ABSTRACT
Background: This study aimed to compare pregabalin and midazolam premedication in pediatric day-case surgery regarding anesthetic and analgesic requirements.Methods: Sixty patients scheduled for day-case surgery were randomly premedicated orally with either pregabalin (Group P) or midazolam (Group M). Intraoperative anesthetic and analgesic requirements were assessed as primary outcomes. Modified Ramsay Sedation Score (RSS), hemodynamic variables, time to eye opening and time to extubation after discontinuation of general anesthesia were measured. Post-anesthesia care unit (PACU) duration of stay, postoperative Pediatric Anesthesia Emergence Delirium (PAED) scale, and Faces Legs Activity Cry Consolability (FLACC) scale, and post-operative analgesic requirements were assessed as secondary outcomes. The study was pre-registered at the Pan African Clinical Trials Registry (registration number: PACTR201907535888819; date of registration: 22/07/2019).Results: We found no significant differences between the studied groups regarding types and duration of surgery, preoperative modified RSS, and postoperative PAED. The mean ranks of sevoflurane and fentanyl consumptions were significantly lower in group P (p < 0.001). Patients in group P showed significantly lower means of heart rate and arterial blood pressure after induction of anesthesia. Postoperative FLACC pain score showed a significantly lower mean rank in group P. The need for rescue analgesics and the mean ranks of rectal diclofenac and meperidine consumption was significantly lower in group P (p < 0.001). The mean ranks of total paracetamol doses were comparable in both groups (p = 0.090). Group P showed a significantly lower duration of stay in PACU and time to open the eye and to extubate, but significantly higher time to first analgesic requirement.Conclusion: Pregabalin significantly reduced the perioperative anesthetic and analgesic requirements, the PACU duration of stay, and the time to open the eye and to extubate, in comparison to midazolam; therefore, its use as a premedication in pediatric day-case surgery is recommended.
1. Introduction
Children are excellent candidates for day-case management as they are usually healthy, and they predominantly require minor or intermediate surgery of short duration [Citation1–3].
An effective premedication not only facilitates a smoother induction and recovery from general anesthesia with minimal hemodynamic alterations, reduces emergence delirium, and minimizes the emotional trauma in children undergoing surgery, but may also ameliorate anesthetic and analgesic requirements [Citation4].
Midazolam is a popular sedative and anxiolytic agent in pediatric surgery [Citation5]. Gabapentinoids, which include gabapentin and pregabalin, is structural analogs of γ-aminobutyric acid with antiallodynic and antihyperalgesic properties. Their efficacy in improving postoperative pain scores has been proven over the last years [Citation6]. Also, the anxiolytic effect of pregabalin may arise through its high-affinity binding to the alpha-2-delta sub-unit of the P/Q type voltage-gated calcium channel, thereby reducing the release of excitatory neurotransmitters. It has a significantly higher potency and binding affinity for the α2–δ subunit compared to that of gabapentin [Citation7]. However, clinical experience with the safety and efficacy of gabapentinoids for the treatment of pediatrics’ pain and agitation is relatively limited [Citation8,Citation9].
This study was conducted to compare pregabalin and midazolam as a premedication in pediatric day-case surgery regarding intraoperative anesthetic and analgesic requirements as primary outcomes and modified Ramsay Sedation Score (RSS), post-anesthesia care unit (PACU) duration of stay, postoperative Pediatric Anesthesia Emergence Delirium (PAED), Faces Legs Activity Cry Consolability (FLACC) scales, and post-operative analgesic requirements as secondary outcomes.
2. Methods
2.1. Ethical considerations
The study protocol was approved by our institutional review board (FWA 000017585, 02/06/2019). We obtained informed written consents from the patients’ guardians. All patients’ data were kept confidential after assigning a code number to each patient known only by the researchers. The study was pre-registered at the Pan African Clinical Trials Registry (registration number: PACTR201907535888819; date of registration: 22/07/2019).
2.2. Study setting, date, design, and eligibility criteria
Sixty patients aged 6 months to 6 years scheduled for day-case surgery in Ain Shams University Hospitals during July 2019 to February 2020 were included in this study. This was a randomized, double-blinded, parallel-group, controlled trial. Patients were randomly allocated to two groups (30 patients each); pregabalin (Group P) and midazolam (Group M). We excluded patients with any of the following: mental retardation or developmental delay that make pain assessment difficult, hypersensitivity to the study medications, use of psychiatric medications, a current diagnosis of neuropathic pain, history of seizure disorders, any serious or uncontrolled systemic disease, intake of central nervous system-active drugs in the past 2 weeks or hyperactivity disorders. Moreover, patients whose parents refused to continue their participation in the study were excluded.
2.3. Sample size calculation
The required sample size was calculated using G*Power software version 3.1.0. Assuming a type I error of 0.05, and 80% power, a sample size of 30 patients in each study group would be enough to detect an effect size (Cohen d) of 0.8 in the primary outcome of interest, considering a dropout rate of 20%.
2.4. Study outcomes
The primary outcomes of the current study were to assess intraoperative anesthetic and analgesic requirements (sevoflurane and fentanyl consumption). Secondary outcomes included preoperative modified RSS (1 = awake and alert, 2,3 = anxiolysis, 4,5 = moderate sedation, 6 = deep sedation, 7,8 = general anesthesia), before and after 30 minutes of premedication [Citation10], time to first analgesic requirement, post-operative analgesic doses (meperidine, oral paracetamol, and rectal diclofenac) required during the first 12 hours, PAED (0 = asleep; 1 = calm; 2 = Crying, but can be consoled; 3 = Crying, but cannot be consoled; 4 = Agitated and thrashing around) was assessed at 10, 20, and 30 min postoperatively [Citation11], FLACC pain score with its 0–10 score range [Citation12], time to eye opening and time to extubate after discontinuation of general anesthesia, and postoperative vomiting.
2.5. Randomization, allocation, and blinding
Simple randomization was achieved by generating random sequence numbers using a computer program. The numbers were written on paper slips and enclosed within opaque envelopes. Patients were randomly allocated using the closed envelopes into two groups equal in size. Both the anesthesia team and the nurse who assessed the outcomes were blinded to the type of intervention.
2.6. Study procedures and interventions
All patients in this study were anesthetized by the same team of anesthesiologists and premedicated orally with either pregabalin syrup 5 mg/kg (100 mg/5 ml syrup) or injectable midazolam 0.75 mg/kg mixed in apple juice 30 minutes before separation from parents. Both syrups were prepared by the pharmacist in equal volumes of 5 ml.
Patients were monitored using standard monitoring (electrocardiography, non-invasive blood pressure, end-tidal carbon dioxide, and pulse oximetry). Both the heart rate (HR) and mean arterial blood pressure (MABP) were recorded before induction of anesthesia and thereafter every 10 minutes till the completion of surgery. All patients were given general anesthesia using sevoflurane in 50% air + 50% oxygen (Drager Anesthesia Machine) through Jackson-Rees’ modification of Ayre’s T piece with appropriate-sized face mask. During induction of anesthesia either a 22- or 24-gauge intravenous cannula was inserted on the dorsum of the hand, and both fentanyl (2 μg/kg) and paracetamol (20 mg/kg) were given intravenously. Endotracheal intubation with appropriate size to the patient’s age was done, with the tube fixed in place, followed by assisted spontaneous ventilation. General anesthesia was maintained with sevoflurane delivered in 50% air + 50% oxygen. An increase in HR or MABP (>20%) with skin incision or at any time during surgery compared with baseline values was treated with an additional dose of fentanyl (1 μg/kg). The response was checked after 10 min; non-responders were treated by an incremental increase of sevoflurane till hemodynamic normalization. Sevoflurane concentration used constantly for more than 50% duration of surgery was taken as the concentration needed for the intraoperative period. After completion of the surgical procedure, patients were awakened, extubated, and transferred to the PACU. Time of eye opening and time of extubation after discontinuation of general anesthesia were measured. Postoperative PAED was assessed at 10, 20, and 30 min postoperatively, and the PACU duration of stay was recorded.
A blinded nurse observer assessed the pediatric FLACC pain scale upon arrival to and at the time of discharge from the PACU, and then every 2 hours till discharge from the hospital or for the first 12 hours after the operation. Paracetamol (15 mg/kg/dose) (250 mg/5 ml oral suspension) was administered as a standard part of the perioperative regimen with a maximum oral daily dose of 60 mg/kg/day and with a minimum interval of 4 hours to achieve FLACC scale score of 3 or less. In the PACU, FLACC score >3 was managed with an intravenous bolus of meperidine 1 mg/kg was given slowly over 4 minutes. After discharge from PACU, patients were observed for 30 minutes after each oral paracetamol dose, if FLACC pain scale score ˃ 3, rectal diclofenac 1 mg/kg/dose was given with a maximum daily dose of 3 mg/kg/day, with a minimum interval of 8 hours. A rescue dose of intravenous meperidine 1 mg/kg was given slowly over 4 minutes, with a minimum interval of 3 hours, if the FLACC pain scale score remained ˃ 3, 30 minutes after rectal diclofenac.
2.7. Statistical analysis
All patients were analyzed in the group that they were originally assigned to (30 patients in each group). Statistical analysis was carried out with the Statistical Package for Social Sciences, version 24 for Windows (SPSS Inc., Chicago, IL, USA). Categorical data were presented as frequencies and percentages, and the association between variables was tested using Chi-square test or Fisher Exact test as appropriate. Numerical variables were checked for normality by the Shapiro-Wilk test. Normally distributed numerical data were expressed as mean and standard deviation, and independent sample t-test was used to test the association between two independent groups. Non-normally distributed numerical data were expressed as the median and interquartile range (IQR) (25th−75th percentiles), and the Mann-Whitney U test was used to compare two independent non-normally distributed variables. Mann–Whitney U test depends on the comparison of mean ranks; instead of using the original measurements, all sample data are ranked from the smallest to the largest and according to its position in the combined data set the rank is assigned; then, the mean of these ranks is calculated. The level of statistical significance was considered at P < 0.05.
3. Results
In the present study, 73 patients were assessed for eligibility, out of whom 13 patients were excluded (9 declined participation and 4 did not meet the inclusion criteria). Sixty patients who underwent day-case surgery were allocated to intervention and were divided into 2 groups, pregabalin group and midazolam group, 30 patients each. Sixty patients were followed up without violation of the protocol at any time of the study ().
Males constituted 51.7%, their age ranged from 8.8 to 65.0 months with a median age of 24.0 (IQR = 14.5–43.0) and their mean weight was 13.0 ± 3.0 kg. There were no significant differences between the studied groups regarding sociodemographic characters (p ˃ 0.05) as well as types and duration of surgery (p ˃ 0.05) ().
Table 1. Sociodemographic characters, types, and duration of surgery
shows that the mean rank of the time (min) of the first analgesic requirement was significantly higher in group P than group M (36.75 vs. 15.41, respectively; p < 0.001). Twenty-six (86.7%) patients in the M group received rectal diclofenac, while only seven (23.3%) patients in the P group received rectal diclofenac with a statistically significant difference (p < 0.001). All patients (100%) in the M group needed an intraoperative rescue dose of fentanyl compared to only 7 (23.3%) patients in the P group (p < 0.001). Likewise, 24 (80%) patients in the M group received intravenous meperidine, while only 2 (6.7%) patients in P group received intravenous meperidine (p < 0.001). Additionally, the mean ranks of the received rectal diclofenac, intravenous fentanyl, and meperidine were significantly lower in group P compared to group M (19.24 vs. 37.78 mg, 17.32 vs. 43.68 µg, and 19.67 vs. 41.33 mg, respectively; p < 0.001). Alternatively, there was no significant difference between group P and group M regarding the total paracetamol dose (p = 0.090). The mean sevoflurane consumption (1.6 ± 0.4 vs. 2.6 ± 0.3%, p < 0.001) was significantly lower in group P compared to group M.
Table 2. Comparison of analgesics and anesthetics requirements in the studied groups
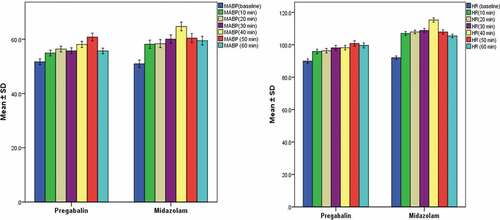
shows significantly higher means of arterial blood pressure (mmHg) at 30 and 40 min after induction of anesthesia in group M compared to group P (60.0 ± 8.6 vs. 55.7 ± 6.0 and 64.7 ± 8.7 vs. 58.1 ± 6.2, respectively). Comparison of the HR (beat per minute) recorded after induction of anesthesia revealed significantly higher means at 10, 20, 30, 40, 50, and 60 min in group M compared to group P (p < 0.05).
Figure 2. The mean arterial blood pressure (MABP) and the heart rate (HR) recorded at the different postoperative times
Comparison of preoperative modified RSS before and after 30 minutes of premedication revealed nonsignificant differences between Pregabalin and Midazolam groups (p ˃ 0.05). Postoperative PAED score calculated at 10, 20, and 30 min in the studied groups revealed nonsignificant differences (p > 0.05). The postoperative FLACC pain score had a significantly lower mean rank at arrival to and discharge from PACU, and at each of 2, 4, 6, and 8 h after operation in group P compared to group M (p < 0.05) ().
Table 3. Comparison of various scores in the studied groups
Ten patients (33.3%) were discharged home after 6 hours in the pregabalin group, while four patients (13.3%) were discharged home after 6 hours in the midazolam group with no significant difference between both groups (p = 0.067) ().
demonstrates that the mean time (min) to open the eye (7.7 ± 1.8 vs. 13.0 ± 2.8, p < 0.001) and the mean time to extubate (9.4 ± 1.8 vs. 16.3 ± 2.6, p < 0.001) were significantly lower in group P than in group M. Furthermore, the median duration of stay in the PACU (17.5 min) was significantly lower in group P than in group M (35.0 min). However, there was no significant difference between the studied groups regarding the incidence of postoperative vomiting.
Table 4. Comparison of time to open the eye, time to extubate, post anesthesia care unit stay, and postoperative vomiting in the studied groups
4. Discussion
Day-case surgery in pediatric surgical patients is a safe and feasible option in developing countries with poor health-related funds. Proper selection of patients and advances in anesthesia care are the cornerstone of good outcomes in the day care surgery [Citation13]. Giving preoperative sedation before day-case surgery is not contraindicated, but careful planning is required to ensure it is given time to work and does not increase the pre-induction time [Citation14].
Preoperative oral midazolam has proved effective in treating preoperative anxiety. In this study, oral administration of injectable midazolam in a dose of 0.75 mg/kg as a premedication shows acceptability, effectiveness, and safety. The combination of the sedative and anxiolytic characteristics is believed to create a calming effect. This makes children more comfortable and less anxious when taken away from their parents and during mask placement [Citation5].
The use of oral midazolam produces very rapid effective sedation and improves behavior at induction without delaying recovery or discharge relatively due to its short duration of action [Citation15,Citation16]. This effect of oral midazolam could be influenced by its low and variable bioavailability of 27–34% resulting from poor gastrointestinal absorption and significant hepatic first-pass metabolism [Citation16,Citation17]. In contrast, the oral bioavailability of pregabalin is greater than or equal to 90% [Citation18].
Pregabalin is a synthetic analog of the inhibitory transmitter gamma-aminobutyric acid. It has anticonvulsant, analgesic, antianxiety, and sleep-modulating effects as it decreases the release of several transmitters, such as serotonin, dopamine, glutamate, noradrenaline, and substance P [Citation19,Citation20]. Safety of pregabalin in children aged 1 month to 16 years was detected in a randomized, placebo-controlled trial done on 65 children with refractory partial seizures [Citation21]. Recently, pregabalin has been demonstrated to be an effective adjunct to multimodal analgesia in several reviews and meta-analyses not only by reducing opioid consumption but also by decreasing the incidence and intensity of both acute and chronic postsurgical pain [Citation8].
Pregabalin anxiolytic and sedative effects were recorded in a randomized double-blinded clinical trial that included 52 children who underwent dental procedures. The reduction of the post-operative visual analog scale for anxiety was significantly greater in the pregabalin group [Citation22]. Another study revealed that gabapentin premedication in children reduced postoperative analgesic consumption and attenuated emergence agitation after sevoflurane anesthesia, which is in accordance with the current study [Citation23]. Moreover, Donmez et al. [Citation24] concluded that gabapentin reduced agitation after circumcision using sevoflurane in pediatrics.
Similarly, several earlier reports found that preoperative administration of gabapentin decreased postoperative pain scores after various types of surgeries [Citation25–27]. Gabapentin appears to effectively manage refractory pain and agitation in pediatrics as it is highly lipophilic, penetrates well through the blood–brain barrier and has a relatively mild adverse effect compared to the grave sedative and addictive properties of opiates and benzodiazepines [Citation9].
On the other hand, Arnold and colleagues [Citation28] reported that pregabalin did not significantly improve the mean pain score in adolescents with fibromyalgia. However, a recent study that compared pregabalin syrup 1.5 mg/kg to placebo syrup given half an hour preoperatively to 60 pediatric patients scheduled for adenotonsillectomy revealed that the pregabalin group had less emergence agitation, less analgesic requirements, and less vomiting, but no significant effects on time to open the eye, time to extubation, or PACU duration of stay compared to the control group [Citation29]. Differences in outcomes in both studies in comparison to the current study might be due to different doses of pregabalin and different characteristics of patients’ groups.
Moreover, the need for rescue analgesics and the mean ranks of rectal diclofenac, intravenous fentanyl, and meperidine consumption was significantly lower in group P. This could be attributed to the pregabalin effect; preventing central sensitization development, which can be rather beneficial in acute postsurgical pain management as part of multimodal analgesia regimen. Furthermore, pregabalin demonstrates predictable and linear pharmacokinetics, making it easy to use in clinical practice [Citation6].
From our results regarding pregabalin effectiveness in reducing preoperative anxiety, in addition to reducing intraoperative anesthetic needs and intra and postoperative analgesic needs, it is worth changing our practice regarding pediatric sedation. However, further studies are required to discuss its use from an economic point of view and its effect on parental satisfaction.
Regarding the limitation of this study, not all patients remained in the hospital for 12 hours to assess the difference in FLACC score and there were variations in the type and site of surgery.
5. Conclusions
Pregabalin significantly reduced the perioperative anesthetic and analgesic requirements, the PACU duration of stay, and the time to open the eye and to extubate, in comparison to midazolam; therefore, its use as a premedication in pediatric day-case surgery is recommended.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bowen L, Thomas M. Paediatric day case surgery. Anaesth Intensive Care Med. 2016;17:274–279.
- Elebute O, Ademuyiwa A, Bode C, et al. Pediatric day case surgical practice at a tertiary hospital in lagos: how have we faired? Ann Med Health Sci Res. 2014;4:559–561.
- Gangadhar S, Gopal T, Sathyabhama K. Rapid emergence of day-care anaesthesia: A review. Indian J Anaesth. 2012;56:336.
- Sheta SA, AlSarheed M. Oral midazolam premedication for children undergoing general anaesthesia for dental care. Int J Pediatr. 2009;2009:2009.
- Jeon S, Lee H-J, Do W, et al. Randomized controlled trial assessing the effectiveness of midazolam premedication as an anxiolytic, analgesic, sedative, and hemodynamic stabilizer. Medicine (Baltimore). 2018;97:e12187–e.
- Raddaoui KRM, Bhar M, Trigui E, et al. Pregabalin for postoperative analgesia after idiopathic scoliosis surgery. Ann Pediatr Child Health. 2018;6:1157.
- Baldwin DS, Ajel K, Masdrakis VG, et al. Pregabalin for the treatment of generalized anxiety disorder: an update. Neuropsychiatr Dis Treat. 2013;9:883–892.
- Egunsola O, Wylie CE, Chitty KM, et al. Systematic review of the efficacy and safety of gabapentin and pregabalin for pain in children and adolescents. Anesth Analg. 2019;128:811–819.
- Sacha GL, Foreman MG, Kyllonen K, et al. The use of gabapentin for pain and agitation in neonates and infants in a neonatal ICU. J Pediatr Pharmacol Ther. 2017;22:207–211.
- Gill M, Green SM, Krauss B. A study of the bispectral index monitor during procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2003;41:234–241.
- Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100:1138–1145.
- Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297.
- Uzair M, Wazir MK, Afridi RAK, et al. Paediatric day case surgery: 3 years experience at private medical centre. KJMS. 2018;11:241.
- Heikal S, Bowen L, Thomas M. Paediatric day-case surgery. Anaesth Intensive Care Med. 2019;20:318–323.
- Brennan LJ, Prabhu AJ. Paediatric day‐case anaesthesia. BJA CEPD Rev. 2003;3:134–138.
- Somri M, Parisinos CA, Kharouba J, et al. Optimising the dose of oral midazolam sedation for dental procedures in children: a prospective, randomised, and controlled study. Int J Paediatr Dent. 2012;22:271–279.
- Paine MF, Shen DD, Kunze KL, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24.
- Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49:661–669.
- Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–1815.
- Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3-isobutyl-gamma-aminobutyric acid block secondary hyperalgesia. Brain Res. 1998;810:93–99.
- Mann D, Liu J, Chew ML, et al. Safety, tolerability, and pharmacokinetics of pregabalin in children with refractory partial seizures: a phase 1, randomized controlled study. Epilepsia. 2014;55:1934–1943.
- Eskandarian T, Eftekharian H, Soleymanzade R. Efficacy and safety of premedication with single dose of oral pregabalin in children with dental anxiety: A randomized double-blind placebo-controlled crossover clinical trial. Dent Res J. 2015;12:528–533.
- Salman AE, Camkiran A, Oguz S, et al. Gabapentin premedication for postoperative analgesia and emergence agitation after sevoflurane anesthesia in pediatric patients. Agri. 2013;25:163–168.
- Dönmez A, Salman AE, Sülemanji D, et al. The effect of gabapentin premedication on the emergence delirium in pediatric patients undergoing circumcision under sevoflurane anesthesia. Turk J Anaesthesiol Reanim. 2012;40:64–70.
- Fassoulaki A, Stamatakis E, Petropoulos G, et al. Gabapentin attenuates late but not acute pain after abdominal hysterectomy. Eur J Anaesthesiol. 2006;23:136–141.
- Turan A, Memis D, Karamanlioglu B, et al. The analgesic effects of gabapentin in monitored anesthesia care for ear-nose-throat surgery. Anesth Analg. 2004;99:375–378.
- Turan A, White PF, Karamanlioglu B, et al. Gabapentin: an alternative to the cyclooxygenase-2 inhibitors for perioperative pain management. Anesth Analg. 2006;102:175–181.
- Arnold LM, Schikler KN, Bateman L, et al. Pregabalin Adolescent Fibromyalgia Study G. Safety and efficacy of pregabalin in adolescents with fibromyalgia: a randomized, double-blind, placebo-controlled trial and a 6-month open-label extension study. Pediatr Rheumatol Online J. 2016;14:46.
- Marouf HM. Effect of pregabalin premedication on emergence agitation in children after sevoflurane anesthesia: A randomized controlled study. Anesth Essays Res. 2018;12:31.