ABSTRACT
Background: Epidural magnesium sulphate provides better analgesia and opioid-sparing effect postoperatively. B-endorphins plasma levels are inversely related to the severity of pain. We investigated the possible relationship between epidural magnesium sulphate, postoperative analgesia and serum beta-endorphin levels in high knee osteotomy orthopedic surgery.
Methods: Sixty patients of both sexes, aged between 18 and 65 years, ASA I or II scheduled for high tibial osteotomy were divided to two groups (30 patients each): control group, received epidural bupivacaine 0.5%, lidocaine 2% and saline; and (Mg group) received epidural bupivacaine, lidocaine and magnesium sulphate (50 mg/10 ml saline). Venous blood samples were withdrawn to measure serum b endorphin levels (SBE), at different time intervals. We recorded time to first analgesic requirement, pain numeric rating score (NRS) and postoperative hemodynamic variables and incidence of complications.
Results: Time to first analgesic request was significantly longer in Mg group (pain score less than 5) compared to control group (P < 0.05) also the frequency and number of analgesic requests were significantly less in the Mg group than in the control group P = 0.000. B endorphins level was significantly lower in the Mg group than in the control group after 4 h of epidural insertion (P = 0.004).
Conclusion: We conclude that the addition of magnesium sulphate 50 mg/10 ml saline to epidural bupivacaine/lidocaine provides better postoperative analgesia as well as reducing serum B endorphins in the postoperative period without increasing the incidence of complications in high knee osteotomy orthopedic surgery postoperatively.
1. Introduction
It has been reported that more than half of postoperative patients receive suboptimal pain control. [Citation1] Surgeons often underestimate the severity and significance of postoperative pain and it could adversely affect clinical outcome and can have significant economic impact. [Citation2] Complications may also be related to the physiologic response to pain and prolonged periods of diminished mobility and these include pulmonary and vascular complications including pneumonia and deep venous thrombosis. Inadequately treated acute pain may ultimately lead to chronic pain via sensitization of the nervous system. [Citation3]
Regional anesthesia is an attractive analgesic modality, which is safe, efficient and of reasonable duration of action. Many studies used different adjuvants with epidural anesthesia that could enhance the duration of regional anesthesia and postoperative pain relief with least complications[Citation4]. Magnesium sulphate has anti-inflammatory, analgesic and opioid-sparing effect is an effective postoperative pain[Citation5]. Its antinociceptive action seems to be associated with the regulation of intracellular calcium flux, [Citation6,Citation7] or modulation of N-methyl-D-aspartate (NMDA) receptors either peripherally or centrally [Citation8,Citation9]. Prior studies had proven that intrathecally administered magnesium prolonged the duration of action of intrathecal opioid without increasing its side effects[Citation5]. These effects have prompted the investigation of epidural magnesium as an adjuvant for postoperative analgesia [Citation4]. Magnesium has been shown to provide analgesia without complication when administered in the epidural space in combination with bupivacaine[Citation7].
Beta-endorphin is an endogenous morphine-like hormone (opioid) produced primarily in the anterior lobe of the pituitary gland. [Citation9]. Pain, as a stress modality, increases plasma beta endorphin titers by augmenting glucocorticoid release and adrenergic stimulation of the hypothalamus-pituitary axis[Citation10].
The primary function of beta-endorphin is antinociception; indeed, beta-endorphin has been shown to be 20 to 33 times more analgesic than morphine[Citation10]. Beta-endorphin is released during stress and pain, and it exerts its analgesic effect in an inhibitory action. B-endorphins suppress neuronal action potential of peripheral somatosensory nerves, especially nociceptive nerve fibers. [Citation11,Citation12] There are other studies that showed that beta-endorphin has inhibitory action in visual, acoustic, and olfactory neural transmission. Other possible effects involve loss of libido, enhanced metabolic catatonic state, and suppressed satiety centers[Citation13]. beta-endorphin is resistance and capacitance vessels vasodilator, with the potential to cause orthostatic hypotension. Plasma beta-endorphin production is elevated during physical activity, and it affects various stages of the immune cascade. [Citation14]
The purpose of this study was to detect the possible relationship between epidural magnesium sulphate, postoperative analgesia and serum beta-endorphin levels in high knee osteotomy orthopedic surgery.
2. Methods
After obtaining the approval of the hospital ethical committee, sixty patients of both sexes, aged between 18 and 65 years, 30 in each group (ASA I or II) scheduled for lower limb orthopedic surgeries (high tibial osteotomy) were enrolled in this randomized, double-blinded controlled study between the period of March 2015 to April 2017. Informed consent was obtained from each patient after thoroughly explaining the epidural technique as well as the Numeric rating scale (NRS; 0: no pain; 10: worst pain) and research plan.
All patients were evaluated with respect to hemodynamics including the heart rate (HR), mean arterial pressure (MAP) and oxygen saturation (SpO2). An epidural catheter was inserted at the L3–L4 or L4–L5 intervertebral space under local anesthetic with the use of loss of resistance technique, and proper position was tested by injection of lidocaine 2% (3 ml).
The study was done in a double-blind method using a sealed envelope technique.
Patients were randomly assigned to one of two equal groups. First group, 30 patients, (control group) received 10 ml saline via epidural catheter followed by injection of saline 2 ml/h during the surgery. Second group, 30 patients, (Mg group) received 50 mg magnesium sulphate (MgSO4) in 10 ml as an initial bolus dose followed by injection of 10 mg/h (2 ml/h) during the surgery. Epidural bupivacaine 0.5% in a dose of 1 ml/segment and lidocaine 2% in a dose of 1–2 ml/segment was given before the surgery to all patients to achieve block from L4-T8 (9 segments). Sensory block was assessed bilaterally by using loss of sensation to pinprick with a short needle. Assessment of motor block was done using a modified Bromage scale (0: no motor block, 1: inability to raise extended legs, 2: inability to flex knees, 3: inability to flex ankle joints). [Citation14]
3. Primary outcome
Patients’ first analgesic requirement times were recorded. The time from end of surgery till the first request of analgesia was defined as the time to the first requirement for postoperative epidural analgesia.
4. Secondary outcome
MAP, HR, and pain score using NRS between 0 and 10 (0 = no pain, 10 = most severe pain) were recorded on arrival to recovery, every hour for 6 h and every 6 h for 24 h in the postoperatively. An NRS of ≤ 4 was considered as acceptable pain control. [Citation15]
Blood samples were drawn to measure serial serum B endorphins (SBE); before placement of the epidural catheter, after complete sensory/motor loss, end of the surgery, every hour for 6 h, then every 6 h for 24 h postoperatively. During the surgery, top ups of epidural bupivacaine 0.5% were given, if needed, to achieve a block up to T8 level. Post-operatively patients discharged to the ward with stable hemodynamic data, fully recovered sensory/motor block, pain-free, and no nausea or vomiting.
Epidural catheter was left in place for 24 h, and used to administer analgesia, if needed, in the form of 5 ml bupivacaine 0.5% boluses, titrated according to the analgesic response or appearance of side-effects. If patients had inadequate analgesia, supplementary rescue analgesia with intramuscular pethidine 50 mg was available. We monitored complications related with the epidural medications Nausea/vomiting, Hypotension, Bradycardia, Respiratory depression. A blinded anesthesiologist who was unaware of the drug given, performed all assessments.
5. Statistical analysis
Sample size was calculated from a previous published data [Citation16] showed that first analgesic request in epidural block for patients undergoing lower limb surgery was 240 minutes, with α error set at 0.05 (two-sided) and 90% for power of the study, the calculated sample size required to detect difference was 48 patients. Another 12 patients were added to the study to compensate for violation of the study protocol.
Data entry and data analysis were done using SPSS version 19 (Statistical Package for Social Science). Data were presented as number, percentage, mean, standard deviation. Chi-square test was used to compare qualitative variables. Mann–Whitney test was used to compare two quantitative variables. Spearman correlation was done to measure correlation between quantitative variables in case of non-parametric data. P-value considered statistically significant when P ≤ 0.05.
6. Results
Patient characteristics showed insignificant differences between the two study groups . Likewise, preoperative and intraoperative hemodynamics showed insignificant difference between the 2 study groups, and . However, mean arterial pressure showed significant difference between the two groups at 4 and 6 h postoperatively, , where, heart rate showed statistical difference at 4, 6, and 18 h postoperative, . First analgesic requirement time for patients after surgery was significantly longer in Mg group compared to control group (P < 0.05) , and the frequency of requesting analgesia was significantly less in magnesium group, .
Table 1. Patient characteristics of the studied groups
Table 2. Mean arterial pressure over the study period (mmHg)
Table 3. Heart rate changes over the study period (beats/min.)
Table 4. Pain numerical rating score and serum B endorphin levels over the study period
Table 5. Post-operative analgesic profile
No patient in the two groups complained of pain during surgery. Whereas the postoperative NRS was significantly less in magnesium group compared to control group at 2, 4 and 6 h postoperatively, . Serum B-endorphins levels showed statistically significant difference at 2, 4 and 6 h postoperatively, , being less in magnesium sulphate group. However, baseline (preoperative) and intraoperative serum B-endorphins showed no statistically significant difference between the studied groups.
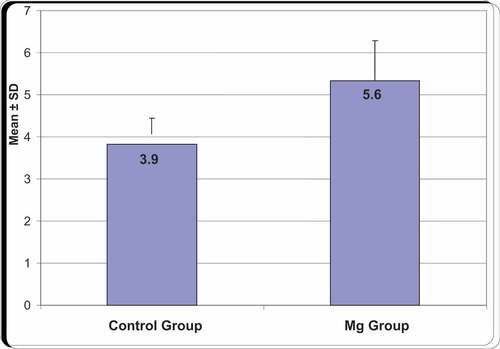
Total amount of pethidine in 24 hours was significantly lower in Mg group than in control group As in Mg group, mean was 105 with SD 15.26, whereas, in control group mean was146.67 with SD 12.69, .
No significant differences were reported regarding the incidence of sedation or side effects between groups, data not show.
7. Discussion
In the present study, we investigated the effect of adding magnesium sulphate as adjuvant to Epidural bupivacaine 0.5% in a dose of 1 ml/segment and lidocaine 2% in a dose of 1–2 ml/segment, on the postoperative pain scores, serum b-endorphins levels and postoperative pain perception. Our study stands out from other studies investigating the role of magnesium sulphate in epidural block as b-endorphins serum levels were estimated along with pain scores in the postoperative period. Plasma BE levels might serve as biomarkers of endogenous antinociceptive capacity. [Citation17]
In line with other studies, [Citation18,Citation19] The results of this study showed that there were insignificant differences between the two groups regarding intraoperative hemodynamics (heart rate, and mean arterial blood pressure). Also, in accordance with other published data, [Citation20] this hemodynamic difference became significant in the postoperative period when patients started regaining somatic sensation.
NRS showed significant reduction the magnesium group in the postoperative period at 2, 4 and 6 hours. This reflects better antinociception control in the magnesium group supported with a statistically significant decrease in the number of patients requesting early postoperative analgesia as well as total pethidine consumption. This in accordance with other studies that showed reduction in both NRS and total opioid consumption in the postoperative period when magnesium sulphate is used in epidural analgesia [Citation21,Citation22]. This Opioid sparing effect was in accordance with other studies done to assess the analgesic effects of adding magnesium sulphate to epidural Bupivacaine in the lower abdominal surgery, [Citation23] combined epidural and intrathecal magnesium sulphate in orthopedic surgeries [Citation24], and continuous intraoperative epidural magnesium sulphate infusion [Citation25]
Plasma BE might act as a biomarker for endogenous opioid analgesic function. [Citation26] In this study there was a statistical difference of BE serum levels being higher in magnesium sulphate group at 2, 4, and 6 h postoperatively. Some studies suggesting that plasma BE levels predict post-operative opioid need and it might also be a marker for more effective endogenous opioid analgesia. Moreover, some clinical researches showed that higher plasma BE predicts greater subsequent acute pain intensity and that higher BE levels could be a marker for enhanced endogenous opioid analgesia. [Citation27,Citation28]
The mechanism of how magnesium exerts it analgesic action is still unclear. A popular theory is that the anti-nociceptive actions is the result of its inhibition of the NMDA receptor. [Citation29] The potentiation of epidural analgesia when magnesium is used as adjuvant has been described in several reports. [Citation30–32] In our study, patients who received magnesium sulphate during the operation showed to have less postoperative pain compared with patients who did not. These observations are in accordance with prior studies results on the analgesia-potentiating effect of magnesium. Nevertheless, there has been a previous report suggesting that the mechanism of magnesium antinociception property might be related to endogenous opioid hyperalgesia. [Citation33,Citation34]
Whether magnesium sulphate could have antinociception action via modulating endogenous opioid analgesia, that beyond the scope of this study and further study is needed to clarify the mechanism of the effect of magnesium on the attention of increased pain intensity.
As regard safety of adding magnesium sulphate to epidural block, this study did not observe significant epidural drugs-related side effects postoperatively. The results in accordance with other studies that have tested the safety of epidural magnesium sulphate in epidural anesthesia. [Citation35]
Our study has potential limitations. First, we did not measure serum b endorphins in the CSF which could reveal another understanding of the pain modulating effect of epidural magnesium sulphate. Second, we did not exclude various pathological states that might have been associated with abnormal endorphin levels e.g., menopausal hormonal changes, as well as migraine and rheumatoid arthritis. Thus, interpreting the results of b-endorphin should be thought of in this context. Finally, we assessed the postoperative rest pain because of the mobility restrictions during the postoperative period. If postoperative pain was evaluated during patient mobility, which could have been considered a stimulus, the results would have been shown magnesium sulphate role in early mobilization, if any.
In summary, we conclude that the addition of magnesium sulphate 50 mg/10 ml saline to epidural bupivacaine/lidocaine provides better postoperative analgesia as well as reducing serum B endorphins in the postoperative period without increasing the incidence of complications in high knee osteotomy orthopedic surgery postoperatively.
Declaration
The Authors declare that there is no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85(4):808–816.
- Rawal N, Hylander J, Nydahl PA, et al. Survey of postoperative analgesia following ambulatory surgery. Acta. Anaesthesiol. Scand. 1997;41(8):1017–1022.
- Filos KS, Lehmann KA. Current concepts and practice in postoperative pain management: need for a change. Eur Surg Res. 1999;31(2):91–107.
- Follin SL, Charland SL. Acute management: operative or medical procedures and trauma. Ann Pharmacother. 1997;31(9):1068–1076.
- Sinatra RS, Torres J, Bustos AM. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg. 2002;10(2):117–129.
- Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001;410(6827):471–475.
- Helal OA, M A W, Yasser MK. Magnesium sulfate versus fentanyl as adjuvant to epidural levobupivacaine in surgeries below umbilicus. Egypt J Hosp Med. 2019;77(2):4987–4992.
- Pavlin DJ, Chen C, Penaloza DA, et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg. 2002;95(3):627–634.
- Omar H. Magnesium sulphate as preemptive adjuvant to levobupivacaine for postoperative analgesia in lower abdominal and pelvic surgeries under epidural anesthesia. Anesth Essays Res.. 2018;12(1):256–261.
- Grape S, Tramer MR. Do we need preemptive analgesia for the treatment of postoperative pain? Best Pract Res Clin Anesthiol.. 2007;21(1):51–63.
- Schulz-Stubner S, Wettmann G, Reyle-Hahn SM, et al. Magnesium as part of balanced general anaesthesia with propofol, remifentenil and mivacurium: a double- blind prospective study in 50 patients. Eur J Anaesthesiol. 2001;18(11):723–729.
- Rashpal SG, Acharya G, Rana A. et al. Comparative evaluation of addition of fentanyl and dexmedetomidine to ropivacaine for epidural anesthesia & analgesia in lower abdominal and lower limb orthopedic surgeries. Anesth Essays Res.. 2016;12(2):572–580.
- Khalili GI, Janghorbani M, Sajedi P. Effects of adjunct intrathecal magnesium sulfate to bupivacaine for spinal anesthesia: a randomized, double-blind trial in patients undergoing lower extremity surgery.. J Anesth. 2011;25(6):892–897.
- Tanmoy G, Chandra G, Malik A, et al. Evaluation of the effect of magnesium sulphate vs. midazolam as adjunct to epidural bupivacaine. Ind J Anesth. 2010;54(4):308–313.
- Boonstra AM, Stewart R, Koke AJA, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal Pain: variability and influence of sex and catastrophizing. Front Psychol. 2016;7(1466):1466-. [1466].
- Jiehao S, Xiuying WU, Xuzhong XU, et al. Comparison of epidural magnesium and/or morphine with bupivacaine for postoperative analgesia after cesarean section. Int. J. Obstet. Anesth .. 2012;21: 310–316.
- Bruehl S, Burns COY, Chont M. What do plasma beta-endorphin levels reveal about endogenous opioid analgesic function? Eur J Pain. 2012 Mar;16(3):370–380.
- Hassan A. APreemptive analgesic effect of low dose n-methyl-d-aspartate. receptor antagonists: ketamine and magnesium in conjunction with spinal anesthesia. AJAIC. 2006;9:1.
- Pflug AE. Halter JB: effect of spinal anesthesia on adrenergic tone and the neuroendocrine responses to surgical stress in humans. Anesthesiology. 1981;55(2):120–126.
- Bilir A, Gulec S, Erkan A, et al. Epidural magnesium reduces postoperative analgesic requirement. Br J Anaesth. 2007;98(4):519–523.
- El-Kerdawy H. Analgesic requirements for patients undergoing lower extremity orthopedic surgery, the effect of combined spinal and epidural magnesium. 2008;19(5):1013–1026. Middle East J Anesth.
- Kandil AHA, Hammad RAE, El Shafei MA, et al. Preemptive use of epidural magnesium sulphate to reduce narcotic requirements in orthopedic surgery. Egypt J Anaesth. 2012;28(1):17–22.
- Farouk S, Ibrahim S. Pre-incisional epidural magnesium provides pre-emptive and preventive analgesia in patients undergoing abdominal hysterectomy. Br J Anaesth. 2008;101(5):694–699.
- Ghatak T, Chandra G, Malik. A, et al. Evaluation of the effect of magnesium sulphate vs clonidine as adjunct to epidural bupivacaine. 2010;54(4):4. Indian Journal of Anaesthesia.
- Tanmoy G, Chandra A, Malik D, et al. Evaluation of the effect of magnesium sulphate vs. Midazolam as adjunct to epidural bupivacaine. 2010 52(308–313); Ind J Anesth.
- Arcioni RS, Palmisani S, Tigano C, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce postoperative analgesic requirements. Acta Anaesthesiol Scand. 2007 April;51(4):482–489.
- Ko SH, Lim HR, Kim DC, et al. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95(3):640–646.
- Koczy B, Stołtny T, Pasek J, et al. Evaluation of β-endorphin concentration, mood, and pain intensity in men with idiopathic hip osteoarthritis treated with variable magnetic field. Medicine (Baltimore). 2019;98(30):e16431.
- McCubbin JA, Wilson JF, Bruehl S, et al. Relaxation training and opioid inhibition of blood pressure response to stress. J Consult Clin Psychol. 1996;64(3):593–601.
- Kawakami H, Mihara T, Nakamura N, et al. Effect of magnesium added to local anesthetics for caudal anesthesia on postoperative pain in pediatric surgical patients: a systematic review and meta-analysis with trial sequential analysis. PLoS One. 2018;13(1):e0190354. .
- Peng YN, Sung FC, Huang ML, et al. The use of intravenous magnesium sulfate on postoperative analgesia in orthopedic surgery. a systematic review of randomized controlled trials. Med (Baltimore). 2018;97(50):e13583.
- Tan X, Shen L, Wang L, et al. Incidence and risk factors for epidural morphine induced pruritus in parturients receiving cesarean section: a prospective multicenter observational study. Medicine (Baltimore). 2019;98(40):e17366.
- Petraschka M, Li S, Gilbert TL, et al. The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience 2007;146(4):1795–1807.
- De Oliveira GSJr Castro-Alves LJ Khan JH McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2013;119(1):178–190.
- Guo BL Lin Y Hu Wet al. Effects of systemic magnesium on post-operative analgesia: is the current evidence strong enough? Pain Physician. 2015;18(5):405–417.