ABSTRACT
Background and Aims: Adequate postoperative analgesia guarantees adequate ventilation and minimizes respiratory complications after thoracotomy. This study compares postoperative outcomes of continuous caudal epidural blockade to systemic intravenous analgesia in neonates undergoing thoracotomy for trachea-esophageal fistula (TOF) repair.
Methods: A randomized, single-center, prospective, controlled study was conducted at El-Shatby University Hospital, from August 2016 to September 2020 where 40 neonates, weighing 2.5–4 kg belonging to the ASA physical Grade II to IV, posted for a TOF repair via thoracotomy. After general anesthesia, in group A, the epidural catheter was inserted through standard caudal epidural technique. Initial bolus of 0.25% bupivacaine (0.2 ml/kg) and postoperatively continuous infusion of 0.125% bupivacaine (0.1 ml/kg/h) was given. In group B, they received fentanyl (2 μg/kg) and paracetamol (10 mg/kg) IV Intraoperatively and postoperatively paracetamol (10 mg/kg/6 h) intravenously.
Results: In group A, good quality of analgesia for 48 h postoperative with a lesser doses of rescue opioids (1 µg/kg fentanyl) required to maintain adequate analgesia, which shortened the length of hospital stay compared to group B.
Conclusion: Very efficient postoperative analgesia can be achieved via continuous caudally inserted epidural blockade compared to intravenous fentanyl in neonatal post-thoracotomy pain.
1. Introduction
Pain experienced by preterm infants has long‐term sequelae on an infant’s behavioral and neurological outcome, and it has been speculated that painful stimulations in the neonatal period had injurious brain involvement [Citation1].
Systemic analgesics are considered the main pillar of most analgesic regimens, including opioids and acetaminophen (paracetamol). Immature metabolic pathways in neonatal period influence pharmacokinetics and pharmacodynamics of analgesics of various potency [Citation2].
Fentanyl is a selective μ-receptor agonist and is considered the most commonly used analgesic opioid in the neonatal intensive care unit as a result of its rapid and predictable onset of action with a short duration of action mostly due to its high lipid solubility; furthermore, it has a greater hemodynamic stability [Citation3].
Muscle rigidity and respiratory depression occur with high doses of fentanyl used in anesthetic induction. High doses of fentanyl can cause neuro-excitation and, rarely, seizure-like activity [Citation4].
Opioid-induced respiratory depression (OIRD) initiated by the activation of µ-opioid receptors expressed on the surface of neurons in brainstem respiratory centers is considered the most serious side effect as it is potentially life-threatening [Citation5].
In infants and children, epidural blockade is frequently accomplished with general anesthesia to provide postoperative analgesia [Citation6]. In neonates and small infants, it could be feasible to pass a catheter from the caudal space to the thoracic dermatomes to provide analgesia for thoracic procedure [Citation7]. The application of US is particularly valuable for epidural block in infants as a result of incomplete vertebral ossification, which facilitates the penetration of the US beam into the posterior vertebral column and makes identification of spinal structures easier [Citation8].
The aim of the present study primarily was to compare the continuous effect of the caudally applied thoracic epidural blockade to systemic intravenous analgesia on post-thoracotomy pain control, while secondarily the length of stay (LOS), supplemental analgesic requirements, duration of surgery, and the incidence of adverse respiratory events were also measured.
2. Methods
This is randomized prospective controlled trial that was piloted at El-Shatby University Uospital from August 2016 to September 2020 after the approval by the Ethics Committee of the Alexandria Main University Hospitals.
A written informed consent from the parents was acquired for the participation of their infants in this study.
Forty neonates of both sex, weighing 2.5–4 kg belonging to the American Society of Anesthesiology physical Grade II–IV, planned for trachea-esophageal fistula repair via thoracotomy were included in the study.
Patients suffering from local infection, neurological disorder, history of allergic reaction to local anesthetics, sacral/vertebral abnormalities, prematurity, renal anomalies, and coagulation disorders were excluded from the study. During the preoperative visit, all patients were assessed.
Premedication with atropine (0.02 mg/kg) will be administered intramuscularly less than 45 min before induction of anesthesia.
In the operation room, all neonates were attached to standard ASA monitor (Penlon PM-9000 Patient Monitor) in the form of continuous electrocardiogram, heart rate, pulse oximeter, and non-invasive arterial blood pressure. End-tidal capnography was attached after insertion of the endotracheal tube (ETT).
Anesthesia was induced with incremental concentrations of inhalational agent sevoflurane (2–4%) and oxygen (3 l/min) using a silicone face mask size 0 connected to a Jackson–Rees circuit while maintaining spontaneous ventilation.
Neonates were in the supine position with a small pillow in-between their shoulders. A 24-gauge intravenous line was secured in place bolus fluids 10 ml/kg lactated Ringer’s administered.
An intentionally main-stemming endobronchial intubation and then slowly withdrawing to the position where until bilateral breath sounds were initially heard. Ideally, spontaneous ventilation was maintained until the fistula is ligated.
Maintenance of anesthesia was done with assisted ventilation (PSV) of PENLON Prima SP2 Anesthesia Machine with oxygen and sevoflurane (2–4%). Patients were randomly allocated into two equal groups using a random number generating software (Research Randomizer Version 4.0).
In group A, after the induction of general anesthesia caudal epidural catheter (B Braun Perifix ONE Paediatric Epidural Set, Germany) was inserted ultrasound guided.
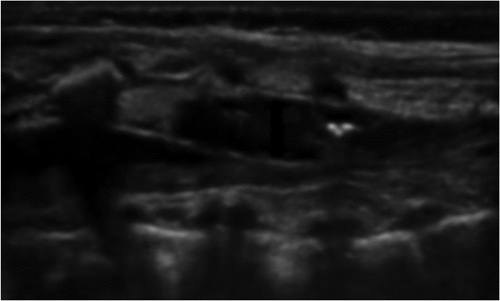
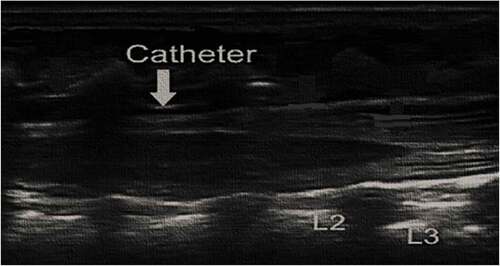
Preparation of appropriated sized US probe (linear 7–13 MHZ) was done and placed generally on the opposite side of the patient to operator. Under complete aseptic sterile technique with drapes, US cover, and sterile gel neonate are placed on left lateral position with knees, hips, and spine flexion. Then we measured the length from caudal space to the desired level of epidural blockade. Caudal epidural space is located using 20-gauge Touhy needle with “loss of resistance” technique.
Under ultrasound guidance we injected a few milliliters of normal saline that were used to widen epidural space and facilitate threading of epidural catheter (). We introduced 24-gauge epidural catheter through caudally inserted 20-gauge Touhy needle to the required level of epidural block under vision using ultrasound (). An initial bolus of 0.25% bupivacaine (0.2 ml/kg) was administered after negative aspiration for cerebrospinal fluid and blood with continuous ECG monitoring. Epidural catheter was safeguarded by looping at the entry site and covering with a clean plastic dressing, which was then directed to the shoulder and fixed to the skin with micropore tape.
While in group B, fentanyl (2 µg/kg)/IV and paracetamol (10 mg/kg)/IV were administered intraoperatively.
Surgery began under general anesthesia maintained with sevoflurane. Thoracotomy was done while maintaining autonomous respiration. Once the surgeon has ligated the fistula, muscle relaxation with rocuronium (0.1 mg/kg) and gentle positive pressure ventilation (PCV) were initiated [Citation9]. At the end of surgery, they were reversed with sugammadex 2 mg/kg and were extubated.
Postoperative epidural analgesia was maintained by a continuous infusion of 0.125% bupivacaine (0.1 ml/kg/h) in group A.
While in group B, paracetamol 10 mg/kg intravenously was given 6 hourly or neonatal infant pain scale (NIPS) score > 4, and the maximum dose 40 mg/kg/day was given.
Hemodynamic measurements including heart rate, mean arterial blood pressure, and oxygen saturation were recorded pre-induction (basal value), immediate post-induction, at starting of surgical manipulation, mean of intraoperative readings with 15 min interval, and at the end of the operation.
Postoperative pain also was assessed by blinded observer every 30 min in the first 2 h then every 2 hs for the next 10 h and thereafter every 4 h interval for the next 36 h using the NIPS scale [Citation10] (), and need for rescue opioid (fentanyl) analgesic use1 µg/kg/dose (first time of rescue and total opioid consumption). These are length of hospital stay (LOS), duration of surgery, incidence of respiratory depression, which is defined as diminished breathing (rate less than 8–10 bpm, SpO2 less than 90%) progresses into irregular (or cyclic) breathing and eventually into apnea (the complete cessation of breathing), and other complications such as systemic toxicity of local anesthetics, sympathetic blockade, hypotension, and bleeding.
Table 1. Neonatal infant pain scale (NIPS) [Citation10]
3. Statistical methods
The sample size was approved to be sufficient by the department of Statistics, Medical Research Institute, Alexandria University, Egypt [Citation11].
Data were analyzed using IBM SPSS software package version 20.0 (IBM Corp, Armonk, NY). The Kolmogorov–Smirnov test was used to validate the normal distribution of variables. Comparisons between groups for categorical variables were measured using the chi-square test (Fisher or Monte Carlo).
Student’s t-test was used to compare two groups for normally distributed quantitative variables while analysis of variance with repeated measures and post hoc test (Bonferroni) was calculated for comparison between different periods. Mann–Whitney test was used to compare between two groups for abnormally distributed quantitative variables, while post hoc test (Dunn’s) for Friedman test was used for comparison between different periods. Significance of the obtained results was judged at the 5% level (*statistically significant at p ≤ 0.05).
4. Results
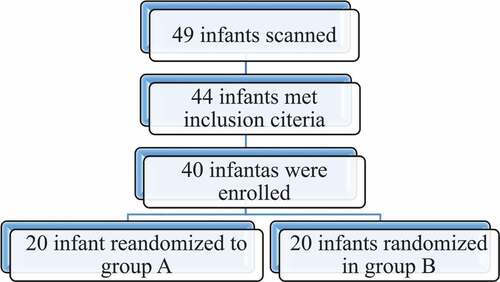
A total of 49 infants were screened for eligibility; 44 patients met inclusion criteria and were approached to participate and 4 parents refused to engage their infant in the study (Flowchart 1). Data were normally distributed within the two equal groups.
There was no statistically significant difference between the two groups regarding demographic parameters (); moreover, surgery lasted from 90 to 120 min without statistically significant difference between both groups.
Table 2. Demographic data of the two groups
Regarding hemodynamic measurement, there was a statistically significant decrease of heart rate in group B compared to group A post-induction (group A = 126.2 ± 6.3, group B = 122.2 ± 2.4, p = 0.012*) and at starting of surgical manipulation (group A = 126.7 ± 5.6, group B = 121.7 ± 2.1, p = 0.001*) but remained hemodynamically stable in the post-induction period ().
Table 3. Comparison between the two studied groups according to hemodynamic measurements
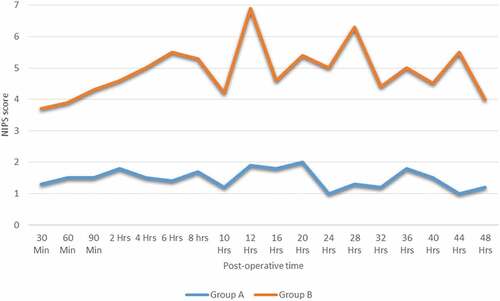
The mean pain score in group A was less than 2 and no need for rescue doses of intravenous fentanyl, whereas in group B, there was above 4 every six hourly and required multiple administration of paracetamol (), Furthermore, the time for first rescue fentanyl dose in group B was in 6.3 ± 0.8 h.
As a result of increased doses of rescue fentanyl needed in group B to reach adequate analgesic level, there was a statistically significant increase of opioid-inducedOIRD incidence (30%) where only one case was severe enough to require postoperative mechanical ventilation, moreover causing a longer hospital stay (11.1 ± 2.1 days) compared to group A (5.1 ± 1 days), but there were no statistically significant differences between two groups regarding incidence of complications (χ2 = 0.004, p = 0.980).
5. Discussion
Thoracotomy frequently causes severe postoperative pain and significant morbidity [Citation12]. Atelectasis, pneumonia, pulmonary embolism, and emergency intensive care admission have all originated from poor analgesia and subsequent immobility [Citation13].
Regarding continuous caudal thoracic epidural in group A the present study showed a good analgesic coverage for 48 h ost-operative with no need for rescue analgesic doses of fentanyl, which leads to shorter duration of hospital stay (5.1 ± 1 days) compared to group B (11.1 ± 2.1 days). In agreement with the present results, Bösenberg et al. reported an earlier return of intestinal motility and a reduced incidence of pneumonia in neonates who received epidural analgesia [Citation14]. Furthermore, Shenkman et al. reported safe use of continuous epidural analgesia in small infants (1400–4300 g) undergoing major surgery [Citation15].
Regional analgesia may also have respiratory stimulant action and associated with reduced need for mechanical ventilation [Citation16].
Regarding incidence of complications, the present study resulted that ultrasound-inserted epidural catheter is considered a safe procedure, in agreement with the current study. Bösenberg et al. studied the use of regional versus systemic analgesia for neonatal surgery and found that neuraxial blockade was not associated with hypotension or hemodynamic instability even in neonates with congenital heart disease [Citation17]; moreover, surgical stress response is more effectively mitigated by regional anesthesia in comparison with systemic opioid and it is also free of immunosuppressive effects of opioids [Citation18,Citation19].
Paracetamol efficacy in mild to moderate pain in neonates is now well documented; however, in the present study severe thoracotomy pain required fixed bolus doses of fentanyl, in agreement with current result where a recent randomized controlled trial (RCT) had documented its opioid-sparing effects in neonates [Citation20].
Opioids are the cornerstone of pain management following a major surgery even in neonates. Fentanyl is the most commonly used opioid in the postoperative period; a large double-blind RCT concluded that fentanyl may be superior to morphine for short-term postnatal analgesia in newborn infants [Citation21].
The present study showed that increased doses of rescue fentanyl needed in group B to reach adequate analgesic level lead to a statistically significant increase of opioid-inducedOIRD incidence (30%). In agreement with the present result Souvik, Maitra et al. found that opioids exhibit a narrow therapeutic window between analgesic doses and the dose that may cause respiratory depression [Citation22].
6. Conclusion
Effective postoperative analgesia could be achieved via continuous caudally inserted epidural blockade compared to intravenous fentanyl in neonatal post-thoracotomy pain. However, this resulted in lesser required rescue doses of fentanyl, lesser incidence of respiratory depression, and moreover shorter LOS.
Availability of data and material
All data supporting the study are presented in the manuscript or available upon request.
Consent for publication
Written informed consent was obtained from the parents for publication of this article and any accompanying tables/images. The copies of the written consents are available for review by the Editor of this journal upon request.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Ethics approval and consent to participate
The present study was approved by the ethical committee of Faculty of Medicine, Alexandria University (IRB no. 00007570, FWA no. 00018702). A consent to participate was obtained as well.
Acknowledgments
Not applicable.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Anand KJS, Scalzo FM. Can adverse neonatal experience alter brain development and subsequent behavior? Biol Neonate. 2000;77:69–82.
- De Lima J, Carmo KB. Practical pain management in the neonate. Best Pract Res Clin Anaesthesiol. 2010;24:291–307.
- Tibboel D, Anand KJ, Van Den Anker JN. The pharmacological treatment of neonatal pain. Semin Fetal Neonatal Med. 2005;10(2):195–205.
- Dewhirst E, Naguib A, Tobias JD. Chest wall rigidity in two infants after low-dose fentanyl administration. Pediatr Emerg Care. 2012;28(5):465–468.
- Niesters M, Overdyk F, Smith T, et al. Opioid-induced respiratory depression in paediatrics: a review of case reports. BJA. 2013;110(2):175–182.
- Tobias JD. Therapeutic applications of regional anesthesia. Paediatr Anaesth. 2002;12:272–277.
- Valairucha S, Seefelder C, Houck CS. Thoracic epidural catheters placed by the caudal route in infants: the importance of radiographic confirmation. Paediatr Anaesth. 2002;12:424–428.
- Kil HK. Caudal and epidural blocks in infants and small children: historical perspective and ultrasound-guided approaches. Korean J Anesthesiol. 2018 Dec;71(6):430–439.
- Andropoulus DB, Rowe RW, Betts JM. Anaesthetic and surgical airway management during tracheo-oesphageal fistula repair. Pediatr Anesthesia. 1998;8(4):313–319.
- Lawrence J, Alcock D, McGrath P, et al. The development of a tool to assess neonatal pain. Neonata Netw. 1993;12(6):59–66.
- Solanki NM, Engineer SR, Vecham P. Comparison of epidural versus systemic analgesia for major surgeries in neonates and infants. J Clin Neonatol. 2017;1(6):23–28.
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs. epidural blockade for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96:418–426.
- Pluijms WA, Steegers MAH, Verhagen AFTM, et al. Chronic post-thoracotomy pain: a retrospective study. Acta Anaesthesiol Scand. 2006;50:804–808.
- Bösenberg AT. Epidural analgesia for major neonatal surgery. Paediatr Anaesth. 1998;8:479–483.
- Shenkman Z, Hoppenstein D, Erez I, et al. Continuous lumbar/thoracic epidural analgesia in low-weight paediatric surgical patients: practical aspects and pitfalls. Pediatr Surg Int. 2009;25:623–634.
- Von Ungern-Sternberg BS, Regli A, Frei FJ, et al. The effect of caudal block on functional residual capacity and ventilation homogeneity in healthy children. Anaesthesia. 2006;61:758–763.
- Bösenberg AT, Jöhr M, Wolf AR. Pro con debate: the use of regional vs systemic analgesia for neonatal surgery. Paediatr Anaesth. 2011;21(12):1247–1258.
- Hollmann MW, Durieux ME, Fisher D. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93(3):858–875.
- Wolf AR, Doyle E, Thomas E. Modifying infant stress responses to major surgery: spinal vs extradural vs opioid analgesia. Paediatr Anaesth. 1998;8(4):305–311.
- Ceelie I, De Wildt SN, Van Dijk M, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309:149–154.
- Saarenmaa E, Huttunen P, Leppäluoto J, et al. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. J Pediatr. 1999;134(2):144–150.
- Maitra S, Baidya DK, Khanna P, et al. Acute perioperative pain in neonates: an evidence-based review of neurophysiology and management. Acta Anaesthesiol Taiwan. 2014;52:30–37.