ABSTRACT
Background: Gabapentin has great efficacy in the treatment of neuropathic pain as a non-opioid alternative. It has been reported to prevent nausea and vomiting postoperatively. Our study compared different dosing regimens to evaluate their effectiveness and frequency of side effects in patients scheduled for laparoscopic abdominal surgery.Methods: 150 adult patients scheduled for elective abdominal laparoscopic surgery were randomly assigned to three equal groups. Group G300 received 300 mg gabapentin orally 1 h before surgery. Group G600 received 600 mg gabapentin orally 1 h before surgery. Group G900 received 900 mg gabapentin orally 1 h before surgery. Incidence and severity of PONV, need for rescue antiemetics, need for rescue analgesia, and incidence of side effects were assessed.Results: Incidence and severity of PONV were statistically higher in G300 versus G600, and G900 (8/50, 4/50, 3/50, respectively) and the total number of patients who received antiemetics, the overall dose of given granisetron, VAS scores for pain, and the total required rescue analgesia were higher in G300 versus G600, and G900. The time needed for first rescue analgesia was statistically longer in G600 and G900 versus G300. G900 was associated with a higher incidence of somnolence/sedation and dry mouth than G300 and G600.Conclusion: Gabapentin 600 mg administered 1 hr before laparoscopic abdominal surgery is as effective as gabapentin 900 mg for PONV control and VAS reduction of 24-hour postoperative pain scores with fewer side effects. On the other hand, gabapentin 300 mg did not demonstrate good control of PONV, or pain control compared to higher doses.
KEYWORDS:
1. Introduction
Postoperative nausea and vomiting (PONV) have an incidence of 40–90% especially after general anesthesia [Citation1]. Severe medical complications could be caused by PONV such as suture dehiscence, subcutaneous emphysema, esophageal rupture, or pneumothorax, which could dramatically increase the overall health care costs and boost patient quality and outcomes through its avoidance. PONV prevention and treatment can be performed by many agents including 5-HT3, dopaminergic, histaminic, and NK1 antagonists. However, the need for cheaper and more reliable treatments cannot be ignored [Citation2]. Gabapentin was initially developed as an efficient anticonvulsant used in the treatment of neurogenic pain, and more recently was researched as a non-opioid alternative to the reduction of morphine requirements as part of a multimodal approach to postoperative pain [Citation3]. Gabapentin has been gradually integrated into “fast-track” packages and enhanced recovery during surgery protocols to prevent unwanted side effects associated with opioid alternatives [Citation4]. Interestingly, it has also been reported that gabapentin decreases the symptoms of chemotherapy-induced nausea [Citation5], resulting in subsequent efficacy results in hyperemesis gravidarum [Citation6] and post-dural puncture emesis [Citation7]. Different dosing regimens were tested to compare control versus either 1-Time preoperative 300 mg, 600 mg, 900 mg, or 1200 mg gabapentin on PONV [Citation8], but according to our knowledge, no research was conducted to compare the different dosing regimens in the same setting to evaluate their effectiveness on PONV and the frequency of side effects as a primary endpoint in patients scheduled for laparoscopic abdominal surgery. The primary goal of our study is to assess the effectiveness of each dose of oral gabapentin given 1 hour before surgery in PONV and its side effects in the first 24 hours, and our secondary goal is to assess the effects of different drug doses on postoperative pain and postoperative opioid requirements to know the most effective dose with the least side effects.
2. Patients and methods
After obtaining institutional ethical committee approval (ethical number FMASU R53/2020), and registration in ClinicalTrials.gov (NCT 04622618), One hundred fifty adult patients scheduled for elective abdominal laparoscopic surgeries were enrolled in the study. Written informed consent was obtained from all patients before randomization. Randomization was done with the help of a computer-generated list of numbers. Patients were divided randomly and equally into three groups (50 patients each). The first group of patients received 300 mg gabapentin orally 1 hour before induction of anesthesia by a sip of water (group G300), the second group of patients received 600 mg gabapentin orally 1 hour before induction of anesthesia by a sip of water (group G600), the third group of patients received 900 mg gabapentin orally 1 hour before induction of anesthesia by a sip of water (group G900). Drug formulation was done by one of the researchers making the dosage of the three groups the same in number and shape of the capsules and then given to an anesthetist who was neither involved nor interested by any mean in the research. The blind grouping was kept to all, including the patients themselves until the completion of the study. Another anesthesiologist performed data collection while being blinded to the given medication during the study, and he was not included in the research team as well.
Our sample patients were aged between 21 and 60 years, ASA I–II, electively scheduled for laparoscopic abdominal surgery. Exclusion criteria included pregnancy or breast-feeding; psychiatric illness; administration of antiemetic or systemic corticosteroids within 24 hours before surgery; vomiting within 24 hours before surgery; alcohol or drug abuse; or known hypersensitivity or contraindications to the drug used in this study; impaired liver or kidney function; a history of motion sickness; anti-depressants; or patients on whom laparoscopic procedure converted into an open technique. All patients were subjected to a thorough medical history, physical examination with thorough airway assessment, laboratory investigations (fasting blood sugar, kidney, liver function tests, serum electrolytes, coagulation profile preoperatively). Counsel was given to them about the anesthetic management, potential complications of the study drug, surgery, and anesthesia. All patients were educated about the explanations of verbal nausea numerical rating scale (VNRS) which is from 0 to 10 cm scale describing nausea severity (0 = no nausea, 10 = worst nausea imaginable, mild nausea 1–3, moderate 4–6, or severe 7–10) and the visual pain analog scale (VAS) from 0 to 10 cm (0 = no pain, 10 = worst intolerable pain, mild pain 1–3, moderate pain 4–6, or severe pain 7–10). All these data were documented. All participants received an equal number of gabapentin capsules with different described doses according to our protocol by a sip of water 1 hour preoperatively in the surgical ward and then all participants were admitted to operating theatre (OR) induction area where patient’s identification was confirmed and an 18-gauge intravenous cannula was inserted to all participants. All patients could take water 2 h before surgery and solid food up to 8 h before surgery. When patients arrived in the operating room, standard monitoring, including five leads electrocardiography, pulse oximetry, non-invasive blood pressure monitor, and capnography were prepared and connected to the patients. Induction of anesthesia was done by IV thiopental sodium 5 mg/kg, 0.6 mg/kg rocuronium bromide, and (1–2) μg/kg fentanyl. Endotracheal intubation was inserted after 3 minutes of bag-mask ventilation with 100% oxygen. Anesthesia was maintained by (1–3) % sevoflurane in oxygen/air mixture 1:1 then Rocuronium 0.1 mg/kg were given for the maintenance of muscle relaxation. Sevoflurane concentration used was aiming to keep the heart rate and blood pressure within 20% above or below baseline values. Adjustment of mechanical ventilation was done to keep an end-tidal carbon dioxide partial pressure of 35–40 mmHg throughout the whole procedure. A bolus dose of 1 μg/kg fentanyl was given 30 minutes before the surgery end for pain control postoperatively. At the procedure ends, discontinuation of sevoflurane and antagonization of neuromuscular blockade were done using a combination of IV atropine 0.02 mg/kg and 0.04 mg/kg neostigmine. Fully awake extubation was performed and Postoperative analgesia protocol for all groups was accomplished by acetaminophen 1 gm/6 hours and ketorolac tromethamine 30 mg intravenous slow injection every 8 hours started immediately post-extubation and if breakthrough pain (VAS>5) a 5 mg Nalbuphine was given.
All patients were assessed for the incidence of PONV episodes (nausea, retching, or vomiting) during three time periods over the first 24 hours: 0–4, 4–12, and 12–24 hr as a primary outcome. As a secondary outcome, we assessed the severity of nausea at the same periods as the episodes of PONV, by using VNRS which is an 11-point verbal-numerical rating scale, the need for rescue antiemetic where granisetron 1 mg if severe nausea or two or more emetic episodes, or upon a request from the patient to be repeated if no response within 1 hour up to 3 mg and how many times rescue antiemetics were given in 24 hours after surgery, Intensity of postoperative pain by using an 11-points VAS at certain time points which is immediately postoperative and at 4, 8, 12 and 24 hours postoperatively and rescue analgesic dose of 5 mg IV nalbuphine was given if VAS >5. The number of rescue analgesic administrations was recorded. Incidence of gabapentin adverse reactions, such as dizziness, headache, and somnolence, were also documented in each group during the first 24 hours postoperatively.
The endpoints of our study were the occurrence of surgical complications such as hemorrhage where re-operation was needed or the conversion of the laparoscopic procedure to conventional surgical technique.
2.1. Sample size justification
The least difference assumption in the nausea rate ranging from 5% and 20%, a 47 patients sample size in each one of the three arms was found to be enough to visualize such difference if true, at 0.05 alpha error and 0.80 power of the test.
2.2. Statistical analysis
The computer received the Data using IBM SPSS software package version 24.0. Description of qualitative data was expressed in terms of number and percent. Chi-square test was used for comparison between different groups regarding categorical variables. Description of quantitative data was done using mean and standard deviation for normally distributed data. Regarding normally distributed data, two independent populations were compared against each other using independent t-test. While more than two population were analyzed, used F-test (ANOVA). Two-tailed probabilities are the method of expression of the test results of significance.5% level was used to judge the significance of the obtained results.
3. Results
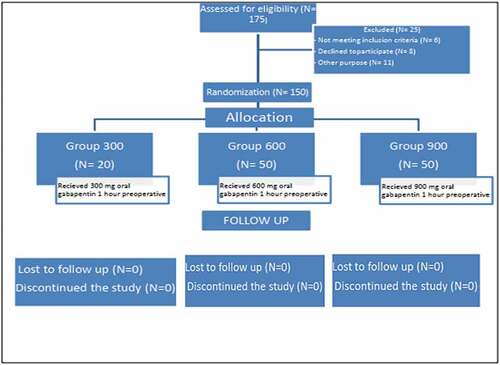
A total of 175 patients had been assessed for eligibility of our study but 6 of them did not meet the inclusion criteria, 8 of them refused to participate in the study, and 11 were excluded from the study due to surgical reasons necessitating a change of surgical approach to conventional open surgical technique ().
Concerning the participants’ demographic data and smoking history, there were no significant differences between the three studied groups. Concerning the duration of surgical, anesthetic, and carbon dioxide insufflation no significant difference between the studied groups with P-value 0.125, 0.098, 0.214, respectively, was found ().
Table 1. Demographic profile of study patients
Regarding the type of laparoscopic procedures performed no statistical difference between the three groups with a p-value of 0.362 was found ().
Table 2. Types of laparoscopic surgical procedures
Regarding the PONV incidence and the severity of nausea recorded in each group independently at a certain time frame. In G300, the total number of patients who experienced PONV was 8 out of 50 over 24 hours following the procedure and it was distributed as 4 patients at 4 hours, 2 patients at 12 hours, and 2 patients at 24 hours after surgery. As regards G600, the total number of patients who experienced PONV was 4 out of 50 over 24 hours following the procedure and it was distributed as 2 patients at 4 hours, and 2 patients at 12 hours and no patient experienced PONV at 24 hours after surgery. In G900, the total number of patients who experienced PONV was 3 out of 50 over 24 hours following the procedure and it was distributed as 2 patients at 4 hours, and 1 patient at 12 hours and no patient experienced PONV at 24 hours after surgery ().
Table 3. Incidence of PONV and severity of nausea at certain time points in G300
Table 4. Incidence of PONV and severity of nausea at certain time points in G600
Table 5. Incidence of PONV and severity of nausea at certain time points in G900
3.1. Values are presented as number and percent
The incidence and severity of PONV were statistically higher in G300 compared to G600, and G900 in the first 24 hours with P-value 0.013, 0.005, respectively, while there was no significant difference between G600 and G900 ().
Table 6. Total incidence of PONV and severity in the first 24 hours among the three study groups
While concerning the number of patients who received rescue antiemetics (granisetron) in the first 24 postoperative hours we found them statistically higher in G300 than in G600 and G900, respectively (P-value 0.023, 0.011). However, there was no statistical difference between G600 and G900 with a p-value of 0.143. As regards the total dose of granisetron administered to the participants, there was a statistical difference between the three groups studied. The G900 group was the least one compared to G300 and G600 with p values of 0.031, 0.025, and 0.01, respectively. G300 was the highest group in consumption of granisetron in 1st 24 postoperative hours compared to G600 and G900 ().
Table 7. Antiemetic requirement for the study patients in the first 24 hours among the three study groups
Regarding postoperative pain as measured by VAS scores in the first postoperative 24 hours, it was statistically higher in G300 at the 4th and 8th hours postoperatively in comparison to G600 with p values 0.019, 0.021, respectively, and also higher VAS scores in G300 compared to G900 at the 4th and 8th hours postoperatively with p values 0.021, 0.022, respectively. While the VAS scores did not show any statistical difference between the three groups studied recorded at the remaining time points ().
Table 8. Visual analog scale (VAS) scores for pain in different time periods among the three study groups in the first 24 hours (mean±SD)
While the times passed till first rescue analgesia was prescribed to patients were significantly shorter in G300 compared to G600 and G900 with P values 0.001, 0.001, respectively, but there was no difference comparing G600 and G900. As regards the total dose needed for rescue analgesia, G300 received higher doses in comparison to G600 and G900 with P-values 0.001, 0.001, respectively, and again there was no difference between G600 and G900 ().
Table 9. Time passed till need rescue analgesia (Nalbuphine) in minutes and its total dose (mg) needed in the first 24 hours
We found that participants in G600 and G900 were associated with a higher rate of sedation/somnolence than Group G300 concerning the incidence of side effects. The participants in group G900 experienced a higher incidence of dry mouth compared to group G300 and G600 with p-value (0.006, 0.103, and 0.01, respectively), but there was no discrepancy between G300 and G600. As regards the incidence of sore throat and headache, there was no statistical difference between all studied groups ().
Table 10. Incidence of side effects in the first 24 hours among the three study groups
4. Discussion
Our results had illuminated that administration of gabapentin in doses of 600 mg or 900 mg 1 hour before surgery was superior to a dose of 300 mg given 1 hour before surgery as regards the prevention and reduction of PONV severity with lower doses of rescue antiemetic needed. It was also noted that the postoperative pain measured by VAS pain rating was lesser in G600, G900 than G300 with longer pain-free times till first rescue analgesia was required and lower total doses of rescue analgesia needed in the first postoperative 24 hours after laparoscopic abdominal surgeries. It was also observed that patients who received 900 mg of gabapentin had a higher rate of sedation, somnolence, and dry mouth than the other two groups.
The mechanism of PONV following laparoscopic abdominal surgeries is not clear, but there are contributing factors to the occurrence of PONV, including CO2 insufflation that causes irritation and stretching of the peritoneum [Citation9]. Gabapentin antiemetic effect is debatable. Some studies suggested that the reduction in calcium conduction in the postrema area [Citation10] and inhibition of tachykinin neurotransmission [Citation11] are responsible for it. Other studies mentioned that the decrease in perioperative inflammation causes a lower incidence of ileus and a lower possibility for the occurrence of PONV [Citation12]. The decrease in opioid consumption by using gabapentin in the protocol of multi-modal analgesia could be one of the mechanisms of gabapentin to prevent PONV [Citation2]. The combination of the above theories could be the mechanism of gabapentin to decrease PONV. anti-hyperalgesic effect of Gabapentin is due to its binding to alpha-2 delta subunits of voltage-gated Ca++channels leading to inhibition of calcium influx with subsequent inhibition of excitatory neurotransmitter (glutamate) release [Citation3].
The pharmacokinetic properties of Gabapentin are unique. Bioavailability is inversely related to the dose given, ranging from 60% for a dose of 300 mg to 40% for a dose of 600 mg and reaching 35% for a dose of 1600 mg three times daily. The saturable transport mechanism is clarified by this [Citation13–15].
A variety of studies designed to determine the impact of preoperative gabapentin on PONV as a primary endpoint have shown that the group of patients receiving gabapentin experienced a substantial statistical reduction in the incidence of PONV and rescue antiemetic requirements during the first 24 hours relative to control groups [Citation16–21]. In contrast, with the above reports, Pandey et al. [Citation22] reported that preoperative administration of 300 mg gabapentin to laparoscopic cholecystectomy patients compared to other groups of patients receiving 100 mg tramadol or placebo had a higher incidence of PONV (24.8%) compared to the other two groups (17.6% and 5.2%, respectively).
These go in correspondence with our study; several reports have shown that preemptive gabapentin with different doses ranging from 300 mg to 1200 mg for acute pain management showed lower VAS scores for pain and lower postoperative analgesic requirements [Citation22–29]. In the study conducted by Pandey et al., when different doses were compared to each other, they suggested that raising the dose of gabapentin by more than 600 mg could not increase the efficacy of decreasing VAS scores or postoperative fentanyl requirements [Citation30].
As regards the side effects associated with preoperative gabapentin, an increased frequency of postoperative sedation/somnolence at doses of 600 and 900 mg compared to 300 mg and a higher incidence of dry mouth was observed. While no previous study showed an association between gabapentin and various side effects, a substantial increase in postoperative sedation/somnolence at higher doses was recorded [Citation8]. There are only two reports commented on the length of stay in the post-anesthetic care unit (PACU) [Citation31,Citation32], but they have not been able to provide evidence of the clinical effects of postoperative somnolence for gabapentin.
In our research, the same anesthetic technique was used in all patients, the length of anesthesia and CO2 insufflation was comparable in all groups, the characteristics of patients like sex and history of smoking were comparable in all groups, and other variables that could influence the incidence of PONV were omitted, such as pregnancy, history of motion sickness and use of antidepressants. The findings obtained are therefore only due to the various doses of gabapentin. We found that a 600 mg dose of gabapentin given 1 hour before surgery is as effective as a 900 mg dose in PONV control and postoperative pain with lower side effects, but we suggest a multicenter study to validate and address the dilemma of different doses.
The limitations of this research are: 1) this study was limited to preoperative gabapentin for general anesthesia in adult patients, and the effect on pediatrics, regional anesthesia, or sedation was not studied [Citation33–36]. 2) We did not research the efficacy of giving gabapentin immediately postoperatively, as previous studies indicate more benefits [Citation37].
5. Conclusion
In our research, gabapentin 600 mg given 1 hour before laparoscopic abdominal surgery is as effective as gabapentin 900 mg for PONV control and VAS reduction of 24-hour postoperative pain scores. Gabapentin 600 mg also has fewer side effects compared to gabapentin 900 mg. On the other hand, gabapentin 300 mg did not demonstrate good control of PONV, or pain control compared to higher doses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Myles PS, Williams DL, Hendrata M, et al. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84(1):6–10. https://pubmed.ncbi.nlm.nih.gov/10740539/
- Achuthan S, Singh I, Varthya SB, et al. Gabapentin prophylaxis for postoperative nausea and vomiting in abdominal surgeries: a quantitative analysis of evidence from randomized controlled clinical trials. Br J Anaesth. 2015;114(4):588–597. https://pubmed.ncbi.nlm.nih.gov/25571932/
- Hurley RW, Cohen SP, Williams KA, et al. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31:237–247.
- Page AJ, Ejaz A, Spolverato G, et al. Enhanced recovery after surgery protocols for open hepatectomy – physiology, immunomodulation, and implementation. J Gastrointest Surg. 2015;19:387–399.
- Cruz FM, Cubero DIG, Taranto P, et al. Gabapentin for the prevention of chemotherapyinduced nausea and vomiting: a pilot study. Support Care Cancer. 2012;20:601–606.
- Guttuso T Jr, Robinson LK, Amankwah KS. Gabapentin use in hyperemesis gravidarum: a pilot study. Early Hum Dev. 2010;86:65–66.
- Erol DD. The analgesic and antiemetic efficacy of gabapentin or ergotamine/caffeine for the treatment of postdural puncture headache. Adv Med Sci. 2011;56:25–29.
- Grant MC, Lee MD, HeeWon MD, et al. A meta-analysis the effect of preoperative gabapentin on postoperative nausea and vomiting. Anesth Analg. 2016;122(4):976–985.
- Wilson EB, Bass CS, Abrameit W, et al. Metoclopramide versus ondansetron in prophylaxis of nausea and vomiting for laparoscopic cholecystectomy. Am J Surg. 2001;181:138–141.
- Stahl SM. Mechanism of action of alpha2delta ligands: voltage sensitive calcium channel (VSCC) modulators. J Clin Psychiatry. 2004;65:1033–1034.
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141.
- Dias JM, De Brito TV, De Aguiar Magalhães D, et al. Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation. 2014;37:1826–1836.
- McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology. 1994;44:S17–S22.
- Vollmer KO, Anhut H, Thomann P, et al. Pharmacokinetic model and absolute bioavailability of the new anticonvulsant gabapentin. Adv Epileptology. 1989;17:209–211.
- Turck D, Bockbrader H, Sedman A. Dose-linearity of the new anticonvulsant gabapentin after multiple oral doses. Eur J Clin Pharmacol. 1989;36(supplement A310):8–11. .
- Pandey CK, Priye S, Ambesh SP, et al. Prophylactic gabapentin for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study.. J Postgraduate Med. 2006;52(2):97–100.
- Bashir F, Mohd K, Qazi S, et al. A randomized, double-blind, placebo-controlled study evaluating preventive role of gabapentin for PONV in patients undergoing laparoscopic cholecystectomy. JK Sci. 2009;11:190–193. Available from: https://www.jkscience.org/archive/volume114/15±Original±Article.pdf
- Khademi S, Ghaffarpasand F, Heiran HR, et al. Effects of preoperative gabapentin on postoperative nausea and vomiting after open cholecystectomy: a prospective randomized double-blind placebo-controlled study. Med Princ Pract. 2010;19:57–60.
- Misra S, Parthasarathi G, Vilanilam GC. The effect of gabapentin premedication on postoperative nausea, vomiting, and pain in patients on preoperative dexamethasone undergoing craniotomy for intracranial tumors. J Neurosurg Anesthesiol. 2013;25:386–391.
- Semira AS, Tandon VR, Bashir A, et al. A prospective, randomized, placebo-controlled trial comparing the effectiveness of gabapentin, ondansetron & dexamethasone in prevention of nausea & vomiting after laparoscopic cholecystectomy. JK Sci. 2013;15:117–121. Available from: http://www.jkscience.org/archives/volume153/Article1.pdf
- Ajori L, Nazari L, Mazloomfard MM, et al. Effects of gabapentin on postoperative pain, nausea and vomiting after abdominal hysterectomy: a double blind randomized clinical trial. Arch Gynecol Obstet. 2012;285:677–682.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51:358–363.
- Fassoulaki A, Patris K, Sarantopoulos C, et al. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer.. Anesth Analg. 2002;95(4):985–991. .
- Rorarius MG, Mennander S, Suominen P, et al. Gabapentin for the prevention of postoperative pain after vaginal hysterectomy. Pain. 2004;110:175–181. .
- Dierking G, Duedahl TH, Rasmussen ML, et al. Effect of gabapentin on postoperative morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind trial. Acta Anaesthesiol Scand. 2004;48:322–327. https://pubmed.ncbi.nlm.nih.gov/14982565/
- Turan A, Memis D, Karamanhoglu B, et al. The analgesic effects of gabapentin in monitored anesthesia care for ear–nose–throat surgery. Anesth Analg. 2004;99:375–378. https://pubmed.ncbi.nlm.nih.gov/15271709/
- Turan A, Karamanhoglu B, Memis D, et al. The analgesic effects of gabapentin after total abdominal hysterectomy. Anesth Analg. 2004;98:1370–1373. https://pubmed.ncbi.nlm.nih.gov/15105217/
- Turan A, Karamanhoglu B, Memis D, et al. Analgesic effects of gabapentin after spinal surgery. Anesthesiology. 2004;100:935–938.
- Eckhardt K, Ammon S, Hofmann U, et al. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg. 2000;91:185–191. https://pubmed.ncbi.nlm.nih.gov/10866910/
- Pandey CK, Navkar DV, Giri PJ, et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blind, placebo-controlled study. J Neurosurg Anesthesiol. 2005;17:65–68. Available from: https://pubmed.ncbi.nlm.nih.gov/15840990/
- Gilron I, Orr E, Tu D, et al. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. Pain. 2005;113:191–200.
- Kinney MA, Mantilla CB, Carns PE, et al. Preoperative gabapentin for acute post-thoracotomy analgesia: a randomized, double-blinded, active placebo-controlled study. Pain Pract. 2012;12:175–183.
- Mayell A, Srinivasan I, Campbell F, et al. Analgesic effects of gabapentin after scoliosis surgery in children: a randomized controlled trial. Paediatr Anaesth. 2014;24:1239–1244.
- Tsai KC, Yang YL, Fan PC. Gabapentin for postoperative vomiting in children requiring posterior fossa tumor resection. Pediatr Neonatol. 2015;56:351–354.
- Kazak Z, Meltem Mortimer N, Sekerci S. Single dose of preoperative analgesia with gabapentin (600 mg) is safe and effective in monitored anesthesia care for nasal surgery. Eur Arch Otorhinolaryngol. 2010;267:731–736.
- Clarke H, Pereira S, Kennedy D, et al. Adding gabapentin to a multimodal regimen does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiol Scand. 2009;53:1073–1083.
- Dauri M, Faria S, Gatti A, et al. Gabapentin and pregabalin for the acute post-operative pain management. A systematic-narrative review of the recent clinical evidences. Curr Drug Targets. 2009;10:716–733.