ABSTRACT
Background: Adequate pain management after knee arthroscopy (KA) under general anesthesia (GA) is often problematic. Therefore, we evaluated the efficacy and safety of lidocaine patch 5% (LP5) supplementation to intra-articular (IA) bupivacaine and dexmedetomidine after KA under GA.Methods: This randomized controlled study was conducted on 40 adult patients undergoing KA under GA. Patients were randomized into two groups (20 patients in each). At the end of the surgery, group A received an IA injection of 10 ml bupivacaine 0.5% and 1 µg/kg dexmedetomidine diluted in 10 ml saline. Group B received as group A, and one LP5 (700 mg) applied at the portal site for 12 h postoperatively. Intravenous pethidine 20 mg was administered if the postoperative visual analogue scale (VAS) was ≥4.Results: The postoperative pethidine consumption in the first 24 h was significantly lower in group B. The time of the first request of analgesia and number of patients discharged at 24 h were significantly higher in group B. Heart rate, mean arterial pressure, and VAS were significantly higher in group A at 6, 8, and 12 h postoperatively. In group B, serum lidocaine levels reached its peak (31.4 ± 3.01 ng/mL) at 12 h and were negatively and strongly correlated with VAS at 12, 18, and 24 hours (r = −0.736 and P < 0.001). All postoperative adverse effects were minimal and mild.Conclusion: After KA, LP5 supplementation to IA bupivacaine and dexmedetomidine decreased the postoperative pain and narcotic requirements safely.
1. Introduction
Knee arthroscopy (KA) is a useful surgical procedure to manage and treat many common knee conditions [Citation1]. The advantage of KA is being a minimally invasive technique allowing an earlier return to daily activities [Citation2].
Most patients have moderate to severe pain in the first 24 hours after KA. Unrelieved pain may delay discharge and prolong hospital stay [Citation3].
Different drugs (such as opioids, non-steroidal anti-inflammatory drugs (NSAIDs), and clonidine) and regimens (such as regional blocks, intra-articular (IA) administration of local anesthetics) were used to provide an effective, safe, and long-lasting analgesia after KA [Citation4–6]. Intra-articular (IA) administration of bupivacaine only showed effectiveness for short periods, and efforts to enhance the quality and duration of analgesia included the use of different adjuvant drugs, like opioids, NSAIDs, magnesium sulphate, dexmedetomidine, ketamine, and neostigmine [Citation7–11].
One of the most commonly prescribed topical analgesics to date is the lidocaine patch 5% (LP5) [Citation12]. LP5 shows short- and long-term tolerability with minimal absorption in the systemic circulation (3 ± 2%), reducing the risk of drug–drug interactions [Citation13].
Although there is a lack of consensus among experts regarding the role of LP5 in the treatment of pain, its use has been reported in a variety of conditions such as osteoarthritis of the knee [Citation14]. Several studies showed the efficacy of LP5 in reducing postoperative pain after knee surgeries [Citation15,Citation16],
However, there is no published study about the use of both LP5 with IA administration of local anesthetics simultaneously. Therefore, this work aimed to evaluate the efficacy and safety of LP5 supplementation to IA bupivacaine and dexmedetomidine after KA under general anesthesia (GA).
2. Materials and methods
This prospective randomized controlled open-label clinical study was conducted on 40 adult patients from both sexes, aged between 18 and 60 years, ASA physical status I–II, undergoing elective KA with a duration of surgery less than 90 minutes. We obtained the approval of the Ethics Committee from the Faculty of Medicine, Tanta University and a written informed consent from all participants in this research. The registry number on clinicaltrial.gov is NCT04322760.
Exclusion criteria were patient refusal, pregnancy, lactation, cardiac, renal, or hepatic diseases, hypertension treated with α methyldopa or beta-adrenergic blockers, opioid use within the previous 24 h, receiving class I antiarrhythmic drugs (such as tocainide and mexiletine), and known history of sensitivity to amide local anesthetics or to any other component of the LP5.
After evaluating the medical and surgical history of every patient, we conducted their clinical examinations and performed routine laboratory investigations. Patients were trained on the use of visual analogue scale (VAS) to determine the intensity of the pain on a scale from (0–100) where 0 = no pain and 10 = the worst pain.
Patients were randomly allocated into two equal groups; 20 patients in each group. Group A (Control group): received IA 10 ml bupivacaine 0.5% and 1 µg/kg dexmedetomidine diluted in 10 ml saline 0.9% (without LP5) and group B (intervention group): as group A plus application of one LP5 for 12 hours.
Randomization was performed by computer-generated random numbers and inserting them into opaque concealed envelopes. It was not applicable to make blind allocations.
2.1. General anesthesia
We administered crystalloid fluids to each patient after establishing an intravenous line (18 G cannula). Monitoring was done by five leads ECG, non-invasive arterial blood pressure system, pulse oximetry, capnography, and temperature probe.
GA was performed by the same anesthesiologist. After pre-oxygenation with 100% O2 for 3–5 minutes, GA was induced by 1 µg/kg fentanyl, 2 mg/kg propofol, and 0.5 mg/kg atracurium, then the trachea was intubated. Anesthesia was maintained by 60% O2, isoflurane at 1 MAC, and boluses of atracurium of 0.1 mg/kg and fentanyl 0.5 µg/kg were given when needed.
2.2. Surgical procedure
Before the surgical incision, a thigh pneumatic tourniquet was applied to all patients on the same side of the surgery, at a pressure of 150 mm Hg above systolic blood pressure. The same surgeon performed all the procedures using a standard surgical technique.
After the end of the surgery, the tourniquet was released then under a complete aseptic technique, patients in group A were administered a 10 ml IA injection consisting of 0.5% bupivacaine and 1 µg/kg dexmedetomidine diluted in 10 ml saline through one of the arthroscopic ports (Total volume 20 ml). Patients in group B (Lidocaine group) received the same IA injection as the previous group, in addition to an LP5 (Lidodermy, Hind Health Care, San Jose, CA) for the first 24 hours postoperative. LP5 contains an adhesive with lidocaine 5% base (700 mg/patch), water, glycerin, d-sorbitol, sodium polyacrylate, sodium carboxymethylcellulose, propylene glycol, and other ingredients on a non-woven polyester backing. The size of a single patch is 10 * 14 cm applied to the skin between the arthroscopic ports. After that, the knee joint area was wrapped with dressings and bandages.
After extubation, patients were transferred to the post-anesthesia care unit (PACU). Patients were not discharged before 24 h for research purposes.
Paracetamol 1 gm intravenous infusion was given every 8 hours as routine analgesia. Intravenous pethidine 20 mg was administered as rescue analgesia if VAS was ≥4.
2.3. Measurements
Heart rate (HR), mean arterial pressure (MAP), respiratory rate (RR), peripheral oxygen saturation (SpO2), and VAS were recorded at PACU, 1, 2, 4, 6, 8, 12, 18, and 24 h postoperatively. In group B, blood samples were withdrawn for determination of lidocaine concentration at the same time of VAS measurement. Besides, we recorded the time of the first postoperative analgesic (pethidine) request and pethidine consumption in the first 24 h. Postoperative adverse effects probably due to lidocaine or other drugs were evaluated: nausea, vomiting, erythema, edema, rash, sedation, or local anesthetic toxicity. The primary endpoint was postoperative pethidine consumption in the first 24 h, and the secondary endpoints were the time of first analgesic request, percent of patients discharged at 24 h, safety of lidocaine, and dexmedetomidine, and correlation between VAS and lidocaine level in group B.
2.4. Sample size calculation and statistical analysis
The sample size was calculated according to a pilot study done before the start of the study (five patients in each group). The mean (±SD) total dose of postoperative pethidine consumption (the primary outcome) was 48.0 ± 10.95 mg in group A and 36.0 ± 8.94 mg in group B. With a 95% confidence limit, 90% power, 1.2 effect size, and 4 cases added to overcome dropout, 20 cases were recruited in each group.
Statistical analysis was done by SPSS v25 (IBM©, Chicago, IL, USA). Shapiro-Wilks test was used to evaluate the normality of the distribution of data. Quantitative parametric data were presented as mean and standard deviation (SD) and were compared by Student t-test. Quantitative non-parametric data were presented as median and range and were compared by Mann Whitney-test. Qualitative data were presented as number and percent and were compared by chi-square (X2) or Fisher’s Exact test when appropriate. Pearson correlation between serum lidocaine level and VAS score was done. P-value <0.05 was considered statistically significant.
3. Results
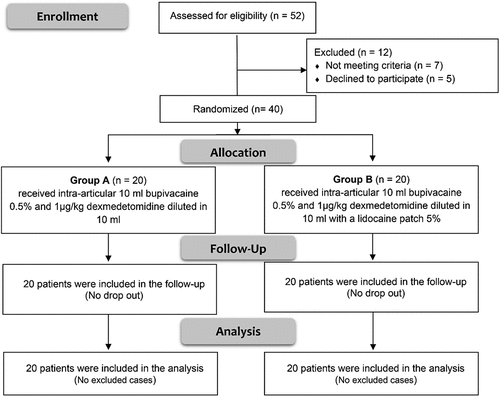
The flowchart of studied patients of both groups is shown in . There was no significant difference in age, sex, weight, ASA physical status, or the type and the duration of the operations between both groups ().
Table 1. Patient characteristics of both groups
The median (IQR) postoperative pethidine consumption in the first 24 hours in group B was significantly lower than group A (40 (15–40) vs. 60 (40–60) mg, P < 0.001). There was a significant increase in the time of the first request of analgesia in group B compared to group A (1064.0 ± 76.19 vs. 397.0 ± 35.63 minutes; P < 0.001). Number of patients discharged at 24 h were higher in group B than group A (17 (85%) vs 9 (45%), P = 0.019). There were insignificant differences in postoperative adverse effects between both groups. ()
Table 2. Comparison of outcomes in both groups
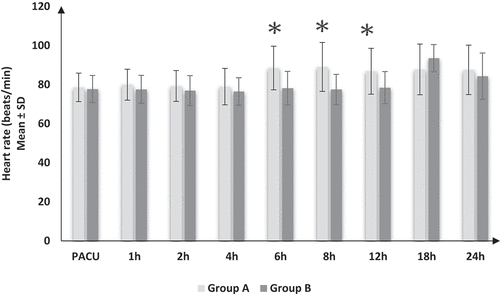
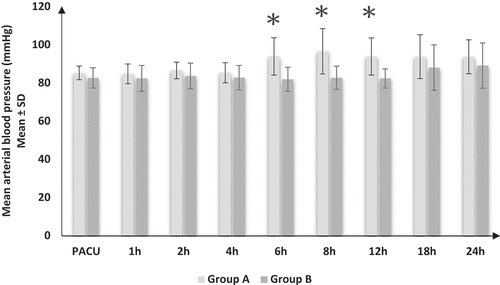
HR (), MAP (), and VAS () showed significant increases in group A compared to group B at 6, 8, and 12 h postoperatively and insignificant differences throughout other times of recordings between both groups. Regarding the RR and SpO2, there were insignificant differences between both groups at all times of recording.
Table 3. Postoperative visual analogue scale (VAS) in both groups
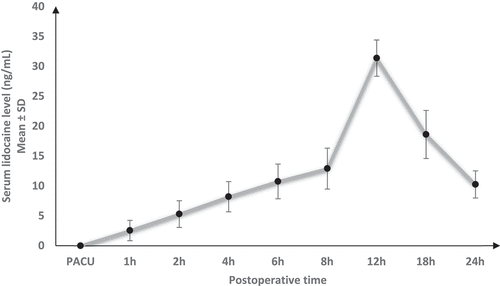
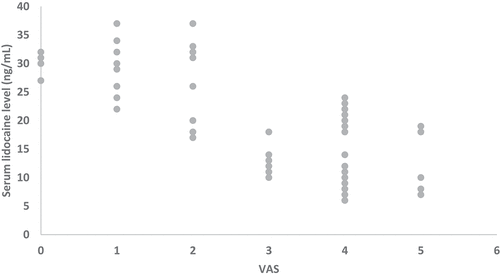
Serum lidocaine levels increased gradually till reached their peak (31.4 ± 3.01 ng/mL) at 12 h then decreased gradually () and were negatively and strongly correlated with VAS at 12, 18, and 24 hours [r = −0.736 (95% confidence interval: −0.837 to −0.588) and P < 0.001]. ()
4. Discussion
Current evidence demonstrates that the administration of a single injection of IA bupivacaine effectively reduces postoperative pain after KA with satisfactory short-term safety. This effect may be due to the inhibition of nerve conduction through C and Aδ fibers, in addition to stimulating the release of peripheral enkephalin-like transmitters [Citation17,Citation18]. IA dexmedetomidine seems to exert its analgesia primarily through direct local action. However, this does not exclude systemic absorption and an accompanying central analgesic effect [Citation11].
The primary aim of this study was to estimate the postoperative pethidine consumption in the first 24 hours after KA under GA, which was significantly lower in group B than group A. There were no previous studies to compare pethidine consumption.
The secondary outcomes were 1) the time of first analgesic request, which was significantly increased in group B compared to group A, 2) safety, which was demonstrated by the minimal side effects, and 3) correlation between VAS and lidocaine level in group B which was negative and strong. All the previous results ensured the efficacy and safety of LP5 application to the intact skin between the portal site in patients undergoing KA under GA.
The novelty in the present study was not only supplementation of LP5 to IA bupivacaine and dexmedetomidine but also results obtained from blood samples withdrawn for determination of serum lidocaine concentrations and correlation with the pain intensity.
Application to injured skin, although not tested, may result in higher blood concentrations of lidocaine from increased absorption. LP5 is only recommended for use on intact skin [Citation12]. Fortunately, the penetration of lidocaine into intact skin after applying LP5 is sufficient to produce an analgesic effect but less than the amount necessary to produce a complete sensory block [Citation19].
In the present study, the authors found that lidocaine levels increased gradually till they reached their peak (31.4 ± 3.01 ng/mL) at 12 h then decreased gradually. IA bupivacaine dexmedetomidine covered the first 6–8 hours postoperative till lidocaine reaches its peak. The peak level of lidocaine is much lower than its dose as an antiarrhythmic drug.
LP5 has an analgesic effect by suppressing the activity of peripheral sodium channels of sensory afferents, thereby reducing ectopic paroxysmal discharge and pain transmission as well as decreasing expression of mRNA in the sodium channel [Citation20]. Another explanation is the suppression of proteolytic release and inflammatory mediators from the surgically damaged tissue, which activates and maintains postoperative pain, thereby inducing antinociception and alleviating the pain [Citation21].
In 1999, LP5 was first approved by the FDA for the relief of pain associated with postherpetic neuralgia (PHN) [Citation22]. Over the past years, the LP5 has emerged as a widely used treatment modality in PHN and other conditions such as lower back pain, carpal tunnel syndrome, diabetic peripheral neuropathy, and osteoarthritis joint pain. A systematic review [Citation23] showed that LP5 was effective in multimodal systemic therapies for specific procedural pain, but future studies are needed to examine its use.
Saber et al. [Citation24] found that LP5 significantly reduced the pain score in patients who underwent laparoscopic ventral herniorrhaphy than the control group but had insignificant lower analgesic use and duration of hospital stay.
Another study [Citation25] showed that the lidocaine patch group reported significantly less pain on coughing over all periods after radical retro-pubic prostatectomy, but the postoperative morphine consumption was not different between the groups.
Furthermore, Kim [Citation26] found that the LP5 provided better pain relief and higher patient and operator postoperative satisfaction after percutaneous endoscopic lumbar discectomy.
Also, Kwon et al. [Citation27] found that placing the lidocaine patch at the laparoscopic port sites reduced postoperative pain, particularly postoperative wound pain after gynecological laparoscopic procedures.
Moreover, Fiorelli et al. [Citation28] showed a significant reduction in pain intensity, both at rest and after coughing, with a decrease in the total morphine consumption in post-thoracotomy pain in the lidocaine group placebo group.
The present study was in disagreement with Khanna et al. [Citation15]. They demonstrated that the LP5 group does not provide significant additional pain relief compared with the control group after total knee replacement.
Similarly, Ingalls et al. [Citation29] concluded that the LP5 does not significantly improve pain control in poly-trauma patients with traumatic rib fractures. Their insignificant results may be explained by that the LP5 delivers lidocaine to the intact skin around the wound instead of directly into the wound as lidocaine diffuses from the epidermis to the deeper parts of skin instead of spreading under the epidermis, or even under the dermis like in wound infiltration [Citation15,Citation29]. Also, the difference in results may be the higher level of pain in total knee replacement and traumatic rib fractures.
The conventional pain control methods used after KA are systemic administration of analgesics, including opioids, NSAIDs, and acetaminophen, which frequently cause side effects [Citation30]. Also, regional blocks (such as sciatic, obturator, and femoral blocks) are reportedly effective in reducing pain but carries the risk of peripheral neuropathy (3 in 10,000) [Citation31]. Hypotension, headache, nausea/vomiting, and shivering are the most common side effects of epidural or subarachnoid block techniques [Citation32].
In contrast, topical anesthetics act directly on the pain area and minimize the incidence of side effects and drug–drug interactions. This was confirmed by results obtained from the present study that showed the hemodynamic stability with no attacks of bradycardia or hypotension in both groups and the low serum level in group B. PONV, edema, erythema, and sedation occurred in a low percentage of patients and were mild. No detected cases of local anesthetic toxicity in patients of both groups.
Our results agree with Rowbotham and Fields [Citation33], who found that the LP5 leads to a better hemodynamic profile and pain relief in postherpetic neuralgia. A recent meta-analysis [Citation34] demonstrated that LP5 was similar to pregabalin in reducing pain in peripheral neuropathic pain with better adverse events profile.
One of the limitations of our study was the use of single LP5, but this is attributed to the small surface area of skin field between the KA portal openings. Another one was the limited time of patch application (12 hours only), but this is attributed to the type of KA as an ambulatory, day case surgery. Therefore, further studies with more than one LP5, for a prolonged time of follow up and without IA administration of local anesthetics are recommended.
5. Conclusion
After KA, LP5 supplementation to IA bupivacaine and dexmedetomidine decreased the postoperative pain and narcotic requirements safely.
Conflict of interest
Nil.
Acknowledgments
Nil
Disclosure statement
No potential conflict of interest was reported by the author(s)
References
- Friberger Pajalic K, Turkiewicz A, Englund M. Update on the risks of complications after knee arthroscopy. BMC Musculoskelet Disord. 2018;19:179.
- Treuting R. Minimally invasive orthopedic surgery: arthroscopy. Ochsner J. 2000;2:158–163.
- Pavlin DJ, Chen C, Penaloza DA, et al. A survey of pain and other symptoms that affect the recovery process after discharge from an ambulatory surgery unit. J Clin Anesth. 2004;16(3):200–206.
- Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth. 2001;13(7):524–539.
- Miskulin M, Maldini B. Outpatient arthroscopic knee surgery under multimodal analgesic regimens. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2006;22: 978–983.
- Johnston DF, Sondekoppam RV, Uppal V, et al. Effect of combining peri-hamstring injection or anterior obturator nerve block on the analgesic efficacy of adductor canal block for anterior cruciate ligament reconstruction: a randomised controlled trial. Br J Anaesth. 2020;124:299–307.
- Ersan O, Akkaya T, Arik E, et al. Intra-articular levobupivacaine, lornoxicam and morphine analgesia after knee arthroscopy: a randomized, controlled trial. Acta Orthop Traumatol Turc. 2012;46:411–415.
- Farouk S, Aly A. A comparison of intra-articular magnesium and/or morphine with bupivacaine for postoperative analgesia after arthroscopic knee surgery. J Anesth. 2009;23:508–512.
- Gentili M, Enel D, Szymskiewicz O, et al. Postoperative analgesia by intraarticular clonidine and neostigmine in patients undergoing knee arthroscopy. Reg Anesth Pain Med. 2001;26:342–347.
- Das PB, Samal S. Efficacy of intra-articular dexmedetomidine versus buprenorphine for postoperative analgesia following knee arthroscopy: a comparative study. Anesthesia: Essays and Researches. 2019;13(2):225–228.
- Devi MM, Gupta S, Amaravathi R, et al. Comparison of efficacy of intra-articular plain bupivacaine and bupivacaine with adjuvants (dexmedetomidine and magnesium sulfate) for postoperative analgesia in arthroscopic knee surgeries: a prospective, randomized controlled trial. Anesth Essays Res. 2018;12:848–854.
- Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. The J Clin Pharmacol. 2003;43(2):111–117.
- Navez ML, Monella C, Bosl I, et al. 5% lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther. 2015;4(1):1–15.
- Gudin J, Nalamachu S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Postgrad Med. 2020;132(1):28–36.
- Khanna M, Peters C, Singh JR. Treating pain with the lidocaine patch 5% after total knee arthroplasty. Pm & R. 2012;4:642–646.
- Sadigursky D, Oliveira M, Macedo JGG, et al. Effectiveness of lidocaine patches for pain treatment after total knee arthroplasty. In: MedicalExpress. 2017. p. 4.
- Sun Q-B, Liu S-D, Meng Q-J, et al. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2015;16:21.
- Wei J, Yang H-B, Qin J-B, et al. Single-dose intra-articular bupivacaine after knee arthroscopic surgery: a meta-analysis of randomized placebo-controlled studies. Knee Surg Sports Traumatol Arthrosc. 2014;22(7):1517–1528.
- Baron R, Mayoral V, Leijon G, et al. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663–1676.
- Ma RSY, Kayani K, Whyte-Oshodi D, et al. Voltage gated sodium channels as therapeutic targets for chronic pain. J Pain Res. 2019;12:2709–2722.
- Lee W, Hahn K, Hur J, et al. Effect of topical lidocaine patch on postoperative pain management in laparoscopic appendectomy: a randomized, double-blind, prospective study. J laparoendoscopic adv surg tech Part A. 2018;28(9):1061–1067.
- Davies PS, Galer BS. Review of lidocaine patch 5% studies in the treatment of postherpetic neuralgia. Drugs. 2004;64(9):937–947.
- Smoker J, Cohen A, Rasouli MR, et al. Transdermal lidocaine for perioperative pain: a systematic review of the literature. Curr Pain Headache Rep. 2019;23:89.
- Saber AA, Elgamal MH, Rao AJ, et al. Early experience with lidocaine patch for postoperative pain control after laparoscopic ventral hernia repair. Int J Surg. 2009;7(1):36–38.
- Habib AS, Polascik TJ, Weizer AZ, et al. Lidocaine patch for postoperative analgesia after radical retropubic prostatectomy. Anesth Analg. 2009;108(6):1950–1953. .
- Kim KH. Use of lidocaine patch for percutaneous endoscopic lumbar discectomy. Korean J Pain. 2011;24(2):74–80.
- Kwon YS, Kim JB, Jung HJ, et al. treatment for postoperative wound pain in gynecologic laparoscopic surgery: topical lidocaine patches. J Laparoendoscopic Adv Surg Tech Part A. 2012;22:668–673.
- Fiorelli A, Pace C, Cascone R, et al. Preventive skin analgesia with lidocaine patch for management of post-thoracotomy pain: results of a randomized, double blind, placebo controlled study. Thorac Cancer. 2019;10(4):631–641. .
- Ingalls NK, Horton ZA, Bettendorf M, et al. Randomized, double-blind, placebo-controlled trial using lidocaine patch 5% in traumatic rib fractures. J Am Coll Surg. 2010;210(2):205–209.
- Rawal N. Analgesia for day-case surgery. Br J Anaesth. 2001;87:73–87.
- Aitkenhead AR. Injuries associated with anaesthesia. A global perspective. Br j anaesthesia. 2005;95(1):95–109.
- Tatikonda CM, Rajappa GC, Rath P, et al. Effect of intravenous ondansetron on spinal anesthesia-induced hypotension and bradycardia: a randomized controlled double-blinded study. Anesth Essays Res. 2019;13(2):340–346.
- Rowbotham MC, Fields HL. Post-herpetic neuralgia: the relation of pain complaint, sensory disturbance and skin temperature. Pain. 1989;39(2):129–144.
- Buksnys T, Armstrong N, Worthy G, et al. Systematic review and network meta-analysis of the efficacy and safety of lidocaine 700 mg medicated plaster vs pregabalin. . Curr Med Res Opin. 2020;36:101–115.