ABSTRACT
Multiple studies have confirmed that erector spinae block is effective in thoracic and breast surgeries. However, studies which investigate the efficacy of this block in cardiac surgery are scarce. This study aimed to compare continuous erector spinae block with multimodal intravenous analgesia in coronary bypass surgery. Methods: Forty patients undergoing coronary bypass surgery were divided into either group A (IV) (n = 20) who received multimodal intravenous analgesia or group B (ES) (n = 20) who had continuous erector spinae block. We compared the two groups regarding Visual Analog Scale (VAS) till 48 h after extubation, total perioperative opioid consumption, post-extubation peak inspiratory flow, duration of mechanical ventilation and ICU stay. Results: Group B showed a significantly lower VAS score than group A. intraoperative fentanyl was significantly less in group B (403.75 ± 44.63) versus (685 ± 99.47) in group A, p = 0.00. Postoperative morphine doses were 50% less in group B; (15.9 ± 2.63) versus (32.3 ± 5.04) in group A, p = 0.00. Peak inspiratory flow was significantly higher in group B after extubation. Duration of ventilation was shorter in group B (4.96 ± 0.71 h) versus (6.08 ± 0.69) in group A, p = 0.00. In addition, ICU stay was also shorter in group B (35.52 ± 3.87 h) versus (47.06 ± 5.08 h) in group A, p = 0.00. No clinically significant adverse effects were recorded. Conclusion: Ultrasound-guided bilateral continuous erector spinae block produced safe and effective analgesia for 48 h after extubation following coronary bypass surgery. It also reduced perioperative opioid consumption and allowed early tracheal extubation without major adverse effects.
1. Introduction
Pain after coronary bypass cardiac surgery is of moderate to severe intensity due to sternotomy, chest tube insertions and internal mammary artery dissection. Derangements of insufficient pain management after open-heart surgeries are hemodynamic instability, increased oxygen consumption and pulmonary atelectasis. [Citation1,Citation2] Acute postoperative pain after cardiac surgery with sternotomy is usually controlled by intravenous opioids. Opioids produce predictable satisfactory analgesia and sedation in postoperative patients but with side effects such as respiratory depression, drowsiness and myocardial depression. Recently, there is a shift toward reducing opioid usage. Opioid-free analgesia can be achieved by combining regional blocks with non-opioid drugs. [Citation3]
Thoracic epidural blockade is the gold standard neuraxial blockade for post-sternotomy pain, but unfortunately it has serious complications. Paraplegia might occur due to epidural hematoma formation after heparinization during cardiac surgery. [Citation4] Although paravertebral blockade is comparable to thoracic epidural regarding analgesic effect in cardiac surgery, it is not widely used as it may cause vascular injuries or pneumothorax. [Citation5,Citation6]
Erector spinae plane block (ESPB) is a recently implemented superficial myofascial plane block. Injecting local anesthetics above the transverse process and below the erector spinae (ES) muscle is a simple and safe technique compared to both paravertebral and thoracic epidural blocks. ESPB has been first described by Forero in 2016 for the treatment of thoracic neuropathic pain and post-mastectomy pain syndrome. [Citation7]
The analgesic effect of bilateral continuous ESPB in cardiac surgery is not fully investigated. We believed that ESPB may be effective as an epidural and paravertebral block as both dorsal and ventral nerve roots are blocked. Moreover, a sympathetic block of rami communicants may lead to visceral analgesia and vasodilatation of internal mammary vessels that are dissected to prepare the arterial graft for coronary vessels. [Citation8] ESPB may also have few merits in comparison with other myofascial blocks that can be used in cardiac surgery like parasternal, transversus thoracic, serratus or pecs blocks [Citation9,Citation10]. ESPB is a superficial and easy to perform block that can be applied preoperatively. Furthermore, the site of catheter insertion is away from the surgical site.
2. Aim of the study
Our study aimed to compare the analgesic effect of bilateral continuous ESPB with multimodal intravenous analgesia in coronary bypass surgery.
3. Methods
This study was a prospective comparative randomized study, and patients were allocated randomly into two groups by a sealed envelope technique after computer-generated randomization. The participants and investigators could not be blinded because of the invasive nature of the study. It was applied from March 2019 to September 2020. After ethical committee approval, the protocol has been registered in the ClinicalTrials.gov: NCT03866733.
4. Sample size
Using STATA program, setting alpha error at 5% and power at 90%, and according to results of a previous study by Krishna et al., which showed that in group I 47.6% of the cases had pain score 3/10 at 10 and 12 h after extubation while none in group II had pain score 3 at the same time point. [Citation11] According to that study, the estimated samples were 20 cases in each group.
Patients included in the study were candidates for elective coronary bypass cardiac surgery via median sternotomy, body mass index <30 kg.m2 and ejection fraction of the left ventricle >50%. Patients with stenosis of the left main coronary artery or on anticoagulants were excluded. Other causes of exclusion were pre-existing respiratory, neurological or renal disease; allergy to local anesthetics; uncooperative or psychiatric patients; and patient's refusal. Intraoperative causes of exclusion were prolonged CPB time (>120 min) or intraoperative inotropic support (dobutamine >5 µg/kg/min or epinephrine infusion >1 µg/min). Patients who had a catheter dislodgement or who developed any postoperative complications such as bleeding, arrhythmias or renal impairment were excluded from the final analysis.
Sixty patients were enrolled in the study but only 20 patients in each group completed the study. All the surgeries were done by the same surgical team. Patient informed consent was obtained from each participant. The Visual Analog Scale (VAS) scores of postoperative pain and pain control methods were explained to each patient during the preoperative visit. [Citation12] Patients in group A received oral pregabalin 150 mg 2 h before surgery.
After insertion of wide pore intravenous access, all patients received 2 mg midazolam and then arterial cannula was inserted with infiltration of local anesthesia. General anesthesia was induced by fentanyl 3 µg/kg followed by propofol (1 mg/kg) and cisatracurium (0.15 mg/kg). Endotracheal tube was inserted, and patients were mechanically ventilated. The central venous line was inserted and secured. Anesthesia was maintained by sevoflurane in a mixture of oxygen and air 1:1 (FIO2 = 50%) and cisatracurium. Fentanyl bolus (1 µg/kg) was given before sternotomy and if systolic arterial blood pressure and/or heart rate increased by more than 20% above baseline in both groups.
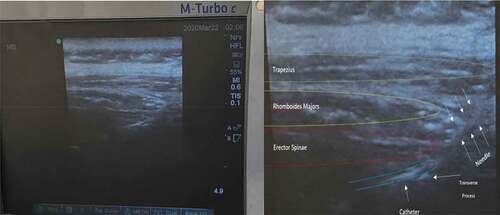
Before induction of anesthesia in group B, we counted and marked spinous processes from C7 to T7 while the patient was in the sitting position. We were guided by bony landmarks and ultrasound scanning. Bilateral ESPB was performed after induction of general anesthesia and positioning the patient in lateral position. Left lateral position was preferred as the radial arterial catheter was often inserted in the left forearm. We used a linear transducer 6–12 MHz (SonoSite M-turbo, USA). The probe was firstly placed in a transverse view over the T5 spinous process and then we moved laterally to view the lamina followed by the transverse process at approximately 3 cm from the median plane. Lastly, we rotated the probe to obtain a longitudinal view of adjacent transverse processes at the paramedian sagittal plane. Three muscles from superficial to deep were seen (trapezius, rhomboids and ES) above the hyperechoic transverse processes. In plane toughy, epidural needle was inserted deep to ES muscle from caudal to cephalic direction. Correct needle location was visualized by saline hydrodissection and then epidural catheter (B Braun Epidural kit) was threaded and secured. The same steps were repeated on the other side ().
Bilateral ESPB was activated in all patients in the supine position while a CV line was inserted and other monitors were applied. After negative aspiration, bupivacaine 0.25%, 15 ml total volume was given in the left catheter for 15 min (5 ml each 5 min) followed by the right catheter. No other boluses were given during the surgery. After ICU transfer, bupivacaine 0.125%, 8 ml/h was infused postoperatively for 48 h after extubation using a silicon balloon infuser (Accufuser, Woo Young Medical co. Korea 300 ml).
Heart rate and mean arterial blood pressure were documented at baseline (after induction of general anesthesia), before skin incision, after skin incision (skin incision was done 20 min after activation of both sides in group B), after sternotomy, 10 min after the end of cardiopulmonary bypass and before ICU transfer.
Hypotension and bradycardia were properly managed by the anesthesia team. Hypotension was defined if mean arterial blood pressure was <65 mmHg off-pump or <50 mmHg on-pump and treated with intravenous noradrenaline 4–8 µg. Bradycardia was defined as a heart rate <50 bpm and treated with intravenous atropine 0.01 mg/kg. If the patient had both hypotension and bradycardia, ephedrine 5–10 mg was given.
After fulfilling extubation parameters, patients were extubated in the ICU. We started acetaminophen 1 g/6 h in both groups, to which ketorolac 30 mg/12 h was added in group A if there is no contraindication to NSAID (renal impairment, gastric ulcer, bleeding tendency and bronchial asthma). Intravenous morphine shots of 0.05 mg/kg were given to patients in both groups by the nurse upon patient's request as rescue analgesia.
Our primary outcome was the postoperative pain score measured by VAS. It was assessed at 0 h (extubation), 4, 8, 12, 24 and 48 h by a nurse not included in the study. Secondary outcomes were intraoperative fentanyl and postoperative morphine consumption, time to extubation, peak inspiratory flow rate at 8, 12, 24 and 48 h using incentive respirometry (1 ball = 600 ml/min, 2 balls = 900 ml/min and 3 balls = 1200 ml/min). The period of ICU stay was also recorded.
Complications as hypotension, bradycardia, catheter-related hematoma or infection were documented. Failure of block was diagnosed if high anesthetic and analgesic requirements were needed during surgery or postoperatively.
5. Results
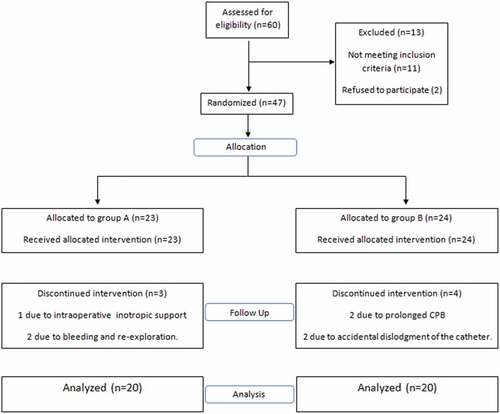
Sixty patients were assessed for enrollment. Thirteen patients were excluded pre-randomization, and seven patients were excluded post-randomization. Finally, 20 patients in each group completed the study ().
6. Statistical analysis
Data were coded and entered into the Statistical Package (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations when their distribution was found parametric. Also, qualitative variables were shown as numbers and percentages. The comparison between the two groups as regards qualitative data was done by using the Chi-square test. The comparison between the two independent groups with quantitative data was done by using an Independent t-test. The confidence interval was set to 95% and the accepted margin of error was set to 5%. There was no statistically significant difference between the two groups in terms of demographic data ().
Table 1. Demographic data of both groups
Intraoperative heart rate () and intraoperative mean arterial blood pressure () were significantly lower after skin incision and after sternotomy in group B, although the difference was not significantly different between the two groups at baseline, just after bypass and before ICU transfer.
Table 2. Intraoperative heart rate (beats/min) at different times of the study
Table 3. Intraoperative Mean arterial blood pressure (mmhg) at different times of the study
VAS was significantly lower in Group B at 0, 4, 8, 12, 24 and 48 h after extubation ().
Table 4. VAS scores in both groups at different times of the study
The total intraoperative fentanyl and postoperative morphine consumption was significantly less in group B. Also, the number of breakthrough episodes was significantly less in group B than in group A ().
Table 5. Intraoperative fentanyl, postoperative morphine and the number of episodes of rescue analgesia in both groups
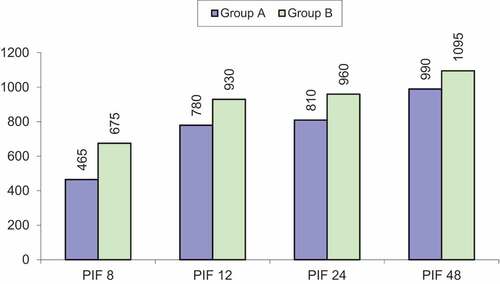
Peak inspiratory flow was significantly higher in group B at 8, 12, 24 and 48 h after extubation (as shown in ).
The length of stay in the ICU and the extubation time were shorter in group B (). Comparing the number of episodes of bradycardia or hypotension in both groups, the difference was not significant ().
Table 6. Extubation time, ICU stay and perioperative complications
Paresthesia of the upper limbs was reported in two patients in Group B but resolved after discontinuation of the local anesthetic infusion. No adverse effects related to catheters, such as hematoma or abscess, were recorded and no signs of bupivacaine toxicity were observed.
7. Discussion
Acute postoperative pain after coronary bypass surgery is related to multiple intraoperative factors as sternotomy, tissue retraction, intercostal nerve trauma and intercostal tube insertion [Citation1]. Opioids provide predictable perioperative satisfactory analgesia for cardiac surgeries but they are not without side effects. The interest of perioperative regional blocks in open cardiac surgery was supported by Bigeleisen et al., who demonstrated that patients may benefit from combinations of different pain control strategies [Citation13]. In addition, the advantages of fast tracking could be the driving force for the usage of these techniques [Citation3].
Thoracic epidural and paravertebral blocks are usually avoided by anesthetists and refused by surgeons in cardiac surgery. Although this concept has changed recently, the fear of hematoma formation with systemic heparinization remains a crucial issue [Citation4,Citation5]. Therefore, the absence of major neurovascular bundles in and around the area of interest renders ESPB safe especially with anticoagulation [Citation14,Citation15]. Despite that, studies investigating the analgesic effect of ESPB in cardiac surgeries with sternotomy are scarce. The present study illustrates the possibility of using continuous ESPB for perioperative analgesia in coronary bypass surgery.
Most of the patients in our study were strongly anxious, highly worried and preferred to be anesthetized before receiving the block. We hope our study will increase the awareness among both surgeons and patients about the safety of ESPB in cardiac surgery. Performing the block under sedation before general anesthesia saves intraoperative time and helps to avoid changing the patient position under anesthesia. Additionally, this facilitates the assessment of cutaneous sensory loss as inter-individual variation of intensity of the ESPB is problematic.
The results of our study show that patients in group B had significantly lower resting pain scores than those in group A; the median VAS score was ≤2 for 48 h after extubation. Krishna et al. had similar results but for a shorter period as patients in their study received single-shot block [Citation11]. Their patients reported pain scores <4/10 for (8.98 ± 0.14 h) in ESPB group.
Despite that, our patients have reported satisfactory pain scores after receiving low volume (3 ml for each dermatome to cover dermatomes from T2 to T6) and low concentration of bupivacaine (0.125%). Using larger bolus volume (20–30) or higher concentration (0.25%) of local anesthetics may produce a better quality of analgesia resembling thoracic epidural block. However, side effects of higher volumes and concentrations should be firstly investigated.
Receiving a high dose of opioids has been a predictor of patient readmission within 30 days after cardiac surgery [Citation16]. In order to decrease opioid consumption after cardiac surgery, Eljezi et al. added ketoprofen to postoperative morphine in the first 48 h [Citation17]. In agreement with the previous research, we gave patients in group A ketorolac postoperatively in addition to acetaminophen. Therefore, postoperative morphine doses in the present study were 32.3 ± 5.04 mg versus 38 (27–45 mg) in the previously mentioned study. Morphine consumption rather decreased by 50% in our patients of group B (15.9 ± 2.63 mg), p = 0.00. Similarly, Bogusław et al. demonstrated that patients who received unilateral ESPB for valve replacement via minithoractomy consumed less postoperative oxycodone [Citation18].
Effective pain management in group B resulted in significantly higher peak inspiratory flow than the flow in group A. Our results go with the results of Nagaraja et al. They concluded that ESPB was effective as an epidural blockade in improving inspiratory capacity following sternotomy in various cardiac surgeries. [Citation19]
Hamilton DL and Manickam B have hinted that ESPB is really an indirect paravertebral block [Citation8]. Multiple studies examined the analgesic effect of the continuous paravertebral block in conventional cardiac surgery. Patients who received the block in those studies experienced early weaning from mechanical ventilation and early transfer to the ward from ICU [Citation20,Citation21]. Concurrent to these findings, extubation time in group B was 4.96 ± 0.71 h in comparison with 6.08 ± 0.69 h in group A, p = 0.00. Moreover, ICU stay of patients in group B was shorter than that in group A, 35.52 ± 3.87 h versus 47.06 ± 5.08 h, p = 0.00.
In the present study, patients in group B did not experience significant hypotension or bradycardia, which suggests that hypotension is not a major risk in those patients. No complications were reported due to needle puncture as postoperative hematoma. Inspection of the catheter was done once daily and no catheter was removed due to inflammation at the puncture point. Catheters were safely removed 48 h after extubation despite starting both aspirin and clopidogrel.
8. Limitation of the study
1-We did not stratify the patients according to the number of grafts required in each patient.
2-We did not report the total doses of vasoactive drugs used during the study in order to investigate their effects on heart rate and arterial blood pressure.
9. Conclusion
This study revealed that continuous bilateral ESPB might provide a safe and satisfactory perioperative pain control after coronary bypass surgery. It decreased perioperative opioid consumption, enhanced early postoperative rehabilitation, and caused early extubation and ICU discharge with a low incidence of adverse events.
10. Recommendations
-Ropivacaine can be used instead of bupivacaine because of its lower toxicity, so higher doses and volumes can be used for better pain control.
Declarations
Ethics approval and consent to participate: The study was approved by the ethical committee of the Ain Shams University (file reference no FMASU R 07/2019). Written consent for all patients was obtained.
Consent for publication: Not applicable.
Availability of data and materials: Please contact the author for data requests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bjornnes AK, Parry M, Lie I. The impact of an educational pain management booklet intervention on postoperative pain control after cardiac surgery. Eur J Cardiovasc Nurs. 2017;16(1):18–27.
- Raksamani K, Wongkornrat W, Siriboon P, et al. Pain management after cardiac surgery: Are we underestimating post sternotomy pain? J Med Assoc Thai. 2013;96(7):824–828.
- Brandal D, Kel.ler MS, Lee C, et al. Impact of enhanced recovery after surgery and opioid-free anesthesia on opioid prescriptions at discharge from the hospital: A historical-prospective study. Anesth Analg. 2017;125(5):1784–1792.
- Hansdottir V, Philip J, Olsen MF, et al. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: A randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology.2006. 104:142–151.
- Fibla JJ, Molins L, Mier JM, et al. Comparative analysis of analgesic quality in the postoperative of thoracotomy: Paravertebral block with bupivacaine 0.5% vs. ropivacaine 0.2%. Eur J Cardiothorac Surg. 2008;33(3):430–434.
- Dhole S, Mehta Y, Saxena H, et al. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15(3):288–292.
- Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: A Novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627.
- Hamilton DL, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth. 2017;118(3):474–475.
- Fujii S, Bairagi R, Roche M, et al. Transversus thoracis muscle plane block. Biomed Res Int. 2019;1(1–6).
- Kaushal B, Chauhan S, Saini K, et al. Comparison of the efficacy of ultrasound-guided serratus anterior plane block, pectoral nerves ii block, and intercostal nerve block for the management of postoperative thoracotomy pain after pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(2):418–425.
- Krishna SN, Chauhan S, Bhoi D. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: A randomized controlled trial. J Cardiothorac Vasc Anesth. 2019;33(2):368–375.
- DeLoach LJ, Higgins MS, Caplan AB, et al. The visual analog scale in the immediate postoperative period: Intrasubject variability and correlation with a numeric scale anesth analg. Anesthesia and Analgesia. 1998;86(1):102–106.
- Bigeleisen PE, Goehner N. Novel approaches in pain management in cardiac surgery. Curr Opin Anaesthesiol. 2015;28(1):89–94.
- Forero M, Rajarathinam M, Adhikary S. Erector spinae plane (ESP) block in the management of post thoracotomy pain syndrome: A case series. Scand J Pain. 2017;17(1):325–329.
- Swisher MW, Wallace AM, Sztain JF. Erector spinae plane versus paravertebral nerve blocks for postoperative analgesia after breast surgery: A randomized clinical trial. Reg Anesth Pain Med. 2020;45(4):260–266.
- Long DR, Lihn AL, Friedrich S, et al. Association between intraoperative opioid administration and 30-day readmission: A pre-specified analysis of registry data from a healthcare network in New England. Br J Anaesth. 2018;120(5):1090–1102.
- Eljezi V, Biboulet C, Boby H, et al. Effects of ketoprofen on dynamic pain after open heart surgery. Pain Physician. 2017;20(6):509–520.
- Nagaraja PS, Ragavendran S, Singh NG, et al. comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21(3):323–327.
- Gawęda B, Borys M, Belina B, et al. postoperative pain treatment with erector spinea plane block and pectoralis nerve blocks in patients undergoing mitral/tricuspid valve repair. A randomized controlled trial. BMC Anesthesiol. 2020;20(1):51.
- Cantó M, Sánchez MJ, Casas MA, et al. Bilateral paravertebral blockade for conventional cardiac surgery. Anaesthesia. 2003;58(4):365–370.
- El Shora HA, El Beleehy A, Abdelwahab GA, et al. Bilateral paravertebral block versus thoracic epidural analgesia for pain control post-cardiac surgery: A randomized controlled trial. Thorac Cardiovasc Surg. 2018;68(5):5.