ABSTRACT
Per- and polyfluoroalkyl substances (PFAS) are persistent environmental pollutants associated with negative health impacts. Assessments of tubing-related measurement bias for volatile PFAS are lacking, as gas-wall interactions with tubing can delay quantification of gas-phase analytes. We use online iodide chemical ionization mass spectrometry measurements to characterize tubing delays for three gas-phase oxygenated PFAS − 4:2 fluorotelomer alcohol (4:2 FTOH), perfluorobutanoic acid (PFBA), and hexafluoropropylene oxide dimer acid (HFPO-DA). Perfluoroalkoxy alkane and high-density polyethylene tubing yielded relatively short absorptive measurement delays, with no clear dependence on tubing temperature or sampled humidity. Sampling through stainless steel tubing led to prolonged measurement delays due to reversible adsorption of PFAS to the tubing surface, with strong dependence on tubing temperature and sample humidification. Silcosteel tubing afforded shorter measurement delays than stainless steel due to diminished surface adsorption of PFAS. Characterizing and mitigating these tubing delays is crucial for reliable quantification of airborne PFAS.
Implications: Per- and polyfluoroalkyl substances (PFAS) are persistent environmental contaminants. Many PFAS are sufficiently volatile to exist as airborne pollutants. Measurements and quantification of airborne PFAS can be biased from material-dependent gas-wall interactions with sampling inlet tubing. Thus, characterizing these gas-wall interactions are crucial for reliably investigating emissions, environmental transport, and fates of airborne PFAS.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of anthropogenic compounds used in numerous industrial applications and consumer products (Glüge et al. Citation2020; Kotthoff et al. Citation2015). The widespread use of PFAS stems from their high thermal and chemical stability. This relative inertness also lends to slow or negligible chemical degradation upon release to the environment. PFAS are therefore capable of building up in the environment as persistent pollutants (Kwok et al. Citation2013; Pickard et al. Citation2018). This stability also makes PFAS resistant to biological breakdown, leading to high bioaccumulation potential (De Silva et al. Citation2021; Lesmeister et al. Citation2021). Exposure to certain PFAS has been linked to various negative health impacts including cancer and liver disease (Espartero et al. Citation2022; Fenton et al. Citation2021; Gaballah et al. Citation2020; Sunderland et al. Citation2019). These health concerns have led to a recent increase in federal and state regulations and monitoring of environmental PFAS in the U.S. (U.S. Congress Citation2021; EPA Citation2021).
Many PFAS are sufficiently volatile under ambient conditions to exist as airborne gas-phase pollutants (D’Ambro et al. Citation2021; Riedel et al. Citation2019). Thus, the atmosphere serves as an important environmental transport and exposure route for these pollutants (Davis et al. Citation2007; D’Ambro et al. Citation2021; Galloway et al. Citation2020; Kirkwood et al. Citation2022). The abundance of volatile PFAS in household consumer products and building materials has also led to their detection in indoor air, thereby serving as an important exposure route in residential and occupational settings (Langer, Dreyer, and Ebinghaus Citation2010; Morales-McDevitt et al. Citation2021; Schlummer et al. Citation2013; Shoeib et al. Citation2005). Despite this, measurements of environmental PFAS have historically focused on condensed phase media, including water and soil. Previous efforts to measure gas-phase PFAS in ambient air and source emissions (i.e., emission stacks from industrial facilities) typically involve offline measurements, which are subject to low time resolution, and require external laboratory analysis of samples (Nakayama et al. Citation2019).
Recent studies have applied online chemical ionization mass spectrometry (CIMS) instrumentation toward real-time measurements of gas-phase PFAS (Riedel et al. Citation2019, Citation2021). When utilizing iodide (I−) reagent ions, CIMS is sensitive and selective toward fluorotelomer alcohols (FTOHs), and perfluorinated carboxylic acids (PFCAs) including perfluoro ether carboxylic acids such as hexafluoropropylene oxide dimer acid (HFPO-DA; C6HF11O3) (Riedel et al. Citation2019, Citation2021). FTOHs are present in fluorosurfactants (Riedel et al. Citation2019), aqueous film-forming foams (AFFFs) (Riedel et al. Citation2019; Titaley et al. Citation2022), and various other consumer products (Buck et al. Citation2011; Gremmel, Frömel, and Knepper Citation2016; Liu et al. Citation2015). They may photochemically transform in the atmosphere to form PFCAs (Ellis et al. Citation2004). Long-chain PFCAs including perfluorooctanoic acid (PFOA; C8HF15O2) have been utilized in manufacturing and other industrial applications, but their use has been phased out due to their adverse health impacts and environmental persistence (Wang et al. Citation2014). Consequently, short-chain PFCAs such as perfluorobutanoic acid (PFBA; C4HF7O2) have recently seen increased use as substitutes for long-chain PFCAs (Wang et al. Citation2014; Chemical & Engineering News Citation2010). Another substitute for long-chain PFCAs is HFPO-DA, a component of GenX which has received attention as an emerging environmental pollutant (Chemical & Engineering News Citation2018). The EPA recently issued a drinking water health advisory for GenX chemicals, including HFPO-DA and its ammonium salt (EPA Citation2022).
Choice of tubing material used for sample inlet construction may substantially impact measurements of gas-phase analytes. Recent studies have demonstrated how sampled gas-phase organic compounds must establish gas-wall equilibrium with tubing, leading to delayed transmission and subsequent quantification (Deming et al. Citation2019; Liu et al. Citation2019; Pagonis et al. Citation2017). The nature of these gas-wall interactions, and resulting measurement delays strongly depend on the type of tubing material used (Deming et al. Citation2019; Pagonis et al. Citation2017). Organics sampled through polymeric tubing undergo absorptive partitioning into tubing walls (Deming et al. Citation2019; Pagonis et al. Citation2017). Resulting measurement delays are dependent on compound volatility, and independent of concentration and humidity (Deming et al. Citation2019; Pagonis et al. Citation2017). Sampling gas-phase organics through metallic or glass tubing result in adsorptive partitioning delays with a strong dependence on analyte concentration, functionality, and sample humidity (Deming et al. Citation2019; Pagonis et al. Citation2017). Additionally, tubing length and diameter, and sample flow rate can influence these measurement delays (Deming et al. Citation2019; Pagonis et al. Citation2017). The online, real-time mass spectrometry methods performed in these studies enabled researchers to probe the rapid (timescale ~minutes) gas-wall partitioning process driving these delays (Deming et al. Citation2019; Liu et al. Citation2019; Pagonis et al. Citation2017).
Analogous assessments of tubing-related measurement bias for gas-phase PFAS are lacking due to the paucity of real-time detection methods for these compounds. Characterizing these biases is essential for performing reliable measurements of gas-phase PFAS. To address this need, we use online CIMS measurements to characterize tubing-related measurement biases for 4:2 FTOH (C6H5F9O), PFBA, and HFPO-DA—three environmentally relevant short-chain PFAS with distinct oxygenated functionalities (Figure S5). We demonstrate how choice of tubing materials, tubing temperature, and sample humidity influence gas-wall interactions between these oxygenated PFAS and the sampling tubing, leading to biased quantification of these analytes.
Experimental methods
Tubing delay experiments
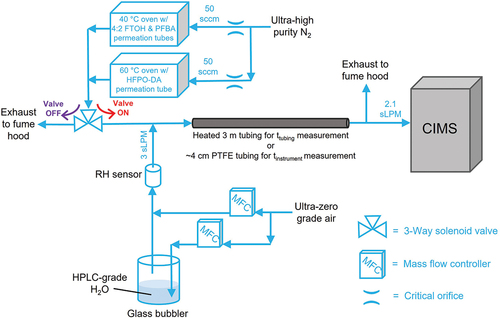
We present a schematic of the experimental setup in . We prepared permeation tubes of neat 4:2 FTOH (99% purity; Synquest Labs), PFBA (98% purity; Oakwood Chemical), and HFPO-DA (97% purity; Synquest Labs). We contained the 4:2 FTOH and PFBA permeation tubes in temperature-controlled oven (Dynacalibrator 190; VICI Metronics Inc.) held at 40°C, and the HFPO-DA permeation tube in a custom-built temperature-controlled oven (60°C). We delivered ~50 sccm dry ultra-high purity nitrogen (UHP N2; Airgas) regulated via a critical orifice to each temperature-controlled oven to generate gas-phase standards of 4:2 FTOH, PFBA, and HFPO-DA.
We directed a mixture of these PFAS standards to a three-way perfluoroalkoxy alkane (PFA) solenoid valve (Cole Palmer). When the valve was configured to the “on” state, PFAS standards were mixed with a dilution flow of 3 sLPM ultra-zero grade air (UZA; Airgas) delivered via MFC mass-flow controller (MFC; Alicat Scientific). UZA dilution flow was either dry (<0.5% relative humidity; RH) or humidified (48. ± 1.% RH at 25°C) with a glass bubbler containing HPLC grade H2O (Sigma Aldrich) (). We monitored UZA dilution RH in-line using a Rotronic Hygropalm HP23AW. This diluted PFAS standard then flowed through 3 m temperature-controlled (30–120°C) tubing (0.476 cm ID, 0.635 cm OD; Clayborn Lab) comprised of either PFA, high density polyethylene (HDPE), stainless steel (type 316), or Silcosteel (Restek) into an online CIMS instrument to monitor sampled PFAS mixing ratios in real-time (Lee et al. Citation2014; Riedel et al. Citation2019). We provide CIMS measurement details in the following section. Diluted PFAS standard mixing ratios sampled by the CIMS were ~1 ppbv 4:2 FTOH, ~5 ppbv PFBA, and ~5 ppbv HFPO-DA. Sampled PFAS flow through the 3 m tubing was laminar (Reynolds number ≤800) and had a residence time of ~ 1 s. Lengths of additional PFA tubing/connections (0.476 cm ID, 0.635 cm OD) in the sampling inlet construction were minimized (<30 cm) to mitigate additional measurements delays. When the solenoid valve was configured to the “off” state, PFAS standard flow was exhausted to a laboratory fume hood, and only dry or humidified UZA was sampled through the temperature-controlled tubing with the CIMS. We toggled this solenoid valve state to alternate between diluted PFAS standard sampling (i.e. tubing passivation), and UZA-only sampling (i.e. tubing depassivation) periods (). We typically performed triplicate passivation and depassivation cycles lasting ≥2 hours for each experimental perturbation tested. We began experiments with instrumental zeros, where CIMS background signals were monitored while sampling UZA for ≥2 hours. Stainless steel tubing required extensive depassivation times (≥12 hours) before experiments to mitigate hysteresis. To assess potential effects of sampling, a PFAS standard mixture versus individual compounds on measurement delays, we repeated experiments involving PFA and stainless steel tubing materials using only 4:2 FTOH standard.
We calculated total measurement delay times (ttotal) for PFAS standards as the sampling time required to achieve 95% of a measured equilibrium (i.e., steady-state) CIMS signal (Seq) during tubing passivation periods. For PFA, HDPE, and Silcosteel tubing, we quantified ttotal and Seq for sampled PFAS standards using double exponential fits to CIMS signal time series data in Igor Pro 7 (WaveMetrics, Inc.). Previous studies involving real-time measurements of gas-phase analytes have reported success in using double exponential functions to parameterize measurement delays attributable to inlet and/or instrument surfaces (Liu et al. Citation2019; Sauer et al. Citation2021; Veres et al. Citation2008). We discuss ttotal calculations for stainless steel tubing experiments in the Supplemental Information (SI). The interior surface of the CIMS ionization source is comprised of electropolished stainless steel, and therefore may act as a sorptive surface for sampled analytes (Krechmer et al. Citation2016; Liu et al. Citation2019). To decouple measurement delays attributable to the 3 m temperature-controlled tubing (ttubing) from those caused by the instrument (tinstrument), we repeated PFAS standard sampling experiments under dry and humidified conditions while replacing the 3 m tubing with a relatively short length (~4 cm) of polytetrafluoroethylene (PTFE) tubing (0.476 cm ID, 0.635 cm OD). From this, we calculated ttubing as the difference between ttotal and tinstrument (Deming et al. Citation2019; Liu et al. Citation2019; Pagonis et al. Citation2017). We expect sorptive delays from the ~4 cm PTFE tubing and PFA solenoid valve (0.476 cm ID, ~4 cm additional sampling length) were insubstantial compared to 3 m tubing lengths.
Chemical ionization mass spectrometry measurements
We performed fast (1 Hz), online measurements of gaseous oxygenated PFAS using a long time-of-flight chemical ionization mass spectrometer (CIMS; Tofwerk AG and Aerodyne Research Inc.) utilizing iodide (I−) reagent ions (Lee et al. Citation2014). We generated I− reagent ions by flowing ~2 sLPM ultra-high purity nitrogen (UHP N2; Airgas) through a temperature-controlled oven (40°C) containing a custom-built iodomethane (CH3I) permeation tube. The output gas mixture from the oven (containing N2 + CH3I) then flowed through a 210Po ionizer (NRD) to yield I− reagent ions, which subsequently entered the ion molecule reactor (IMR) of the CIMS. We sampled analytes into the IMR at a flow rate of ~2.1 sLPM, regulated via critical orifice. Analytes of interest (M) reacted with I− reagent ions in the IMR to form iodide-analyte adducts ([I+M]−), which were subsequently detected in real-time via time-of-flight mass spectrometry. We calibrated CIMS instrumental responses toward 4:2 FTOH, PFBA, and HFPO-DA following Riedel et al. (Citation2019). From these calibrations, we derived instrumental sensitivities of (1.0 ± 0.2) × 104, (1.0 ± 0.2) × 104, and (5. ± 1.) × 104 Hz ppbv−1 for 4:2 FTOH, PFBA, and HFPO-DA, respectively. We acquired CIMS mass spectra at 1 Hz using Acquility software (v2.3.13; Tofwerk AG) running in Igor Pro 7 (WaveMetrics Inc.). We acquired CIMS mass spectra between 30 and 1000 mass-to-charge (m/z) units. Mass spectral peak resolution (m/Δm) was ≥12000, and mass accuracy was typically <2 ppm.
We processed CIMS mass spectral data using Tofware (version 3.2.3; Tofwerk AG) running in Igor Pro 7. Briefly, this software performs mass calibration, mass spectral baseline calculation, and high-resolution peak fitting algorithms to extract CIMS signal time series from individual mass spectral peaks. We typically included I(H2O)−, I(CH2O2), I(HNO3), I2−, I3−, I(C4F7O2H)− and I(C6F11O3H)− as CIMS mass calibration peaks, as they were typically fully resolved, and persistent in instrumental background measurements. We performed CIMS mass calibrations using a three-parameter (px) fit (p1*(m/z)^p3+p2) to convert ion time-of-flight (ns) to m/z. We performed all further analyses of CIMS data in Igor Pro 7, including CIMS data normalization and background signal subtraction. We ensured changes in CIMS signal intensity were not driven by changes in reagent ion signal by normalizing CIMS time series data to total reagent ion signal. Here, we multiplied CIMS signals by the ratio of the averaged total reagent ion (I− + I(H2O)−) signal during instrumental background measurements to the real-time total reagent ion signal during periods of analyte measurement (Bertram et al. Citation2011).
Results and discussion
We demonstrate measurement delays associated with stepwise changes in sampled PFAS concentrations in . Sampling through PFA and HDPE tubing generally resulted in the shortest tubing delays (ttubing) for all compounds, while stainless steel produced the longest delays ( and S1a). Tubing delays were typically lowest for 4:2 FTOH, and highest for HFPO-DA (). We observed an analogous trend for instrument delays (tinstrument) (Figure S2). Instrument delays often contributed substantially to total measurement delays (ttotal), accounting ≤60% of ttotal for 4:2 FTOH, ≤87% of ttotal for PFBA, and ≤76% of ttotal for HFPO-DA. We briefly discuss tubing depassivation timescales in the SI, but focus our discussion of experimental results on comparing tubing passivation timescales.
Figure 2. CIMS signal time series for 4:2 FTOH (orange traces), PFBA (blue traces), and HFPO-DA (green traces) during (a) PFA tubing passivation sampling, (b) PFA tubing depassivation sampling, and stainless steel tubing passivation experiments under (c) dry conditions, and (d) humidified conditions. CIMS signals are normalized to signal magnitudes achieved at gas-wall equilibrium (i.e., Seq). Sinusoidal oscillations in signal observed in panel (d) are due to the heating cycle of the temperature-controlled tubing (see also Figure S4c).
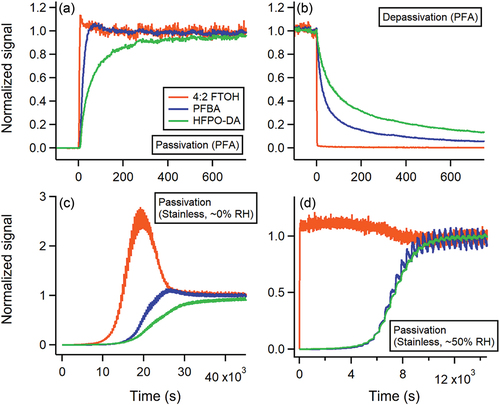
Figure 3. Tubing delay times (ttubing) for 4:2 FTOH (orange markers), PFBA (blue markers), and HFPO-DA (green markers) calculated for 3 m of various tubing materials, tubing temperatures, and humidity conditions while sampling (a) the three-compound mixture (i.e., 4:2 FTOH + PFA + HFPO-DA), and (b) sampling 4:2 FTOH only. Error bars correspond to ± one standard deviation of calculated ttubing. Horizontal dashed lines on plots visually indicate break in vertical axes. We provide a base-10 logarithm scaling of the vertical axis in Figure S1.
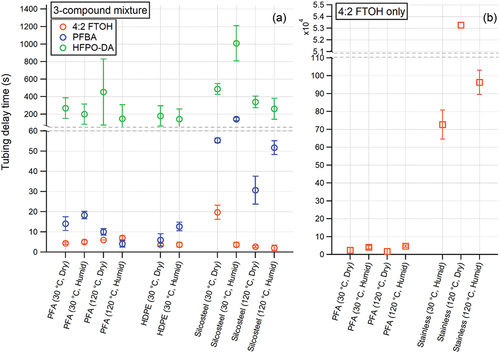
We report relatively short tubing delays for PFA and HDPE across all tubing temperature and humidification conditions tested, with ttubing ranging from 2 to 7 s for 4:2 FTOH, 4–18 s for PFBA, and 100–500 s for HFPO-DA (). This suggests the different chemical makeup of these tubing materials (fluoropolymer versus polyethylene polymer) did not substantially influence gas-wall equilibrium of the sampled PFAS mixture. These ttubing values increased with decreasing compound volatility (Table S1) (EPA Citation2018; Bhhatarai and Gramatica Citation2011; Krusic et al. Citation2005), and did not exhibit a clear dependence on sampled humidity, consistent with absorptive partitioning behavior expected between polymeric tubing material and gas-phase analytes (Deming et al. Citation2019; Pagonis et al. Citation2017). Interestingly, we did not observe a clear trend in ttubing as a function of tubing temperature for PFA. Absorptive delay times of organic molecules are diminished with increasing compound volatility, and augmented with increasing mass of polymeric tubing available for absorptive partitioning to take place (Deming et al. Citation2019; Pagonis et al. Citation2017). While increasing tubing temperature from 30 to 120°C would increase volatility of sampled PFAS, we hypothesize this was accompanied by an increase in absorptive mass available due to thermally-driven changes to the physical properties of the polymeric tubing, resulting in a “canceling out” of these two temperature effects on absorptive delay times (Deming et al. Citation2019; Pagonis et al. Citation2017). Sampling only 4:2 FTOH through PFA tubing yielded similarly low ttubing (<10 s) compared to the PFAS mixture, with no clear dependence on sampled humidity or tubing temperature ( and S1b). We observed transient spikes in 4:2 FTOH and PFBA signals above their respective Seq following initial passivation of PFA tubing. This is unlikely due to competitive displacement of 4:2 FTOH and PFBA by HFPO-DA, as transient spikes above Seq also occur while sampling 4:2 FTOH only through PFA tubing under dry and humidified conditions (Figure S6). Rather, a sampling artifact, e.g., transient pressurization of sampling lines upon switching of the PFA solenoid valve state may explain this phenomenon.
We compare experimental ttubing for PFBA and HFPO-DA through PFA tubing with values derived from the absorptive tubing delay model of Pagonis et al. (Citation2017). This model relates absorptive gas-wall partitioning to compound saturation vapor concentration (C*), or the ambient concentration at which a compound exists equally in gaseous and condensed phases (e.g., absorbed into walls of polymer tubing) (Pagonis et al. Citation2017). We estimated C* values of 7 × 107 and 4 × 106 µg m−3 for PFBA and HFPO-DA, respectively, as model inputs. We report additional model details in the SI. Modeled PFBA and HFPO-DA absorptive delays through PFA tubing were considerably underestimated compared to experimental values (Figure S3). Reproducing experimental PFBA and HFPO-DA tubing delays in the model required lowering C* by approximately two orders of magnitude. We speculate this discrepancy was influenced by uncertainties in the structure-activity relationship method for estimating C* for PFAS (D’Ambro et al. Citation2021). Additionally, it is unclear to what extent aqueous solubility of these PFAS influenced experimental tubing delays, which the model does not explicitly account for. For instance, Liu et al. (Citation2019). demonstrated that tubing delays of certain small polar molecules are driven by their solubility into thin films of water present on Teflon tubing surfaces, rather than by C*. However, a detailed mechanistic elucidation of these absorptive tubing delays lies beyond the scope of this work.
Sampling the PFAS mixture through stainless steel tubing resulted in prolonged measurement delays compared to PFA and HDPE. We visually compare time series from these experiments, and do not explicitly calculate ttubing for sampled compounds (). Sampling under dry conditions resulted in volatility-driven competitive adsorptive behavior, consistent with previous observations of gas-phase organics sampled through stainless steel tubing () (Deming et al. Citation2019; Pagonis et al. Citation2017). Here, we observe a transient increase in measured 4:2 FTOH above its gas-wall equilibrium level signal (i.e., Seq) as it is competitively displaced by PFBA, followed by a slight displacement of PFBA by HFPO-DA. Sampling times of >3 × 104 s would be necessary to mitigate tubing-related measurement biases of these compounds under these experimental conditions. Further, we observed negligible transmission (<1% of Seq) of sampled PFAS during the first 5 × 103 s of sampling. Sample humidification considerably decreased these measurement delays through stainless steel, due to the competitive adsorption of water molecules on active surface sites () (Deming et al. Citation2019). Here, we observed a relatively rapid equilibration in 4:2 FTOH signal (<60 s), followed by a slower rise in PFBA and HFPO-DA signals. While notably faster than dry conditions, the humidified PFAS mixture still required >2.5 hours to fully establish gas-wall equilibrium with stainless steel tubing.
We quantitatively evaluate the effects of sample humidity and tubing temperature on stainless steel tubing delays while sampling 4:2 FTOH only ( and S1b). We calculated ttubing of 5.3 × 104 s for 4:2 FTOH sampled through stainless steel tubing at 120°C under dry conditions. We observed <1% transmission of 4:2 FTOH in the first 1.2 × 104 s of sampling (Figure S4). We did not perform a corresponding dry experiment at a 30°C tubing temperature, but expect a lower tubing temperature would further augment ttubing due to a reduction in 4:2 FTOH volatility. Humidifying the sample greatly diminished this tubing delay, resulting in ttubing of 100 s. Interestingly, we observe an increased ttubing when raising tubing temperature from 30°C to 120°C under humidified conditions. We suspect this is due to the increased vapor pressure of water at higher tubing temperatures. The enhanced volatility of water would decrease its propensity to interact with the stainless steel surface, thereby reducing the strong surface-displacing effect of humidity on sampled PFAS.
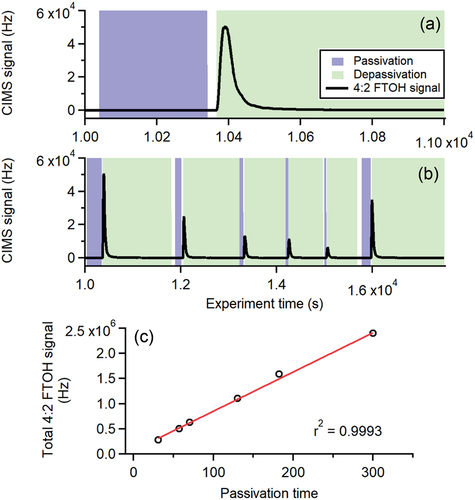
We demonstrate the reversible nature of adsorptive partitioning of sampled PFAS to stainless steel in . Here, we passivated the stainless steel tubing with 4:2 FTOH under dry conditions for varying periods of time (30–300 s), which coincided with insubstantial transmission (and therefore detection via CIMS) of 4:2 FTOH (). Following passivation periods, we depassivated the stainless steel tubing with humidified UZA (). Sampled water molecules competitively displaced the adsorbed 4:2 FTOH, leading to a spike and subsequent decay in measured 4:2 FTOH. The integrated 4:2 FTOH signal (i.e., total desorbed 4:2 FTOH) during depassivation directly correlated with passivation time (i.e., total sampled 4:2 FTOH) (). This indicates effectively all sampled 4:2 FTOH adsorbed to stainless steel tubing during these short passivation times, preceding competitive desorption by another species.
Figure 4. (a-b) CIMS signal time series for 4:2 FTOH (black traces) during systematic adsorption-desorption experiments with stainless steel tubing. Shaded blue periods correspond to 4:2 FTOH passivation under dry conditions, and shaded green periods correspond to 4:2 FTOH depassivation under humidified conditions as described in the main text. (c) Scatter plot demonstrating linear relationship between total (e.g., integrated) 4:2 FTOH CIMS signal, and passivation time. Red line corresponds to linear fit to data.
Tubing delays through Silcosteel were considerably shorter than stainless steel, but generally longer than PFA or HDPE (). The silicon-based coating on the tubing pre-passivates the stainless steel surface, presumably reducing adsorption and therefore measurement delays of sampled PFAS. Unlike stainless steel, the dependence of gas-wall equilibrium on sampled humidity is unclear for Silcosteel. We speculate the coated surface of this tubing also inhibits the adsorption of water molecules, thereby mitigating the humidity-dependent measurement delays observed for stainless steel tubing. However, temperature strongly impacted this gas-wall equilibrium for Silcosteel. We observed substantially lower ttubing for all sampled PFAS at a tubing temperature of 120°C compared to 30°C during both dry and humidified experiments, supportive of volatility-dependent gas-wall interactions of sampled PFAS with the Silcosteel tubing.
Conclusion
Our results highlight the importance of selecting appropriate inlet tubing materials when sampling airborne PFAS. Adsorption to stainless steel tubing led to prolonged measurement delays/losses of sampled FTOHs and PFCAs. These adsorptive delays were substantially impacted by tubing temperature and sampled humidity. Polymeric tubing materials such as PFA or HDPE are potentially more suitable for these measurements. Silcosteel tubing, and potentially other “pre-passivated” adsorptive tubing materials may be a suitable alternative to stainless steel if the use of polymeric tubing for PFAS sampling is untenable. For instance, PFA and other fluoropolymer tubing materials may outgas fluorinated organic molecules (Brophy and Farmer Citation2015; Veres et al. Citation2010), which could potentially interfere with gas-phase PFAS measurements.
This work serves as the first in-depth assessment of tubing material-dependent measurement biases of airborne PFAS. Given the selective scope of this work, future studies could explore effects of additional tubing materials/dimensions, additional PFAS functional groups, and PFAS concentrations on these measurement delays. Further, more realistic atmospheric matrices containing variable humidity and other traces gases which may influence gas-wall partitioning of sampled PFAS should be investigated. Mitigating tubing-related measurement bias is crucial for reliably investigating emissions, environmental transport, and fates of airborne PFAS.
PFAS_Tubing_Delay_-_Supporting_Information__revised_.docx
Download MS Word (1.5 MB)Acknowledgment
The U.S. EPA through its Office of Research and Development supported the research described here. It has been subjected to Agency administrative review and approved for publication but may not necessarily reflect official Agency policy. Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the U.S. Environmental Protection Agency. EPA and its employees do not endorse any commercial products, services, or enterprises. J.M.M. was supported by the Oak Ridge Institute for Science and Education (ORISE) Research Participation Program for the U.S. Environmental Protection Agency (EPA).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in ScienceHub at http://doi.org/10.23719/1527927, reference number D-0p2r.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10962247.2023.2174612.
Additional information
Notes on contributors
James M. Mattila
James M. Mattila is an Oak Ridge Institute for Science and Education (ORISE) postdoctoral fellow at the U.S. Environmental Protection Agency Office of Research and Development. He received his PhD in Chemistry form Colorado State University in 2021. His research focuses on studying airborne PFAS using online mass spectrometry instrumentation.
Emily Y. Li
Emily Y. Li is a Research Chemist with the U.S. Environmental Protection Agency Office of Research and Development. Her current research focuses on developing methods for characterization and mitigation solutions for air pollutant sources to assess and control emerging air emissions.
John H. Offenberg
John H. Offenberg is a Research Chemist at the U.S. Environmental Protection Agency Office of Research and Development. His research focuses on photochemical transformations in the atmosphere.
References
- Bertram, T. H., J. R. Kimmel, T. A. Crisp, O. S. Ryder, R. L. N. Yatavelli, J. A. Thornton, M. J. Cubison, M. Gonin, and D. R. Worsnop. 2011. A field-deployable, chemical ionization time-of-flight mass spectrometer. Atmos. Meas. Tech. 4 (7):1471–79. doi:10.5194/amt-4-1471-2011.
- Bhhatarai, B., and P. Gramatica. 2011. Prediction of aqueous solubility, vapor pressure and critical micelle concentration for aquatic partitioning of perfluorinated chemicals. Environ. Sci. Technol. 45 (19):8120–28. doi:10.1021/es101181g.
- Brophy, P., and D. K. Farmer. 2015. A switchable reagent ion high resolution time-of-flight chemical ionization mass spectrometer for real-time measurement of gas phase oxidized species: Characterization from the 2013 southern oxidant and aerosol study. Atmos. Meas. Tech. 8 (7):2945–59. doi:10.5194/amt-8-2945-2015.
- Buck, R. C., J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. De Voogt, A. A. Jensen, K. Kannan, S. A. Mabury, and S. P. van Leeuwen. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 7 (4):513–41. doi:10.1002/ieam.258.
- Chemical & Engineering News. 2010. Fluorochemicals Go Short. Accessed July 14, 2022. https://cen.acs.org/articles/88/i5/Fluorochemicals-Short.html
- Chemical & Engineering News. 2018. What’s GenX still doing in the water downstream of a Chemours plant? Accessed July 14, 2022. https://cen.acs.org/articles/96/i7/whats-genx-still-doing-in-the-water-downstream-of-a-chemours-plant.html
- D’Ambro, E. L., H. O. Pye, J. O. Bash, J. Bowyer, C. Allen, C. Efstathiou, R. C. Gilliam, L. Reynolds, K. Talgo, and B. N. Murphy. 2021. Characterizing the air emissions, transport, and deposition of per-and polyfluoroalkyl substances from a fluoropolymer manufacturing facility. Environ. Sci. Technol. 55 (2):862–70. doi:10.1021/acs.est.0c06580.
- Davis, K. L., M. D. Aucoin, B. S. Larsen, M. A. Kaiser, and A. S. Hartten. 2007. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 67 (10):2011–19. doi:10.1016/j.chemosphere.2006.11.049.
- Deming, B. L., D. Pagonis, X. Liu, D. A. Day, R. Talukdar, J. E. Krechmer, J. A. de Gouw, J. L. Jimenez, and P. J. Ziemann. 2019. Measurements of delays of gas-phase compounds in a wide variety of tubing materials due to gas–wall interactions. Atmos. Meas. Tech. 12 (6):3453–61. doi:10.5194/amt-12-3453-2019.
- De Silva, A. O., J. M. Armitage, T. A. Bruton, C. Dassuncao, W. Heiger-bernays, X. C. Hu, A. Kärrman, B. Kelly, C. Ng, and A. Robuck. 2021. PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem. 40 (3):631–57. doi:10.1002/etc.4935.
- Ellis, D. A., J. W. Martin, A. O. De Silva, S. A. Mabury, M. D. Hurley, M. P. Sulbaek Andersen, and T. J. Wallington. 2004. Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 38 (12):3316–21. doi:10.1021/es049860w.
- Espartero, L. J. L., M. Yamada, J. Ford, G. Owens, T. Prow, and A. Juhasz. 2022. Health-related toxicity of emerging per-and polyfluoroalkyl substances: Comparison to legacy PFOS and PFOA. Environ. Res. 212:113431. doi:10.1016/j.envres.2022.113431.
- Fenton, S. E., A. Ducatman, A. Boobis, J. C. DeWitt, C. Lau, C. Ng, J. S. Smith, and S. M. Roberts. 2021. Per-and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 40 (3):606–30. doi:10.1002/etc.4890.
- Gaballah, S., A. Swank, J. R. Sobus, X. M. Howey, J. Schmid, T. Catron, J. McCord, E. Hines, M. Strynar, and T. Tal. 2020. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ. Health Perspect. 128 (4):047005. doi:10.1289/EHP5843.
- Galloway, J. E., A. V. Moreno, A. B. Lindstrom, M. J. Strynar, S. Newton, A. A. May, and L. K. Weavers. 2020. Evidence of air dispersion: HFPO–DA and PFOA in Ohio and West Virginia surface water and soil near a fluoropolymer production facility. Environ. Sci. Technol. 54 (12):7175–84. doi:10.1021/acs.est.9b07384.
- Glüge, J., M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, X. Trier, and Z. Wang. 2020. An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ. Sci. Process Impacts 22 (12):2345–73. doi:10.1039/D0EM00291G.
- Gremmel, C., T. Frömel, and T. P. Knepper. 2016. Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets. Chemosphere 160:173–80. doi:10.1016/j.chemosphere.2016.06.043.
- Kirkwood, K. I., J. Fleming, H. Nguyen, D. M. Reif, E. S. Baker, and S. M. Belcher. 2022. Utilizing pine needles to temporally and spatially profile per-and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol. 56 (6):3441–51. doi:10.1021/acs.est.1c06483.
- Kotthoff, M., J. Müller, H. Jürling, M. Schlummer, and D. Fiedler. 2015. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 22 (19):14546–59. doi:10.1007/s11356-015-4202-7.
- Krechmer, J. E., D. Pagonis, P. J. Ziemann, and J. L. Jimenez. 2016. Quantification of gas-wall partitioning in Teflon environmental chambers using rapid bursts of low-volatility oxidized species generated in situ. Environ. Sci. Technol. 50 (11):5757–65. doi:10.1021/acs.est.6b00606.
- Krusic, P. J., A. A. Marchione, F. Davidson, M. A. Kaiser, C.-P. C. Kao, R. E. Richardson, M. Botelho, R. L. Waterland, and R. C. Buck. 2005. Vapor pressure and intramolecular hydrogen bonding in fluorotelomer alcohols. J. Phys. Chem. A. 109 (28):6232–41. doi:10.1021/jp0502961.
- Kwok, K. Y., E. Yamazaki, N. Yamashita, S. Taniyasu, M. B. Murphy, Y. Horii, G. Petrick, R. Kallerborn, K. Kannan, and K. Murano. 2013. Transport of perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: Implications for sources. Sci. Total Environ. 447:46–55. doi:10.1016/j.scitotenv.2012.10.091.
- Langer, V., A. Dreyer, and R. Ebinghaus. 2010. Polyfluorinated compounds in residential and nonresidential indoor air. Environ. Sci. Technol. 44 (21):8075–81. doi:10.1021/es102384z.
- Lee, B. H., F. D. Lopez-Hilfiker, C. Mohr, T. Kurten, D. R. Worsnop, and J. A. Thornton. 2014. An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: Application to atmospheric inorganic and organic compounds. Environ. Sci. Technol. 48 (11):6309–17. doi:10.1021/es500362a.
- Lesmeister, L., F. T. Lange, J. Breuer, A. Biegel-Engler, E. Giese, and M. Scheurer. 2021. Extending the knowledge about PFAS bioaccumulation factors for agricultural plants–a review. Sci. Total Environ. 766:142640. doi:10.1016/j.scitotenv.2020.142640.
- Liu, X., B. Deming, D. Pagonis, D. A. Day, B. B. Palm, R. Talukdar, J. M. Roberts, P. R. Veres, J. E. Krechmer, and J. A. Thornton. 2019. Effects of gas–wall interactions on measurements of semivolatile compounds and small polar molecules. Atmos. Meas. Tech. 12 (6):3137–49. doi:10.5194/amt-12-3137-2019.
- Liu, X., Z. Guo, E. E. Folk IV, and N. F. Roache. 2015. Determination of fluorotelomer alcohols in selected consumer products and preliminary investigation of their fate in the indoor environment. Chemosphere 129:81–86. doi:10.1016/j.chemosphere.2014.06.012.
- Morales-McDevitt, M. E., J. Becanova, A. Blum, T. A. Bruton, S. Vojta, M. Woodward, and R. Lohmann. 2021. The air that we breathe: Neutral and volatile PFAS in indoor air. Environ. Sci. Technol. Lett. 8 (10):897–902. doi:10.1021/acs.estlett.1c00481.
- Nakayama, S. F., M. Yoshikane, Y. Onoda, Y. Nishihama, M. Iwai-Shimada, M. Takagi, Y. Kobayashi, and T. Isobe. 2019. Worldwide trends in tracing poly-and perfluoroalkyl substances (PFAS) in the environment. TrAc. Trends. Anal. Chem. 121:115410. doi:10.1016/j.trac.2019.02.011.
- Pagonis, D., J. E. Krechmer, J. de Gouw, J. L. Jimenez, and P. J. Ziemann. 2017. Effects of gas–wall partitioning in Teflon tubing and instrumentation on time-resolved measurements of gas-phase organic compounds. Atmos. Meas. Tech. 10 (12):4687–96. doi:10.5194/amt-10-4687-2017.
- Pickard, H. M., A. S. Criscitiello, C. Spencer, M. J. Sharp, D. C. Muir, A. O. De Silva, and C. J. Young. 2018. Continuous non-marine inputs of per-and polyfluoroalkyl substances to the High Arctic: A multi-decadal temporal record. Atmos. Chem. Phys. 18 (7):5045–58. doi:10.5194/acp-18-5045-2018.
- Riedel, T. P., J. R. Lang, M. J. Strynar, A. B. Lindstrom, and J. H. Offenberg. 2019. Gas-phase detection of fluorotelomer alcohols and other oxygenated per-and polyfluoroalkyl substances by chemical ionization mass spectrometry. Environ. Sci. Technol. Lett. 6 (5):289–93. doi:10.1021/acs.estlett.9b00196.
- Riedel, T. P., M. A. G. Wallace, E. P. Shields, J. V. Ryan, C. W. Lee, and W. P. Linak. 2021. Low temperature thermal treatment of gas-phase fluorotelomer alcohols by calcium oxide. Chemosphere 272:129859. doi:10.1016/j.chemosphere.2021.129859.
- Sauer, J. S., R. Simkovsky, A. N. Moore, L. Camarda, S. L. Sherman, K. A. Prather, and R. S. Pomeroy. 2021. Continuous measurements of volatile gases as detection of algae crop health. Proc. Natl. Acad. Sci. U.S.A. 118 (40):e2106882118. doi:10.1073/pnas.2106882118.
- Schlummer, M., L. Gruber, D. Fiedler, M. Kizlauskas, and J. Müller. 2013. Detection of fluorotelomer alcohols in indoor environments and their relevance for human exposure. Environ. Int. 57-58:42–49. doi:10.1016/j.envint.2013.03.010.
- Shoeib, M., T. Harner, B. H. Wilford, K. C. Jones, and J. Zhu. 2005. Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: Occurrence, partitioning, and human exposure. Environ. Sci. Technol. 39 (17):6599–606. doi:10.1021/es048340y.
- Sunderland, E. M., X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner, and J. G. Allen. 2019. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 29 (2):131–47. doi:10.1038/s41370-018-0094-1.
- Titaley, I. A., J. Khattak, J. Dong, C. I. Olivares, B. DiGuiseppi, C. C. Lutes, and J. A. Field. 2022. Neutral Per-and Polyfluoroalkyl Substances, Butyl Carbitol, and Organic Corrosion Inhibitors in Aqueous Film-Forming Foams: Implications for Vapor Intrusion and the Environment. Environ. Sci. Technol. doi:10.1021/acs.est.2c02349
- U.S. Congress. 2021. H.R.2467 - PFAS Action Act of 2021. Accessed July 14, 2022. https://www.congress.gov/bill/117th-congress/house-bill/2467?q=%7B
- U.S. EPA. 2021. Contaminant Candidate List (CCL) and Regulatory Determination. Accessed July 14, 2022. https://www.epa.gov/ccl/regulatory-determination-4
- U.S. EPA. 2018. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN 62037-80-3) Also Known as “GenX Chemicals”.
- U.S. EPA. 2022. Drinking Water Health Advisory: Hexafluoropropylene Oxide (HFPO) Dimer Acid (CASRN 13252-13-6) and HFPO Dimer Acid Ammonium Salt ( CASRN 62037-80-3), Also Known as “GenX Chemicals”.
- Veres, P., J. M. Roberts, I. R. Burling, C. Warneke, J. de Gouw, and R. J. Yokelson. 2010. Measurements of gas-phase inorganic and organic acids from biomass fires by negative-ion proton-transfer chemical-ionization mass spectrometry. J. Geophys. Res.: Atmos 115 (D23). doi:10.1029/2010JD014033.
- Veres, P., J. M. Roberts, C. Warneke, D. Welsh-Bon, M. Zahniser, S. Herndon, R. Fall, and J. de Gouw. 2008. Development of negative-ion proton-transfer chemical-ionization mass spectrometry (NI-PT-CIMS) for the measurement of gas-phase organic acids in the atmosphere. Int. J. Mass. Spectrom 274 (1–3):48–55. doi:10.1016/j.ijms.2008.04.032.
- Wang, Z., I. T. Cousins, M. Scheringer, R. C. Buck, and K. Hungerbühler. 2014. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environ. Int. 70:62–75. doi:10.1016/j.envint.2014.04.013.