC solutions due to high viscosity. The procedure was repeated for the 4% CMC solution, with the addition of CuI crystals or biochars as previously described.
Siloxane
Siloxane (as Ge® Silicone II 100% silicone caulking gel) was added to 200 mL ethyl acetate in sufficient quantity to make 1%, 5%, 10%, and 50% (w/v) solutions, and stirred with a glass rod until silicone was dissolved and a homogenous mixture was obtained. The solutions were poured over the discs with the use of a glass spreader to ensure even coverage. The discs were placed in a fume-hood overnight to allow the ethyl acetate to evaporate. The procedure was repeated using the addition of CuI crystals and biochar as previously described. The ratio of ingredients can be summarized as 1:1:1 (SiO2:CuI*: Coir w/w). 1
Hg exposure and quantification
GEM capture per formulation
Approximately 50 g liquid Hg was placed in a small beaker seated in a water bath and heated to 50°C to facilitate off-gassing of GEM. Raw and NaOH treated fibers, as well as all other modified coir fiber samples, were sequentially placed over the mouth of the beaker to allow for direct exposure to the GEM plume emanating from the beaker. Exposure time was 1 h, except for 50% siloxane-coated fiber with CuI, which was also exposed for 24 h. Post-exposure samples were placed in sterile 15 mL tubes and kept away from sunlight and excessive heat until analyzed using a pre-calibrated Milestone DMA-80 direct mercury analyzer (EPA Method 7473) with reference material being NIST San Joaquin 1400 ± 80 ng g−1 Hg.
GEM capture per Siloxane plus CuI formulation
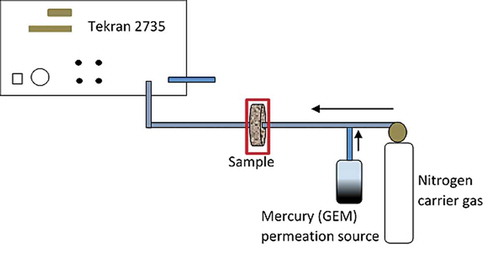
Liquid mercury in a Teflon flask was secured in a water bath heated to 50°C. Fibers were tightly packed in short lengths of polytetrafluoroethylene (PFTE) tubing that were snugly secured over the flask opening to facilitate exposure to GEM vapor in the headspace. Mercury content of exposed fibers was measured using the DMA-80. Surface area of top of liquid Hg was approximately 28 cm2, with exposed front of samples about 5 cm from the surface. A range of exposure times per sample were conducted. Samples were tested for mercury content using a DMA-80 direct mercury analyzer. A second experiment measured real time Hg concentrations of vapor before and after passing through the treated fibers using an automatically calibrated Tekran® 2735X mercury vapor analyzer, with exposure per sample 45 h. (Tekran®, Toronto, Canada) as depicted in . Tubing and sample holders were made of 100% Teflon®.
Figure 1. Abridged experimental set up using Tekran 2735X mercury vapour analyser. Permeation source was liquid mercury secured in a thermally controlled water bath at 50°C. Sample carrier gas was pure nitrogen. Tekran internal carrier gas was pure argon, with sample flow rate 0.5 L min-1, samples dimensions were 28 cm2 of lineally compressed 5 mm thick discs of coir treated with non cross- linked polymeric siloxane and CuI.
Imagery
Scanning electron microscopy (SEM) was undertaken of platinum sputter coated samples using an S-4700 II Scanning Electron Microscope (Hitachi) at the Electron Microscopy & Microanalysis Facility, University of Nevada, Reno, Nevada.
Results and discussion
Coating and adhesion
Poly-isoprene coated coir fiber was manually inspected to view coating homogeneity characteristics and to compare flexural changes between coated vs uncoated fibers. It was found that the most appropriate coating was a 10% solution of polyisoprene dissolved in THF. The 1% solution did not tend to cover the fibers well enough. The 5% solution left some small portion of fibers uncoated (agitating the media while drying may resolve this, although this was not attempted here). The 10% solution easily coated all fibers, and did not leave pores in the matting poly-isoprene matrix through which GEM might escape without interacting with the fibers. All subsequent work used the 5% and 10% solutions.
The dissolved CMC proved difficult to work with in terms of suitable coating characteristics. It was difficult to obtain an even coating with all solutions below 4%, while the 4% solution, although producing an improved coating outcome, was very difficult to make and administer due to low solubility and high viscosity, respectively. All coatings were uneven and tended to coagulate upon drying rather than producing a homogenous coating. It was also noted that these CMC coatings became contaminated over time with bacterial or fungal micro-organisms. This is problematic in terms of end use of the product, because the concept of coating is not only to provide a matrix for GEM binding additives but also to protect fibers from degradation while employed as a geotextile. Although no attempt was made to identify contaminating species, the fact the CMC was so readily colonized suggests this is a poor surface coating for the intended purpose without further efforts to decrease susceptibility to contamination by micro-organisms.
Siloxane coatings were found to be the most suitable in any dilution in terms of homogeneity of the coating. However, the 1% and 5% solutions left pore spaces in the matrix through which GEM could pass without interacting with the fibers. The 10% siloxane solution proved the most effective in terms of overall coverage, and the flexural characteristics were little changed compared to the uncoated fibers in that the matting was not noticeably stiffer. However, it proved very difficult to retain CuI crystals in suspension using any concentrations during the solvent evaporation step as they tended to drop out of the solution and remained as detritus. The 50% solution proved better in retaining CuI crystals in solution, but was difficult to coat using pre-formed matting due to higher viscosity and so matting was teased apart prior to coating. This also had undesirable flexural and structural characteristics upon drying. summarizes the results. GEM capture is discussed in more detail later.
Table 1. Summary results. Treatments as follows: (1) poly-isoprene, (2) CMC (3) poly-siloxane, (4) untreated fibers, (5) solvent and NaOH treated fibers, (6) 10% polyisoprene and biochar (7) 10% poly-siloxane and biochar, (8)(a) 10% poly-siloxane +0.5 g CuI g−1 coir, (b) +1.0 g CuI g−1 coir, (c) +2.0 g CuI g−1 coir.
Irrespective of coverage, raw fibers tended to produce poor adhesion outcomes and some portion of the coatings could readily be removed by rubbing through fingers. In contrast, dewaxed and NaOH treated fibers produced much better adhesion results, and coatings were not easily removed through rubbing between fingers or other manual stresses such as folding, rubbing, and chafing. However, it was noted that a 10% NaOH tended to reduce the structural integrity of fibers, whereas the 5% solution did not alter these characteristics.
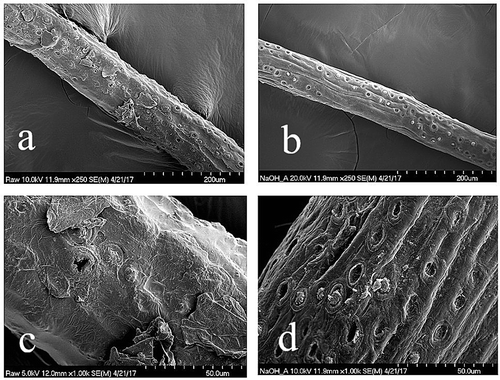
clearly shows the difference in surface morphology between untreated coir fiber and dewaxed and 5% NaOH treated coir fiber. These results are similar to those obtained by Rout et al. (Citation2001), with treated fibers showing greatly reduced surface waxes and oils, greater pore morphology, and increased fibrillation together with removal of tyloses. These characteristics should all improve coating adhesion, and this does seem to be the case in this set of trials. Coating dewaxed and 5% NaOH treated coir fibers with either polyisoprene, CMC or silicone resulted in much better adhesion characteristics, although CMC-coated fibers proved less favorable in that some portion of the coating could still be readily removed through friction.
Figure 2. SEM images of untreated coir fiber at magnification x250 (a) and x1000 (c), and Soxhlet dewaxed and 5% NaOH treated coir fiber at magnification x250 (b) and x 1000 (d).
The addition of CuI crystals resulted in a much greater surface area that might be exposed to GEM as compared to biochar or activated charcoal additive, as seen in . This was in part due to higher size fraction of the biochar particles, estimated to be >150 µm on average as compared to <10 µm for CuI crystals, also aided by greater surface area from siloxane morphology. Coverage might be helped through crushing biochar into a smaller particle size prior to use, although no attempt was made in the current work to obtain smaller uniform particle sizes. Further, overall coverage appeared to be better for CuI crystals, as also shows crystals () are more homogenously coated on the fiber as compared to biochars ()). Also noted was a loss of biochar when exposed to rubbing and chafing, as compared to no loss for CuI crystals when manually rubbed between fingers. Greatly increased loss of both biochar and CuI was noted for CMC-coated fibers, strongly indicating this is not an ideal matrix for coating or holding GEM capture additives such as CuI crystals or biochar particles.
Figure 3. SEM images of (a) 10% polyisoprene with biochar addition and (b) 10% polyisoprene with CuI crystal addition and (c) 10% polysiloxane with CuI (magnification x1000).

When coated with 10% siloxane ()), a denser CuI distribution was achieved as compared to using poly-isoprene ()) or lower siloxane concentrations. This is in part due to the nature of the dissolved product applied to the fibers, whereby the lower viscosity solutions resulted in a good proportion of the CuI crystals in the suspension settling out as particulates, and were left as detritus in the glass trays after evaporation of the solvent. This was a similar problem for biochar suspensions. Even so, 10% solution of siloxane proved difficult to directly coat, as much of the CuI crystals also settled out as detritus during the solvent evaporation stage. The solution found to rectify this problem was to add CuI crystals during the drying process. Ideal coating material was found to be a 2:1 solution of ethyl acetate and siloxane (33% solution), followed by addition of a mass of CuI equal to the mass of siloxane used, as this appeared to give the most even and dense coating while reducing any unbound fraction to a minimum. This was applied to a mass of coir fiber equal to the mass of siloxane used. is an image of a typical sample prepared in this manner.
GEM capture per treatment
Upon visual inspection of the samples after initial exposure, it was clear that the siloxane plus CuI formula had caused a visual discoloration, as seen in which represents a sample exposed to mercury vapor for 24 h. This color change was in accordance with expectations, as copper(I) tetra-iodide mercurate has a reddish brown color as compared to white CuI crystals; however, the vivid discoloration was not expected. No other color variation was detected by eye on other samples only exposed for 1 hr to the GEM plume.
Figure 5. Unexposed coir containing siloxane plus CuI (left) compared to coir exposed to GEM for 24 h (right). A clear color change is noted following exposure, indicating the formation of copper(I) tetra-iodide mercurate.

Please note, although mercury speciation was not characterized, in the discussion following it is assumed emissions are GEM, although it is likely there is trace gaseous oxidized mercury and other minor mercury constituents, but these are ignored at this scale as they likely represent pg m−3 concentrations. indicates that fibers that had not undergone solvent extraction had high intrinsic mercury content, of about 400 to 500 ppb Hg, compared to those that had undergone extraction and NaOH treatment, where Hg levels were reduced to 40 to 50 ppb. This indicates prior Hg contamination, either during growing or manufacturing processes to produce raw coir matting. The solvent extraction process removes existing mercury to roughly 90% efficiency. It was also noted that all samples retained some capacity to adsorb more mercury, irrespective of treatment. This might be related to existing surface oils and waxes having some capacity to adsorb mercury, as when these natural waxes and oils had largely been removed, GEM adsorbance is lower.
Figure 6. Mercury capture per treatment. Raw and 10% silicone used non pre-treated coir, while 5% NaOH and [R2SiO]n + CuI were solvent and NaOH treated prior to coating application. Samples exposed to a highly concentrated plume of GEM vapors for 60 min.
![Figure 6. Mercury capture per treatment. Raw and 10% silicone used non pre-treated coir, while 5% NaOH and [R2SiO]n + CuI were solvent and NaOH treated prior to coating application. Samples exposed to a highly concentrated plume of GEM vapors for 60 min.](/cms/asset/03745447-4041-4e7e-9ffb-9bcf94ae4f8d/uawm_a_1748141_f0006_b.gif)
It also appears that while silicone can adsorb mercury, it is not as efficient as natural waxes and oils, but more efficient than solvent and NaOH treated samples. also indicates that by far the best performing in terms of GEM capture was the siloxane plus CuI samples, where post-exposure mercury levels were much greater than for other samples. This data suggests coir fiber coated with a poly-siloxane coating containing CuI crystals may be a good way to capture GEM emissions.
The data in should be treated cautiously, as the sampling caused problems with the DMA-80, likely due to either high silicone or copper contamination, which got progressively worse until sampling could not continue, and explains the truncated results presented. The dataset shown is the average of triplicate successful measurements, over two replicate experiments, also averaged to give final figures as presented. An attempt was made to overcome issues with the DMA-80 contamination problem using a Tekran® Series 2600, through solubilizing mercury, however, continued contamination problems were encountered, again likely due to high copper content, so surmised due to the distinctive dark discoloration of reagents, and the continued failure of the instrument.
To better evaluate the adsorbance characteristics of the most promising material, an experiment was set up to measure the GEM concentration of a gaseous mercury flow in real time, and compare that after passing it through a volume packed with fibers coated with silicone + CuI of varying concentration. shows the results of those experiments. Note the log10 y-axis scale is used to present the data. As can be seen, the filters were very effective in capturing GEM, with the best performing being the filters coated with 2.0 g CuI g−1 coir, capturing close to 100% of extremely high emissions over a 45 h period. The data presented are from a single trial whereby some breakthrough could be detected for the 1.0 and 2.0 g CuI versions. Other trials showed no such breakthrough. Breakthrough for the 0.5 g CuI sample was similar for all trials.
Figure 7. Concentration of GEM after passing through coir-based filters. The permeation source averaged 3973 ng m-3 Hg h-1, and the flow rate was 0.5 L min-1. Filters were constructed from solvent and NaOH treated coir, with a coating of poly-siloxane with 0.5, 1.0, and 2.0 g CuI g-1 coir, respectively. Carrier gas was nitrogen (Hg), set to an ultra-low flow rate. Tubing, fixtures, and sample housing were made from 100% Teflon®. Exposure time per sample was 45 h. Concentrations measured using a Tekran® 2735X mercury vapor analyzer.
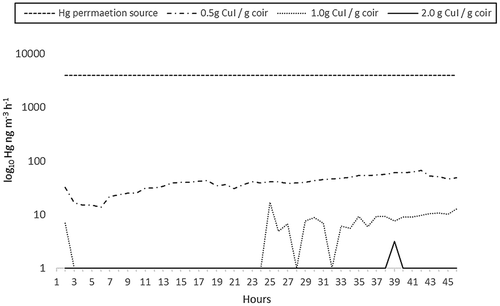
We interpret this to represent under-coverage of the available surface area at 0.5 g CuI g−1 coir. Further, samples were compressed tightly in the holding chambers, meaning some distortion of the substrate may have taken place to induce patches of less homogenous thickness and reactant coverage. When two similar filters were housed together linearly, no such breakthrough was detected after 45 h, even for the 0.5 g CuI g-1 coir samples. Of course, it is envisaged actual filters could be made of any geometric shape, density, and volume to suit.
The next best performing were those filters coated with 1.0 g CuI g−1 coir formula, which had a small breakthrough after 24 h of about 10 ng m−3 h−1, however considering the starting concentration of the gas, this represents a negligible fraction. The filters using 0.5 g CuI g−1 coir consistently performed worst, although still captured approximately 99% of the mercury. Somewhere between 1 and 2 g CuI g−1 coir appears to be the optimal level of CuI, but somewhat below 2 g CuI g−1 coir, as it was noted that there was residual CuI that could be readily shaken off prepared samples at this level, while no such problem was encountered with those prepared at 1 g CuI g−1 coir. It should be noted that coverage will also depend heavily on application method. The stoichiometry suggests even at low efficiency, each gram of CuI could potentially react with and capture at least tens of mg Hg. No attempt was made to ascertain sorption capacity of the filters due to time constraints; however, it is estimated at 125 mg Hg g−1 CuI at 50% efficiency. Estimated total exposure (Q) was 5.4 µg Hg, given Q = FCT, where F is sample flow rate m−3 h, C is concentration GEM ng m−3, T is exposure time (hours).
Once the reaction takes place to form copper(I) tetra-iodide mercurate, the Hg is bound in a virtually insoluble and stable solid form. This is particularly important for large-scale outdoor applications such as matting, as they are exposed to weather. Hg in this form will not re-volatilize readily and is strongly bound to the silicone coating. This is critical, as CuI has serious deleterious effects to aquatic life, as does Hg. Manufacturing processes could mitigate against this problem, but in environmental applications of scale, management of any risk by loss of CuI from the substrate would be important.
While it was difficult to explore the full characteristics and operating parameters here, future exploration and detailed comparison under thermal and chemical environments likely to be encountered in industrial settings are the obvious extension to this proof of concept work. These more complex environments, such as hot, evolving, and chemically complex flue gasses would require some detailed analysis regarding the GEM capture performance of the proposed technology; however, this might be well worth exploring given the expense and limited efficiency of the current technology, where employed.
Much research has been conducted into capture of industrial GEM emissions, largely focusing on catalytic oxidation followed by appropriate scrubbing. Similarly, activated carbons have been the subject of detailed examination for their intrinsic and induced mercury capture properties, although the detailed mechanisms are still the subject of debate. However, little research has been applied to the capture of GEM without the need for prior oxidation. If the technology described in this work is used as a geotextile matting, for example, the product could be used to capture GEM at large scale, such as in gold mining operations, or in the remediation of soils. Alternatively, one could envisage artisanal gold mining applications, where GEM emissions are known to have serious consequences for those participants. Such devices might include packed bed columns or face masks, among other configurations. Similarly, for point source industrial emissions including from energy production, a wide variety of applications could potentially be explored.
Conclusion
Coir fiber was investigated as a substrate to construct a GEM capture device capable of working in ambient conditions without prior catalytic oxidation of mercury. Over 99% of Hg was effectively scrubbed from a plume containing 4000 ng Hg m−3. The product is made from a renewable resource available in bulk at low cost, with readily available and inexpensive additives. These results suggest this technology shows potential for application in a variety of settings, and can be manufactured in a facile manner with existing technology.
Note
Patent Cooperation Treaty (PCT) International Application No.: PCT/AU2018/000169. Intellectual property owned by Macquarie University, Australia.
Acknowledgments
The authors wish to thank Prof. Glenn Miller, Applied Research Facility, Dept. Natural Resources and Environmental Science, University of Nevada, Reno, and Zachary Karmiol, Department of Chemical and Materials Engineering, University of Nevada, Reno, for SEM imagery. Support (non-grant) for this work came from the Australian Government Research Training Program (RTP) and Macquarie University, Sydney, Australia, as well as logistical support from University of Nevada, Reno. Funding provided via Macquarie University Research Training Program Scholarship (MQRTP).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Damien N. McCarthy
Damien N. McCarthy is a researcher with the Department of Earth and Environmental Sciences, Faculty of Science and Engineering, Macquarie University, 2109, NSW, Australia. He can be contacted at [email protected] or [email protected]
Grant C. Edwards
Grant C. Edwards is now deceased. He was a senior lecturer in atmospheric sciences at Macquarie University, Department of Earth and Environmental Sciences, Faculty of Science and Engineering.
References
- Amos, H. M., D. J. Jacob, D. G. Streets, and E. M. Sunderland. 2013. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Global Biogeochem. Cycles 27:410. doi:10.1002/gbc.20040.
- Balan, K., (1995) Studies on engineering behaviour and uses of geotextile with natural fibres. Ph.D. Thesis, Indian Institute of Technology Delhi, India.
- Chandrasekar, M., M. R. Ishak, S. M. Sapuan, Z. Leman, and M. Jawaid. 2017. A review on the characterisation of natural fibres and their composites after alkali treatment and water absorption. Plast. Rubber Compos. 46 (3):119–36. doi:10.1080/14658011.2017.1298550.
- Dash, H. R., and S. Das. 2012. Bioremediation of mercury and the importance of bacterial mer genes. Int. Biodeterior. Biodegrad. 75:207–13. doi:10.1016/j.ibiod.2012.07.023.
- Finkelman, R. B., (1981) Modes of occurrence of trace elements in coal. United States Geological Survey. Open File Report No. OFR-81-99. pp. 301
- Galloway, J., S. Thornton, H. Volchok, and R. McLean. 1982. Trace metals in atmospheric deposition: A review and assessment. Atmos. Environ. 16 (7):1677–700. doi:10.1016/0004-6981(82)90262-1.
- Gao, Y., Z. Zhang, J. Wu, L. Duan, A. Umar, L. Sun, Z. Guo, and Z. Wang. 2013. A critical review on the heterogeneous catalytic oxidation of elemental mercury in flue gases. Environ. Sci. Technol. 47:10813−10823. doi:10.1021/es402495h.
- Lekha, K. R. 2004. Field instrumentation and monitoring of soil erosion in coir geotextile stabilized slopes: A case study. Geotext. Geomembr. 22 (5):399–413. doi:10.1016/j.geotexmem.2003.12.003.
- Lindberg, S., R. Bullock, R. Ebinghaus, D. Engstrom, X. Feng, W. Fitzgerald, N. Pirrone, E. Prestbo, and C. Seigneur. 2007. A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 19–32. doi:10.1579/0044-7447(2007)36[19:ASOPAU]2.0.CO;2.
- Liu, W., A. K. Mohanty, L. T. Drzal, and M. Misra. 2004. Effects of alkali treatment on the structure, morphology and thermal properties of native grass fibers as reinforcements for polymer matrix composites. J. Mater. Sci. 39 (3):1051–54. doi:10.1023/B:JMSC.0000012942.83614.75.
- Muntean, M., G. Janssens-Maenhout, S. Song, N. E. Selin, J. G. Olivier, D. Guizzardi, R. Maas, and F. Dentener. 2014. Trend analysis from 1970 to 2008 and model evaluation of EDGARv4 global gridded anthropogenic mercury emissions. Sci. Total Environ. 494:337–50. doi:10.1016/j.scitotenv.2014.06.014.
- Nicholas, D. D. 1982. Wood deterioration and its prevention by preservative treatments: Degradation and protection of wood, Vol. 1. New York: Syracuse University Press.
- Norrby, L. J. 1991. Why is mercury liquid? Or, why do relativistic effects not get into chemistry textbooks? J. Chem. Educ. 68 (2):110. doi:10.1021/ed068p110.
- Prabhakar, G., A. Sorooshian, E. Toffol, A. F. Arellano, and E. A. Betterton. 2014. Spatiotemporal distribution of airborne particulate metals and metalloids in a populated arid region. Atmos. Environ. 92 (9):339–47. doi:10.1016/j.atmosenv.2014.04.044.
- Punyamurthy, R., D. Sampathkumar, C. V. Srinivasa, and B. Bennehalli. 2012. Effect of alkali treatment on water absorption of single cellulosic abaca fiber. BioResources 7 (3):3515–24.
- Rout, J., S. S. Tripathy, S. K. Nayak, M. Misra, and A. K. Mohanty. 2001. Scanning electron microscopy study of chemically modified coir fibers. J. Appl. Polym. Sci. 79 (7):1169–77. doi:10.1002/1097-4628(20010214)79:7<1169::AID-APP30>3.0.CO;2-Q.
- Si, L., and P. A. Ariya. 2018. Recent advances in atmospheric chemistry of mercury. Atmosphere 9 (2):76. doi:10.3390/atmos9020076.
- Slemr, F., G. Schuster, and W. Seiler. 1985. Distribution, speciation, and budget of atmospheric mercury. J. Atmos. Chem. 3:401–34. doi:10.1007/BF00053870.
- Streets, D. G., Z. Lu, L. Levin, A. F. Ter Schure, and E. M. Sunderland. 2018. Historical releases of mercury to air, land, and water from coal combustion. Sci. Total Environ. 615:131–40. doi:10.1016/j.scitotenv.2017.09.207.
- Suni, S., N. Unnikrishnan, and L. Mathew. 2016. Experimental investigations on biological resistance of surface modified coir geotextiles. Int. J. Geosynth. Ground Eng. 2 (4):31. doi:10.1007/s40891-016-0073-3.

