ABSTRACT
Background: Intrathecal morphine can be considered as a gold standard for analgesia following cesarean section (CS), which is not devoid of complications namely postoperative nausea and vomiting. We evaluated the antiemetic effect of intravenous dexamethasone combined with intrathecal atropine after CS.
Methods: 120 parturient undergoing elective CS under spinal anesthesia were randomized into three groups. Dexamethasone group (D): Received intrathecal hyperbaric bupivacaine (0.5% in 2 ml) mixed with morphine (200 µg in 0.5 ml) and normal saline (0.5 ml as placebo) and intravenous (iv) dexamethasone (8 mg in 2 ml). Atropine group (A): Received hyperbaric bupivacaine (0.5% in 2 ml) mixed with morphine (200 µg in 0.5 ml) and atropine (100 µg in 0.5 ml), in addition to iv normal saline (2 ml as placebo). Dexamethasone and Atropine group (DA): Received intrathecally as group A, and iv dexamethasone (8 mg in 2 ml). Follow-up of both nausea and vomiting was done during the first 24 hours postoperatively.
Results: Nausea was noticed in 7 patients (17.5%) in group D, 8 patients (20%) in group A, and one patient (2.5%) group DA, with significant differences between DA and D (p = 0.025) and DA and A (p = 0.013). Regarding vomiting, there were 5 patients (12.5%) in group D, 4 patients (10%) in group A only, with significant differences between DA and D (p = 0.021) and DA and A (p = 0.041).
Conclusions: Combination of intravenous dexamethasone and intrathecal atropine has additive antiemetic effect after spinal anesthesia for cesarean delivery using bupivacaine and morphine.
1. Introduction
Pain is a major problem during the postoperative period and can be considered as a challenge for the anesthesiologist. Proper post-operative pain alleviation improves the prognosis, decreases the morbidity, and increases patient satisfaction [Citation1]. Neuraxial morphine can be considered as a gold standard for analgesia following cesarean section (CD). Intrathecal (IT) morphine is not devoid of complications namely pruritus, and post-operative nausea and vomiting (PONV) [Citation2,Citation3].
Studies have noted that minor opioid-related drawbacks after neuraxial morphine injection are not related to the dose and can even occur with very small doses of morphine. The incidence of PONV in patients who received IT opioids is 60% – 80% [Citation4,Citation5]. The other risk factors for the development of PONV include female gender, non-smoker status, general anesthesia with inhalational anesthetics, and surgical factors (duration and type of surgery) [Citation6].
A lot of modalities for prevention and treatment of neuraxial opioid-induced PONV have been evolved. Antagonists of 5‐HT3 e.g. ondansetron and granisetron have good efficacy, but their cost limits their use [Citation7]. Dopamine receptor antagonists e.g. droperidol, prochlorperazine, and metoclopramide are commonly used, but they carry the risk of extrapyramidal symptoms [Citation8].
On the other hand, corticosteroids have been proven to be effective for prevention of PONV, which could be associated with general anesthesia [Citation8], and reduction of PONV in women undergoing pelvic surgery and CS under epidural anesthesia with morphine as an adjuvant [Citation9]. Intrathecal atropine also carries a significant antiemetic effect, and this can make it a valuable pharmacologic modality for the prevention of intrathecal opioid-related PONV [Citation10,Citation11].
Based on these studies, we have established our hypothesis to investigate the utility of intravenous (iv) dexamethasone combined with intrathecal (IT) atropine for prevention of morphine-induced PONV as a primary outcome in parturient undergoing CS under spinal bupivacaine anesthesia plus morphine sulfate as an adjuvant.
2. Materials and methods
2.1. Study design
This double-blinded randomized controlled clinical trial was carried out at Woman Health Hospital (Assiut University, Egypt), during the period between March and August 2017. The study was established in accordance with the CONSORT Statement for Reporting Trials as shown in . It was firstly approved by Faculty of Medicine, Assiut University local ethics committee under the number of IRB00009916 and registered in clinical trials under the number of NCT03387956. Informed written consent has been obtained from each participant.
2.2. Inclusion criteria
One hundred twenty parturient, ASA physical status I–II, undergoing elective CS under bupivacaine spinal anesthesia in conjunction with IT morphine were included in this study. Exclusion criteria included patients with systemic diseases, e.g. gastric, esophageal, renal or hepatic problems, those with a history of chronic cough, smoking, morbid obesity, retching, vomiting, moderate to severe nausea in 24 hours preceding anesthesia. Patients who have contraindications for IT injection e.g. coagulopathy, and or infection at the suspected injection site were excluded as well.
2.3. Randomization
The participants were equally randomized into three groups through a web-based randomizer (https://www.randomizer.org) and randomization tables were generated and enclosed in a sealed envelope. Just prior to anesthesia, an anesthetist (Not sharing in the study) has prepared the intravenous and intrathecal solutions (The solution volume was equal in all groups; 3 ml for intrathecal injection, and 2 ml for iv injection) in color-coded syringes under completely sterile conditions according to the group which patient’s number assumed to be in it. The solutions were then given to the anesthetist who was kept blind to the definite mixture of the prepared solutions and responsible for anesthesia. The outcome assessing physician and the patients also remained blind to the grouping until the end of the study. Decoding of the collected data results (group-based) was finally done by the statistician.
2.4. Anesthesia technique
Upon arrival to the operating room, patients were monitored with non-invasive blood pressure, ECG, and pulse oximetry. An intravenous 18-gauge cannula was inserted in a peripheral vein for fluid administration [Ringer’s lactate solution (10 ml/kg)]. After careful antiseptic preparation with the patient was kept in a sitting position, the subarachnoid injection was done through a 25-gauge Whitacre spinal needle at L4–L5 interspaces. Then the patient was allowed to lay down in bed with a 15° tilting of the patient to the left by a wedge placed under the right hip. A prophylactic iv ephedrine sulfate was given in a bolus dose of 5 mg. Urinary bladder catheter was inserted to all the parturient after intrathecal injection.
2.5. Study groups
40 patients in each group as follows:
Dexamethasone group (D): Patients received intrathecal hyperbaric bupivacaine, (Marcaine spinal heavy 0.5% by Astra Zeneca, Buyukdere Cad.) in a dose of 10 mg (2 ml) mixed with morphine (Morphine Sulfate by Dawaya, Egypt) in a dose of 200 µg (0.5 ml was withdrawn from a syringe containing 4 mg morphine sulfate diluted in 10 ml normal saline) and normal saline (0.5 ml) as placebo [Total volume 3 ml], followed by iv dexamethasone (Dexamethasone by AMRIYA, Egypt) 8 mg (2 ml).
Atropine group (A): Patients received intrathecal hyperbaric bupivacaine 10 mg (2 ml 0.5%) mixed with morphine 200 µg (0.5 ml) and atropine (Atropine sulfate CID by Dawaya, Egypt) 100 µg (0.5 ml was withdrawn from a syringe containing 2 mg atropine sulfate diluted in 10 ml normal saline) [Total volume 3 ml], followed by iv injection of normal saline (2 ml as placebo).
Dexamethasone and Atropine group (DA): Patients received intrathecal hyperbaric bupivacaine in a dose of 10 mg (2 ml) mixed with morphine 200 µg (0.5 ml) and atropine 100 µg (0.5 ml) [Total volume 3 ml], followed by iv injection of dexamethasone 8 mg (2 ml).
All episodes of PONV during the follow-up period as a primary goal monitored through a direct questionnaire and recorded according to scales shown in [Citation12]. Grade 2 or more, nausea and/or vomiting with stable hemodynamic were treated by iv injection of 4 mg ondansetron. Incidence of postoperative itching was monitored and treated with 25 mg iv diphenhydramine. Respiratory depression (RR <8 breaths/min or SPO2 < 90%) was managed with oxygen therapy, non-invasive or invasive ventilation as appropriate. Pain intensity was assessed by using a visual analog pain scale (VAS with 0 = no pain and 10 = the worst imaginable pain) every two hours during the first 24 hours postoperatively. Patients were not being awakened during sleep (VAS was considered to be < 3). Rescue analgesia was given in the form of iv ketorolac tromethamine (30 mg) if the visual analog scale (VAS) has reached ≥3.
Table 1. Grading of nausea and vomiting severity.
Non-invasive blood pressure was controlled after spinal anesthesia every two minutes until the fetus delivery, then every five minutes until the end of operative intervention. Heart rate, systolic, diastolic and mean arterial blood pressures and respiratory rate were recorded before intrathecal injection and every two hours during the first 6 hours, then every 6 hours along the next 18 hours. The sensory level was checked bilaterally regarding the blunt pinprick and temperature (frozen plastic ampules of sterile water) along the midclavicular line. The motor block was evaluated through the modified Bromage scale (0 = extended leg lift, 1 = just knee flexion, 2 = foot movement only, 3 = no movement absolutely). After intrathecal injection, the onset and duration of sensory were recorded. Intraoperative hypotension was defined as systolic blood pressure <100 mmHg or a decrease of it >20% from the baseline. Episodes of intraoperative hypotension were treated with iv ephedrine sulfate boluses of 5 mg as required. After the end of the follow-up period, the mean of each hemodynamic parameter and respiratory rate were calculated and compared.
2.6. Statistical analysis
A sample size of 38 patients per group was calculated and guided by a previous study [Citation10] which estimated the incidence of morphine induce PONV as 56%. This sample size could be sufficient to detect a 20% reduction in the incidence of PONV after adding atropine to intrathecal morphine with a study power of 90% and at the significance level of 5%. We added two patients in each group to compensate for dropouts. All data were analyzed by computer program IBM, SPSS (Statistical Package for Social Sciences), Version 23, 2015, and firstly tested through the Anderson-Darling test for normality and homogeneity of variances. Categorical variables were described as a number (ratio), whereas continuous variables described as mean ± standard deviation (SD). Parametric continuous variables were compared by the one-way ANOVA test, whereas non-parametric variables by Kruskal-Wallis test. Chi-square test was used to compare categorical variables. For all statistical tests the p-value <0.05 was considered a statistically significant difference (confidence interval 95%).
3. Results
As shown in the CONSORT flow-chart 120 patients were included and completed the study period (). The patients in the three groups were comparable regarding their demographic and operative data (p > 0.05) as shown in .
Table 2. Demographic and operative data in the three groups.
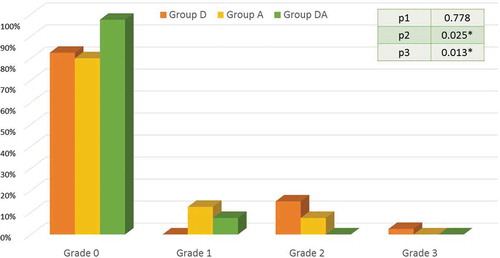
Postoperative nausea in the first 24 hours postoperatively has occurred in 7 patients 17.5% (95% CI = 5.1% – 29.8%) in group D (grade 2 = 6 patients and grade 3 = 1 patient), 8 patients 20% (95% CI = 7.0% – 32.9%) in group A (grade 1 = 5 patients and grade 2 = 3 patient), and one patients 2.5% (95% CI = (−2.5% – 7.5%) in group DA had nausea (grade 1 = 1). There were significant differences between DA and D groups, as well as, DA and A groups as shown in .
Figure 2. The incidence and grades of nausea in the three study groups.
Caption: Data are expressed as percentage. Group D = Dexamethasone group. Group A = Atropine group. Group DA = Dexamethasone and atropine group. P1 between groups D and A, P2 between groups D and DA, P3 between groups A and DA. p < 0.05 = *significant difference.
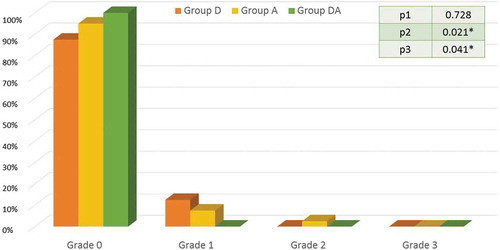
Five patients 12.5% (95% CI = 1.7% – 23.2%) in group D had vomiting (grade 1), 4 patients 10% (95% CI = 0.2% – 19.7%) in group A had vomiting (grade 1 = 3 patients and grade 2 = 1 patient) and no patient 0.0% in group DA had vomiting. There were significant differences between DA and D groups, as well as, DA and A groups as shown in .
Figure 3. The incidence and grades of vomiting in the three study groups.
Caption: Data are expressed as percentage. Group D = Dexamethasone group. Group A = Atropine group. Group DA = Dexamethasone and atropine group. P1 between groups D and A, P2 between groups D and DA, P3 between groups A and DA. p < 0.05 = *significant difference.
shows statistically insignificant differences between the three groups as regards to the mean 24 hours cardiorespiratory parameters and VAS pain score. The incidence of intraoperative hypotension showed non-significant differences between the three study groups. The hypotension episodes were not correlated to nausea and vomiting. On the other hand, the incidence of postoperative itching was significantly higher in group A when compared to group D and group DA. No recuse analgesia was requested during the 1st postoperative day and VAS was almost always ≤ 2 in all patients.
Table 3. Cardiorespiratory parameters, characteristics of sensory block, visual analog pain scale, and itching in the three study groups.
4. Discussion
This study involved 120 female patients undergoing cesarean delivery under spinal anesthesia. Our results revealed that the incidence of PONV in the iv dexamethasone combined with IT atropine DA group was 2.5% for nausea and 0.0% for vomiting and no patient required iv ondansetron compared to both iv dexamethasone group D and IT atropine group A. Dexamethasone group D has the incidence of 17.5% and 12.5% for nausea and vomiting respectively when compared to atropine only group A, which has nausea and vomiting incidence of 20% and 10% in consequence.
Up to our knowledge, this is the first clinical study which used both dexamethasone and atropine in one group and compared the effect of such combination upon PONV in comparison to atropine or dexamethasone in another two separate groups. Additionally, one study was done by Bagomedov et al. and compared three intravenous medications for prevention of intraoperative nausea, vomiting, and abdominal discomfort during caesarian section under spinal anesthesia in three groups of 150 women. They mentioned that intravenous atropine in a small dose (0.006–0.009 mg/kg) was superior to dexamethasone (0.04–0.1 mg/kg) and droperidol (0.08–0.12 mg/kg) [Citation13]. The slight differences between our results and this study could be assumed to the variability of the dose and route of atropine administration.
Dexamethasone exerts its antiemetic action through its effect on glucocorticoid receptors which found in the nucleus of the solitary tract, the raphe nucleus, and the area postrema [Citation14]. These nuclei are well known to have significant neuronal activity in the regulation of nausea and vomiting. Dexamethasone has been reported to be effective for PONV prevention in patients undergoing tonsillectomy [Citation15], thyroidectomy [Citation8], cholecystectomy [Citation16], and cesarean section under epidural [Citation17] or spinal anesthesia with morphine [Citation18]. It is also effective in preventing nausea and vomiting associated with chemotherapy [Citation19]. D’Souza et al. noticed that a single dose of dexamethasone was safe, effective, had a lesser cost and could be a good alternative to single dose ondansetron for the PONV prevention after the gynecologic laparoscopic intervention [Citation20].
Interestingly, Baciarello and his colleagues had mentioned that a small-dose IT atropine significantly reduced the relative risk of PONV, and they mentioned that IT atropine exerts its central nervous system effects with minimal or no involvement of extra-neural muscarinic receptors [Citation10]. Based on these observations and the availability of a preservative-free preparation, the obstetric anesthesia team of our department began a prospective exploratory analysis using atropine as an adjunct to standard subarachnoid anesthesia as prophylaxis for PONV after the approval of our institution’s medical direction board and the local Ethics Committee.
In 1996, Ramaioli described the benefit of adding atropine (20µg) to intrathecal morphine to prevent post-operative emesis [Citation11]. On the other hand, iv atropine as a postoperative antiemetic was earlier described by Chhibber et al. in 1999. They observed that the reversal of neuromuscular blockade with atropine and neostigmine was associated with a lower incidence of postoperative emesis compared with glycopyrrolate and neostigmine in children undergoing tonsillectomy [Citation21]. Additionally, a study done by Ozcan et al. concluded that the premedication with diazepam and atropine sulfate decreases nausea and vomiting after strabismus surgery [Citation22].
Gehling and his colleges’ study results demonstrated a clear and clinically significant antiemetic effect of atropine when administered with IT bupivacaine and morphine for the scheduled Cesarean section. The IT atropine was significantly valuable in comparison to the preoperative intravenous atropine administration as regards the problem of PONV [Citation23].
Hypotension could be the most important cause of intraoperative nausea and vomiting that occurs during this type of surgeries under spinal anesthesia. Hypotension can induce emetic symptoms by leading to cerebral hypoperfusion [Citation24]. According to our results, there were insignificant differences between groups regarding hemodynamic parameters, and the changes which occurred within each group were within clinically accepted ranges. No participant has requested rescue analgesia during the follow-up period. Our results regarding such two interests are in agreement with the previously mentioned studies [Citation10,Citation23].
4.1. Limitations
The total consumption of diphenhydramine and ondansetron were not recorded. Diphenhydramine has an antiemetic effect and could have some conflicts with the study of drugs. We have used regular morphine with preservative, and actually, this is the only available form in our country.
5. Conclusion
Combination of IV dexamethasone (8 mg) and IT atropine (100 µg) had additive antiemetic effect after spinal anesthesia for cesarean delivery using bupivacaine with morphine (0.3 mg). This combination showed a safety profile upon hemodynamics and did not affect the analgesic efficacy of morphine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Araujo R. Pain management, local infection, satisfaction, adverse effects and residual pain after major open abdominal surgery: epidural versus continuous wound infusion (PAMA Trial). Acta Med Port. 2017;30:683–690.
- Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114:813–822.
- Kumar K, Singh SI. Neuraxial opioid-induced pruritus: an update. J Anaesthesiol Clin Pharmacol. 2013;29:303–307.
- Raffaeli W, Marconi G, Fanelli G, et al. Opioid-related side-effects after intrathecal morphine: a prospective, randomized, double-blind dose-response study. Eur J Anaesthesiol. 2006;23:605–610.
- Szarvas S, Chellapuri RS, Harmon DC, et al. A comparison of dexamethasone, ondansetron, and dexamethasone plus ondansetron as prophylactic antiemetic and antipruritic therapy in patients receiving intrathecal morphine for major orthopedic surgery. Anesth Analg. 2003;97:259–263.
- Rüsch D, Eberhart LHJ, Wallenborn J, et al. Nausea and vomiting after surgery under general anesthesia: an evidence-based review concerning risk assessment, prevention, and treatment. Dtsch Arztebl Int. 2010;107:733–741.
- Fujii Y, Tanaka H, Toyooka H. Granisetron-dexamethasone combination reduces postoperative nausea and vomiting. Can J Anaesth. 1995;42:387–390.
- Wang JJ, Ho ST, Lee SC, et al. The prophylactic effect of dexamethasone on postoperative nausea and vomiting in women undergoing thyroidectomy: a comparison of droperidol with saline. Anesth Analg. 1999;89:200–203.
- Ho ST, Wang JJ, Tzeng JI, et al. Dexamethasone for preventing nausea and vomiting associated with epidural morphine: a dose-ranging study. Anesth Analg. 2001;92:745–748.
- Baciarello M, Cornini A, Zasa M, et al. Intrathecal atropine to prevent postoperative nausea and vomiting after Cesarean section: a randomized, controlled trial. Minerva Anestesiol. 2011 Aug 1;77(8):781.
- Ramaioli F, De Amici D. Central antiemetic effect of atropine: our personal experience. Can J Anaesth. 1996;43:1079.
- Han DW, Hong SW, Kwon JY, et al. Epidural ondansetron is more effective to prevent postoperative pruritus and nausea than intravenous ondansetron in elective cesarean delivery. Acta Obstet Gynecol Scand. 2007;86:683–687.
- Bagomedov RG, Slepushkin VD, Omarova K. Prophylaxis of intraoperative nausea, vomiting and abdominal discomfort due to spinal anaesthesia for caesarian operation. Anesteziol Reanimatol. 2014;1:38–40.
- Morimoto M, Morita N, Ozawa H, et al. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269.
- Splinter WM, Roberts DJ. Dexamethasone decreases vomiting by children after tonsillectomy. Anesth Analg. 1996;83:913–916.
- Wang JJ, Ho ST, Liu YH, et al. Dexamethasone reduces nausea and vomiting after laparoscopic cholecystectomy. Br J Anaesth. 1999 Nov 1;83(5):772–775.
- Wang -J-J, Ho S-T, Wong C-S, et al. Dexamethasone prophylaxis of nausea and vomiting after epidural morphine for post-Cesarean analgesia. Can J Anaesth. 2001;48:185.
- Cardoso MM, Leite AO, Santos EA, et al. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section: a randomised, placebo-controlled, double-blind trial. Eur J Anaesthesiol. 2013;30:102–105.
- Italian Group for Antiemetic Research. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med. 1995;332:1–5.
- D’Souza N, Swami M, Bhagwat S. Comparative study of dexamethasone and ondansetron for prophylaxis of postoperative nausea and vomiting in laparoscopic gynecologic surgery. Int J Gynaecol Obstet. 2011;113:124–127.
- Chhibber AK, Lustik SJ, Thakur R, et al. Effects of anticholinergics on postoperative vomiting, recovery, and hospital stay in children undergoing tonsillectomy with or without adenoidectomy. Anesthesiology. 1999;90:697–700.
- Ozcan AA, Gunes Y, Haciyakupoglu G. Using diazepam and atropine before strabismus surgery to prevent postoperative nausea and vomiting: a randomized, controlled study. J Aapos. 2003;7:210–212.
- Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. 2009;64:643–651.
- Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth. 2005;14:230–241.