ABSTRACT
Koji mold, which belongs to the Aspergillus section Nigri, is used in the production of shochu. The section Nigri is composed of very morphologically similar members that in some cases produce mycotoxins, which rises concerns as to whether the presence of mycotoxin-producing fungi in shochu producing sites can compromise consumer safety. Thus, we examined the presence of mycotoxin-producing sec. Nigri fungi in six shochu factories (named A–F) in Japan. Airborne fungal levels in the factories were determined, and a traditional koji called “kona-koji” made from the mold naturally present in factory C (Aogashima village) was analyzed. Isolates of sec. Nigri fungi were identified morphologically and confirmed via cytochrome b gene analysis. In factory A (Nago city), airborne fungal levels of sec. Nigri were 4,000 and 100 cfu/m3 in the koji-making and fermentation rooms, respectively. In factories B, C, and D, the levels were 40, >104 cfu/m3, and 100 cfu/m3, respectively. In factory F (Iki city), there were high levels of airborne white-koji mold (a white mutant of Asp. luchuensis). The most dominant fungal species of sec. Nigri was isolated and identified as Asp. luchuensis via genetic analysis. This is likely to have originated from the commercial fermentation culture used. Asp. niger and Asp. luchuensis were isolated from kona-koji. Mycotoxin production (ochratoxin and fumonisin B2) by Asp. luchuensis (eight strains) and Asp. niger (three strains) was virtually inexistent; only one strain of Asp. niger was positive for fumonisin B2. This study clearly shows that mycotoxin-producing fungi are not dominant in the fungal flora present in the shochu factories examined and therefore, that the liquor can be safely fermented.
Implications: In this study, we examined the presence of mycotoxin-producing Aspergillus sec. Nigri fungi in six shochu (Japanese distilled beverage) factories. The most dominant fungal species of sec. Nigri was isolated and identified as Aspergillus luchuensis (black-koji mold). The proportion of mycotoxin-producing Aspergillus niger and Aspergillus carbonarius was very small. In addition, the Asp. niger isolated from koji mold did not have the ability to produce ochratoxins or fumonisin B. This study clearly shows that shochu can be safely fermented.
Introduction
Aspergillus luchuensis, one of the koji molds, is used as a fermentation starter in the production of Japanese single distilled shochu, which is fermented using ingredients such as barley, sweet potato, and rice. This filamentous fungus belongs to the Aspergillus section Nigri (Park et al. Citation2019) and is similar in morphology to Asp. niger, Asp. carbonarius, and Asp. tubingensis, which are classified in the same section. Fungi belonging to Asp. section Nigri are also called “black aspergilli” because they produce black conidia (Huang et al. Citation2020). Some strains of Asp. niger and Asp. carbonarius are known to produce ochratoxin A (OTA) (Abarca et al. Citation1994; Wicklow et al. Citation1996), and Asp. niger also produces fumonisin B2 (FB2) (Frisvad et al. Citation2007). Asp. niger and Asp. carbonarius are known to cause mycotoxin contamination of food (Huang et al. Citation2020). In the past, the classification of black-koji mold was confusing. Although black-koji was sometimes reported as synonym of Asp. niger (Kozakiewicz Citation1989), recent genetic analysis has shown that this is incorrect and that black-koji mold is a different species (Hong et al. Citation2013; Yamada et al. Citation2011). Subsequently, the multiple scientific names that were used for black-koji mold could be integrated under a single species name, Asp. luchuensis (Yamada et al. Citation2011). Asp. luchuensis, unlike Asp. niger, is a genetically confirmed non-OTA producing fungus whose safety has been proven (Yamada et al. Citation2011).
The purpose of this study was to investigate the presence and abundance of mycotoxin-producing black aspergilli in the fungal flora of shochu production sites. To that aim, we visited between 2011 and 2013 six factories (A–F) located in Japan to sample and identify the airborne fungi present in the indoor factory environment. In factory C (Aogashima village, Tokyo), we were able to investigate the fungal flora of “kona-koji” (Okada et al. Citation1999a, Citation1999b, Citation1999c), a unique koji made not from a commercially available mold but from the mold naturally present in the koji-making room. The fungus is either directly inoculated onto or aerially exposed to crushed or milled ingredients in order to initiate the fermentation process. Currently, the factory on Aogashima is the only one making kona-koji; thus, little is known about kona-koji. Here, we report the airborne fungal flora of shochu factories from each region of Japan, and that of the unique kona-koji. Moreover, we demonstrate that shochu production occurs in a safe environment.
Methodology
Details of the surveyed distilleries
The location, specific rooms, and date of examination for each shochu factory are shown in . Factory A is located in Nago city and produces awamori, which is a traditional rice shochu in Okinawa. Factories B, C, and D are located in Aogashima and produce ao-chu, one of the sweet potato and barley shochu. While factories B and C have koji-making rooms owned by the master brewer, the fermentation and distillation are performed at factory D using koji that is produced by master brewers in their own koji-making room. Factory E is located in Hachijo town and produces barley shochu and sweet potato barley blended shochu. Factory F is located in Iki city and produces barley shochu. In each factory, we examined the koji-making and/or fermentation rooms.
Table 1. Details of factories surveyed in this study
Isolation and morphological identification of fungi
Airborne fungi were sampled in each factory as described by AIJES-A0002-2013 (AIJ Citation2013), which is an official analytical method approved by the Japanese Architectural Institute. For air sampling, SAS IAQ (Pbi International, Italy) conforming to ISO 14698–1 was used, and dichloran 18% glycerol agar (DG18, Merck) according to ISO 21527 was selected as the medium. Samples were collected onto DG18 plates at a height of 1.5 meter using the air sampler, and 100 L of air (flow rate 100 L/min) was sampled per plate. Collected fungi were cultured at 25°C for 7 days and subsequently counted. In factories B, C, and D, samples were collected using a single plate in the center of the room. In factories A and E, 6 different locations within each factory were sampled, while for factory F, 10 different locations were sampled within the factory. Average values were calculated for each room. The detection limit at one location was 10 cfu/m3. To confirm the sterility of the medium, blank plates were used in each series of measurements and incubated at 25°C for 7 days. The air sampler and incubator as well as all laboratory equipment used in our study were regularly checked.
In factory A, fungi were also isolated directly from the wall using a swab method (PF-2002, Eiken Chemical), which was performed according to the manufacturer’s instructions. A 100 cm2 fermentation room wall, which had turned black with mold, was wiped off with a swab, and the sample solution was streaked on DG18 medium and cultured at 25°C for seven days. One piece of kona-koji was placed on potato dextrose agar and cultured at 25°C for five days. The grown colonies were extracted using an inoculation loop, suspended in phosphate-buffered saline, streaked on PDA medium, and cultured at 25°C for seven days.
Isolates were identified on the basis of their micro-macro-morphological characteristics after subculturing on potato dextrose agar (PDA, Nissui), Malt extract agar (MEA, Difco), and Czapek yeast extract agar (CYA, Difco) plates. Aspergillus species were identified as described in the literature (Klich Citation2002; Kitahara and Yoshida Citation1949; Samson et al. Citation2004a). Other fungal species were identified as described by Samson et al. (Citation2004b).
When sec. Nigri species were detected, one strain was isolated from each sampling point and gene analysis was performed. Since two morphological patterns of sec. Nigri were detected in factory C, we decided to analyze multiple colonies. Two strains from air and four strains from kona-koji were isolated and gene analysis was performed.
DNA analysis of black aspergilli isolates
Black aspergilli isolates were identified using mitochondrial cytochrome b (cytb) gene analysis (Wang et al. Citation1998). This study was based on the method of Wang et al. (Citation1998) with some modifications. Briefly, isolates were cultured in potato dextrose broth at 25°C for 3 days, harvested, fixed with 75% (v/v) ethanol, and stored at 4°C. Cells were analyzed using a DNA extraction kit (GenTLE TM for yeast, TAKARA) following the manufacturer’s instructions. Amplification of the cytb gene was performed using the primers E1m4 and rE2m4 (Yokoyama et al. Citation2001) and illustra Hot Start Mix RTG (GE Healthcare UK Ltd.). This is a novel universal primer developed by Yokoyama et al. (Citation2001) that can amplify the cytb gene of Asp. sec. Nigri. The cytb amplicon obtained using this primer was approximately 400 bp. PCR, electrophoresis, and PCR product purification were carried out as previously reported (Wang et al. Citation1998). The amplified DNA strands were sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and ABI 3130xl genetic analyzer. Evolutionary and phylogenetic tree analyses were conducted in MEGA6 (Tamura et al. Citation2013). Although Wang et al. (Citation1998) used the UPGMA method for sequence analysis, we used the Neighbor-Joining method (Saitou and Nei Citation1987), which is currently the most established and widely used method for phylogenetic analysis of closely related species.
LC-MS/MS analysis of mycotoxins
Ochratoxin and fumonisin B production was analyzed following the method described by Hashimoto et al. (Citation2015). Fungal strains were incubated on a PDA slant at 25°C for 7 days. Conidia were harvested. The resultant conidial suspensions (108 cfu/mL) were prepared in sterilized distilled water containing 0.01% (v/v) Tween 80. Each conidial suspension was inoculated into two types of grain to test for either OT or FB. OT production was examined by inoculating a conidial suspension into 15 g of barley grains followed by an incubation period of 7 days at 20°C. Thirty milliliters of an acetonitrile/water mixture (6:4 v/v) was added to the barley culture and OTs were extracted. FB production was examined by inoculating a conidial suspension into 20 g of rice grains followed by an incubation period of 7 days at 25°C. Fifty milliliters of methanol/water mixture (3:1 v/v) was added to the rice culture and FBs were extracted. The mycotoxins were analyzed using LC-MS/MS according to a previous report (Hashimoto et al. Citation2015).
Chromatographic separation was performed using a Waters alliance liquid chromatograph coupled with a SCIEX API 3000 triple mass spectrometer with a turbo spray ion source (ESI). For OTA and OTB, a 150 × 2.1 mm i.d. (3 μm) Inertsil ODS-3 V column (GL Sciences, Tokyo) was used for separation. The mobile phase was programmed with a 30–90% acetonitrile linear ramp in 5 mM ammonium acetate for 10 min at a flow rate of 0.20 mL/min. MS/MS measurements were carried out with ESI at negative polarity in multiple reaction monitoring (MRM) mode with the following transitions: OTA quantifier m/z 402.1 -> 357.9 and qualifier m/z 402.1 -> 166.9; OTB quantifier m/z 368.1 -> 324.0 and qualifier m/z 368.1 -> 280.1, respectively. The limits of detection and quantification were approximately 0.1 and 0.4 μg/kg, respectively.
For FB1 and FB2, a 150 × 2 mm i.d. (3 μm) TSK gel ODS 100 V column (Tosoh, Tokyo) was used. The mobile phase was programmed with a 30–90% acetonitrile linear ramp in 0.1% formic acid for 10 min at a flow rate of 0.20 mL/min. MS/MS measures were carried out with ESI at positive polarity and in MRM mode; FB1 quantifier m/z 722.5 -> 334.5 and qualifier 722.5 -> 352.2; FB2 quantifier m/z 706.3 -> 336.4 and qualifier m/z 706.3 -> 318.4, respectively. The limits of detection and quantification were 0.003 and 0.01 mg/kg, respectively.
Results and discussion
Airborne fungal levels
The airborne fungal levels and isolate identification results are shown in . The concentration of airborne fungi with correction values was obtained by converting the number of isolated colonies using Feller’s formula (Karwowska Citation2005) (). According to “AIJES-A0002-2013” (AIJ Citation2013), which is the standard used by the Architectural Institute of Japan, airborne fungal levels in the working area of a food factory in Japan should be maintained at a desirable level below 1000 cfu/m3. This standard value is used to maintain the building. Although factories A, B, C, and F exceeded this threshold (1000cfu/m3), the figures were greatly influenced by the samples collected from the koji-making rooms. Overall, the fungal concentrations were higher in the koji-making room than in the fermentation room, and the koji-making rooms of A, C, and F exceeded the upper limit of quantification (>104 cfu/m3). The koji-making room is a facility for culturing the koji molds that are used for fermenting ingredients such as barley, sweet potato, and rice. Since the koji-making room has an environment that favors fungal growth, koji mold is present in the air and on the surfaces of many shochu factories. Thus, fungi are not seen as contaminants. In addition, the type of ventilation possibly affected the fungal concentration in the fermentation room. Compared with the fermentation room of factory A, which had natural ventilation, the fermentation rooms of factories D, E, and F, which had ventilation equipment, tended to have lower fungal concentrations.
Table 2. Airborne fungal concentrations (cfu/m3) of airborne fungi isolated from Japanese shochu factories
The average airborne fungal concentration of Aspergillus spp. in Japanese food factories has been reported to be <10 cfu/m3 (Morozumi et al. Citation2004), which is significantly lower than the concentration of sec. Nigri in all factories surveyed in this study. The greater airborne fungal concentration in these factories can be attributed to the fact that the fungi are growing in situ. In particular, the concentration of sec. Nigri exceeded 104 cfu/m3 in the koji-making rooms of factories C and F. The sec. Nigri isolated from factories A–D were typical “black aspergilli,” which produce black conidia. Therefore, genetic analysis is essential to differentiate between these species. In factory F, many of the sec. Nigri isolates were Asp. kawachii (Asp. luchuensis mut. kawachii), a white-koji mold used in shochu production. As its name suggests, white-koji Asp. kawachii is easily distinguished from black aspergilli by the color of its conidia (Kitahara and Yoshida Citation1949).
In factory E, black aspergilli were not isolated and the total concentration of airborne fungi was the lowest among all factories. A possible explanation for these findings is that there was very little disturbance to the airflow and little distribution of airborne spores on the day of the survey, as it was a holiday and the factory was not in operation. This is contrast with the results from factories C and F, in which high concentrations of sec. Nigri were recorded (>104cfu/m3), as the factories C and F were in operation on the day of the survey.
Identification of black aspergilli isolates
. and . show the identification of 11 black aspergilli isolates based on cytochrome b gene analysis. The strains isolated from factory F were excluded from the analysis, as Asp. kawachii could be identified based on the morphological features. The airborne black aspergilli IFM 60590 and IFM 60589 isolates from the koji-making and fermentation rooms of factory A were identified as Asp. luchuensis. In addition, IFM 60592, a strain isolated from the wall, was also identified as Asp. luchuensis. Asp. luchuensis is not usually isolated from common indoor environments in Japan (Ara et al. Citation2004; Hashimoto, Fujii, and Kawakami Citation2019; Hashimoto and Kawakami Citation2018; Takahashi Citation1997). Since factory A uses commercially available black-koji mold (Asp. luchuensis) for fermentation, the isolates were identical to black-koji mold. These results indicated that Asp. luchuensis grows abundantly in situ in the indoor environment of factory A.
Table 3. Species identification by cytb gene analysis and the mycotoxin production by Aspergillus section Nigri (black aspergilli) strains
Figure 1. Phylogenetic tree of Aspergillus section Nigri identified by mitochondrial cytochrome b gene analysis. The evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The evolutionary distances were computed using the Tamura 3-parameter method and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution. There were a total of 397 positions in the final dataset. T indicates a type strain
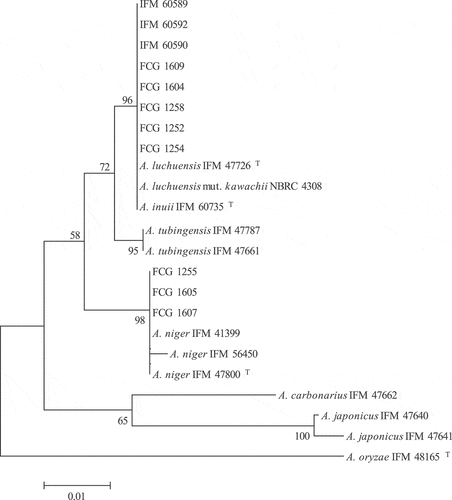
In Aogashima, the airborne black aspergilli isolated in factories B and D were also identified as Asp. luchuensis. In Factory C, although black aspergilli were isolated from air and koji, the genetic analysis revealed the presence of two species, Asp. luchuensis and Asp. niger. The koji molds used in the production of shochu are Asp. luchuensis, Asp. kawachii, or Asp. oryzae, and there are no known cases of Asp. niger use. The kona-koji of factory C is unique because it does not utilize commercially available koji mold, but utilizes the koji mold that is present in the koji-making room. This traditional method (Okada et al. Citation1999a) of making koji may have produced the characteristic microbial flora of kona-koji. According to Okada et al. (Citation1999a), there are three types of kona-koji in Aogashima that are discernable by their color: yellow, black, and mixed (yellow and black). An examination of the yellow type demonstrated that Asp. oryzae group is the most common species, followed by Asp. niger group (Okada et al. Citation1999a). In this study, the kona-koji of factory C was identified as a black type (), and most of the isolates were black aspergilli. Asp. oryzae group (Aspergillus section Flavi) was isolated from all three factories in Aogashima (). Okada et al. (Citation1999b) surveyed the fungal flora of soil and air in the koji-making rooms of Aogashima and their surroundings, and found that the soils contained species such as Asp. oryzae group, Asp. niger group, and Rhizopus, while the airborne fungi were mostly composed of Asp. oryzae group, Asp. niger group, Rhizopus, Penicillium, Mucor, and Alternaria. These results are in agreement with the results of this study (). The airborne concentration of sec. Flavi has previously been reported to be <1.0 cfu/m3 (Hashimoto and Kawakami Citation2018; Takahashi Citation1997), while in this study, the concentrations in factories B–D were >100 cfu/m3. This result suggested the uniqueness of the Aogashima environment compared to other areas in Japan.
Mycotoxin production by the identified strains
OTA, OTB, FB1, and FB2 production by the 11 strains of black aspergilli found in this study is shown in . None of the six strains of Asp. luchuensis isolated from the air and walls produced OT or FB. It has previously been reported that Asp. luchuensis and Asp. kawachii, which are used for fermentation, do not produce either OTA or FB (Hashimoto et al. Citation2013; Yamada et al. Citation2011) and that approximately 70% of Asp. niger strains can produce FB2 (Nielsen et al. Citation2009). Hashimoto et al. (Hashimoto et al. Citation2013, Citation2015) reported 0.2–278 μg/g of FB2 production by Asp. niger on barley. In this study, Asp. niger FGG 1255, one of the strains isolated from the air at the koji-making room in factory C, produced 17 μg/g of FB2, and its production range was similar to that reported in previous studies (0.2–278 μg/g) (Hashimoto et al. Citation2013, Citation2015). Two strains of Asp. luchuensis and two strains of Asp. niger isolated from kona-koji did not produce OTA or FB2. Mycotoxins are removed during the distillation process to obtain the final shochu end-product. Nagatomi et al. (Citation2012) artificially added 14 types of mycotoxins, such as OTA, FB1, and FB2, to the fermentation mash (moromi) that is obtained in the process of producing barley shochu, distilled it, and analyzed the distillate using LC-MS/MS. The results showed that none of the mycotoxins were transferred to the distillate. Furthermore, Inoue et al. (Citation2010) conducted similar experiments with pesticides and reported that pesticides with low molecular weight added to the fermentation mash are removed in the distillation process and are therefore not transferred to shochu. Thus, we believe that although mycotoxin-producing fungi were present in the air of some of the surveyed factories, such concentrations are unlikely to cause issues concerning product safety.
Acknowledgments
The authors are grateful to all the distilleries for their cooperation and support of this study. The authors would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
Notes on contributors
Kazuhiro Hashimoto
Kazuhiro Hashimoto, Yuji Kawakami and Hisayuki Oda are researchers at the Laboratory of Integrated Pest Management, FCG Research Institute Inc., Tokyo, Japan.
Ruiko Hashimoto
Ruiko Hashimoto is a researcher at the Chiba Prefectural Institute of Public Health, Chiba, Japan.
Yohei Kitaoka
Yohei Kitaoka and Yoshiki Onji are researchers at the Nara Prefectural Institute of Public Health, Nara, Japan.
Maiko Watanabe
Maiko Watanabe and Haruo Takahashi are researchers at the National Institute of Health Science, Kanagawa, Japan.
Koji Yokoyama
Koji Yokoyama is a researcher at the Medical Mycology Research Center, Chiba University, Chiba, Japan.
References
- Abarca, M. L., M. R. Bragulat, G. Castella, and F. J. Cabanes. 1994. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 60:2650–52. doi:https://doi.org/10.1128/AEM.60.7.2650-2652.1994.
- AIJ, Architectural Institute of Japan. 2013. AIJES-A0002-2013. Standards for design and maintenance on indoor air pollution by microbe. Tokyo, Japan: AIJ.
- Ara, K., M. Aihara, M. Ojima, Y. Toshima, C. Yabune, H. Tokuda, S. Kawai, N. Ueda, T. Tanaka, K. Akiyama, et al. 2004. Survey of fungal contamination in ordinary houses in Japan. Allergology Int. 53:369–77. doi:https://doi.org/10.1111/j.1440-1592.2004.00356.x.
- Frisvad, J. C., J. Smedsgaard, R. A. Samson, T. O. Larsen, and U. Thrane. 2007. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 55:9727–32. doi:https://doi.org/10.1021/jf0718906.
- Hashimoto, K., H. Fujii, and Y. Kawakami. 2019. Genetic identification of dematiaceous fungi isolated from washing machine in Japan, and considering of fungal removal methods. Biocontrol Sci. 24:89–96. doi:https://doi.org/10.4265/bio.24.89.
- Hashimoto, K., and Y. Kawakami. 2018. Effectiveness of airborne fungi removal by using a HEPA air purifier fan in houses. Biocontrol Sci. 23:215–21. doi:https://doi.org/10.4265/bio.23.215.
- Hashimoto, R., H. Nakagawa, Y. Onji, K. Asano, K. Yokoyama, and H. Takahashi. 2015. Mycotoxin contamination of Vietnamese coffee beans caused by Aspergillus sections Nigri and Circumdati. JSM Mycotoxins 65:1–6. doi:https://doi.org/10.2520/myco.65.1.
- Hashimoto, R., K. Asano, T. Tokashiki, Y. Onji, M. Hirose-Yasumoto, R. Takara, T. Toyosato, A. Yoshino, M. Ikehata, L. Ying, et al. 2013. Mycotoxin production and genetic analysis of Aspergillus niger and related species including the kuro-koji mold. JSM Mycotoxins 63:179–86. doi:https://doi.org/10.2520/myco.63.179.
- Hong, S. B., M. Lee, D. H. Kim, J. Varga, J. C. Frisvad, G. Perrone, K. Gomi, O. Yamada, M. Machida, J. Houbraken, et al. 2013. Aspergillus luchuensis, an industrially important black Aspergillus in East Asia. PLoS One 8:e63769. doi:https://doi.org/10.1371/journal.pone.0063769.
- Huang, X., Z. Xiao, F. Kong, A. J. Chen, G. Perrone, Z. Wang, J. Wang, and H. Zhang. 2020. Diversity and ochratoxin A-fumonisin profile of black Aspergilli isolated from grapes in China. World Mycotoxin J. 13:225–34. doi:https://doi.org/10.3920/WMJ2019.2505.
- Inoue, T., Y. Nagatomi, T. Kinami, A. Uyama, and N. Mochizuki. 2010. Fate of pesticides in a distilled spirit of barley shochu during the distillation process. Biosci. Biotechnol. Biochem. 74:2518–22. doi:https://doi.org/10.1271/bbb.100543.
- Karwowska, E. 2005. Microbiological air contamination in farming environment. Polish J. Environ. Stud. 14:445–49.
- Kitahara, K., and M. Yoshida. 1949. On the so-called Awamori white mold part III. (1) Morphological and several physiological characteristics. J. Ferment. Technol. 27:162–66.
- Klich, M. A. 2002. Identification of common Aspergillus species. Utrecht, Netherlands: Centraalbureau voor Schimmelcultures.
- Kozakiewicz, Z. 1989. Mycotoxins: Aspergillus species on stored products. Mycological Papers (No. 161). Kew, UK: CAB International Mycological Institute.
- Morozumi, S., H. Fujikawa, T. Wauke, and T. Chiba. 2004. Fungal contamination and its control in foods. Annu. Rep. Tokyo Metropolitan Inst. Public Health 55:3–12.
- Nagatomi, Y., T. Inoue, A. Uyama, and N. Mochizuki. 2012. The fate of mycotoxins during the distillation process of barley shochu, a distilled alcoholic beverage. Biosci. Biotechnol. Biochem. 76:202–04. doi:https://doi.org/10.1271/bbb.110639.
- Nielsen, K. F., J. M. Mogensen, M. Johansen, T. O. Larsen, and J. C. Frisvad. 2009. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 395:1225–42. doi:https://doi.org/10.1007/s00216-009-3081-5.
- Okada, T., Y. Maeda, M. Kanauchi, T. Kakuta, M. Suzuki, and T. Koizumi. 1999c. Characteristics of Kona koji for Imo-shochu production in aogashima studies of Kona-koji (Part.3). J. Brewing Soc. Japan 94:849–52. doi:https://doi.org/10.6013/jbrewsocjapan1988.94.849.
- Okada, T., Y. Maeda, T. Kakuta, M. Suzuki, and T. Koizumi. 1999a. Characteristics of koji making and the mold flora of Kona-koji for Imo-shochu production in Aogashima. Studies of Kona-koji (Part 1). J. Brewing Soc. Japan 94:150–57. doi:https://doi.org/10.6013/jbrewsocjapan1988.94.150.
- Okada, T., Y. Maeda, T. Kakuta, M. Suzuki, and T. Koizumi. 1999b. The origin of the main microbe (mold) in Kona-koji for Imo shochu production in Aogashima. Studies of Kona-koji (Part 2). J. Brewing Soc. Japan 94:674–81. doi:https://doi.org/10.6013/jbrewsocjapan1988.94.674.
- Park, J., W. Kwon, B. Zhu, A. Mageswari, I. B. Heo, K. H. Han, and S. B. Hong. 2019. Complete mitochondrial genome sequence of the food fermentation fungus, Aspergillus Luchuensis. Mitochondrial DNA Part B 4:945–46. doi:https://doi.org/10.1080/23802359.2018.1547160.
- Saitou, N., and M. Nei. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–25. doi:https://doi.org/10.1093/oxfordjournals.molbev.a040454.
- Samson, R. A., E. S. Hoekstra, and J. C. Frisvad. 2004b. Introduction to food and airborne fungi. Utrecht, Netherlands: Centraalbureau voor Schimmelcultures.
- Samson, R. A., J. A. M. P. Houbraken, A. F. Kuijpers, J. M. Frank, and J. C. Frisvad. 2004a. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 50:45–61.
- Takahashi, T. 1997. Airborne fungal colony-forming units in outdoor and indoor environments in Yokohama, Japan. Mycopathologia 139:23–33. doi:https://doi.org/10.1023/a:1006831111595.
- Tamura, K., G. Stecher, D. Peterson, A. Filipski, and S. Kumar. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30:2725–29. doi:https://doi.org/10.1093/molbev/mst197.
- Wang, L., K. Yokoyama, M. Miyaji, and K. Nishimura. 1998. The identification and phylogenetic relationship of pathogenic species of Aspergillus based on the mitochondrial cytochrome b gene. Med. Mycol. 36:153–64. doi:https://doi.org/10.1080/02681219880000231.
- Wicklow, D. T., P. F. Dowd, A. A. Alftafta, and J. B. Gloer. 1996. Ochratoxin A: An antiinsectan metabolite from the sclerotia of Aspergillus carbonarius NRRL 369. Can. J. Microbiol. 42:1100–03. doi:https://doi.org/10.1139/m96-141.
- Yamada, O., R. Takara, R. Hamada, R. Hayashi, M. Tsukahara, and S. Mikami. 2011. Molecular biological researches of Kuro-Koji molds, their classification and safety. J. Biosci. Bioeng. 112:233–37. doi:https://doi.org/10.1016/j.jbiosc.2011.05.005.
- Yokoyama, K., L. Wang, M. Miyaji, and K. Nishimura. 2001. Identification, classification and phylogeny of the Aspergillus section Nigri inferred from mitochondrial cytochrome b gene. FEMS Microbiol. Lett. 200:241–46. doi:https://doi.org/10.1111/j.1574-6968.2001.tb10722.x.

