?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: The COVID-19 pandemic created a remarkable impact on healthcare providers (HCP) both physically and psychologically. Perceived psychological stress (PSS) influences the homeostatic equilibrium, involving activation of the sympathetic nervous system and hypothalamus pituitary adrenal (HPA) axis. Copeptin, C-terminal portion of Vasopressin (AVP) precursor is stable; however, evidence about impact of PSS on copeptin levels is limited.
Aim: The aim of this study was to estimate the influence of psychological stress on copeptin levels among HCP working in intensive care unit (ICU).
Methods: A total of 70 HCP served in quarantine ICU participated in this prospective study; 35 physicians (28 males and 7 females) and 35 nurses (10 males and 25 females). A control group of 40 HCP matched age, BMI and specialty in non- quarantine hospitals. Fasting morning blood samples were withdrawn for determination of copeptin, cortisol, insulin at three points; pre-quarantine at ICU. Second point at end of first week and third point was two weeks’ post quarantine. A questionnaire was conducted to all participants to assess stress (PSS). Cortisol was determined by a chemiluminescence immunoassay while insulin and Copeptin were measured by ELISA.
Results: Baseline plasma copeptin level pre-quarantine was significantly increased 15.76 ± 8.6 pmol/l (P = 0.001*) and was positively correlated with high stress PSS score mean 66.9 ± 18.3. Post-quarantine copeptin was markedly reduced 3.98 ± 1.28 pmol/l and mean PSS was 23.0 ± 7.95 (P = 0.001*). Also, there was positive correlation between plasma copeptin and PSS, systolic blood pressure and serum insulin. On the other hand, there was no correlation between copeptin and serum cortisol.
Conclusion: Our finding suggested that copeptin may be used a potential biomarker for physiological strain during work in a stressful environment.
KEYWORDS:
1. Introduction
The recent coronavirus (SARS-CoV-2) disease 2019 (COVID-19) pandemic has generated an extraordinary impact on healthcare providers (HCP) both physically and psychologically worldwide [Citation1].Conventionally, it is well documented that global health workforce encounters high levels of stress and stress-related health problems, due to fear of acquiring infection, concern of incompetence to provide effective and sufficient care for patients given limited resources, worry of carrying the virus home and delivery of disease to comrades and family members [Citation1,Citation2]. Particularly, those working in intensive care unit (ICU), are subject to unique environment, looking after critically ill patients with specific medical needs, encompassed by their ability to apply stringent personal protection equipment (PPE) and other infection prevention and control (IPC) measures [Citation3]. Nevertheless, lack of appropriate experience for that crucial tragedy, feeling of insecurity and uncertainty exposed medical staff to emotional distress [Citation4]. Furthermore, increased need for human and resources both material supplies and funds might have added weight of work concerns [Citation5].
Perceived psychological stress (PSS) is defined as feelings or thoughts individuals experience regarding extent of stress they confront at a specified situation over a particular time period [Citation6]. It reflects the interaction between individuals and environment specifically threatening or constraining their capabilities in a way supposedly upsets their wellbeing [Citation7]. Perceived occupational stress attributes has two major dimensions: (a) Physiological reactions of the body reflected as headache, migraine, fatigue, palpitation, sleep disturbance and muscle ache, as well as digestive symptoms, sleeping disturbance and smoking habits [Citation7]. (b) Psychological consequences often viewed as both emotional reactions; in terms of irritability, anxiety and depression and behavioral symptoms; as neglecting responsibilities, hypnotic usage and nervous habits [Citation1,Citation8].
Furthermore, psychological stress influences the homeostatic equilibrium of the body, comprising a cascade of substantial events through activation of the sympathetic nervous system and hypothalamic pituitary adrenal axis (HPA) triggering glucocorticoid secretion, which is considered a major contributor in stress response [Citation9]. Through neurons in the paraventricular nucleus (PVN) of the hypothalamus releasing two neuropeptides; corticotrophin‐releasing factor (CRF) and arginine vasopressin (AVP) into the hypophysial portal blood. Both peptide hormones exhibit anxiogenic and depressive effects [Citation10]. AVP is derived from a larger precursor molecule along with two other peptides; neurophysin II and copeptin [Citation11]. However, AVP measurement has limitations due to its instability and short half-life. Alternatively, Copeptin the C-terminal portion of the AVP precursor is stable, and established to be sensitive surrogate biomarker for AVP release [Citation12].
This study was conducted to investigate possible relationship between circulating copeptin concentration and PSS among HCP working in ICU during COVID-19 pandemic.
2. Subjects and methods
This prospective study was conducted at University Quarantine Hospitals during the period from April to November 2020. The study was approved by the Ethics Committee of the Faculty of Medicine University (Protocol ID 0304842). An informed consent was obtained from all participants included in the study after full explanation of the study purpose, and registered at clinicaltrials.gov (identifier NCT04757285).
A total of 70 HCP comprised study group; 35 physicians (27 males and 8 females) and 35 nurses (12 males and 23 females). All participants were in good physical health, Exclusion criteria included hypertension, diabetes mellitus, individuals under treatment with glucocorticoids, psychotropic drugs, with HPA axis alterations or a previous diagnosis of mental health disorders. Also, obesity BMI ≥30, subjects with serum sodium ≤135 or ≥ 145 mmol/L at baseline or females receiving contraceptive pills.
Taking into consideration that all medical staff was replaced every two weeks’ quarantine in ICU. PCR (polymerase chain reaction) testing of a nasopharyngeal swab was performed for each HCP before admission into quarantine and at the end of quarantine period in ICU.
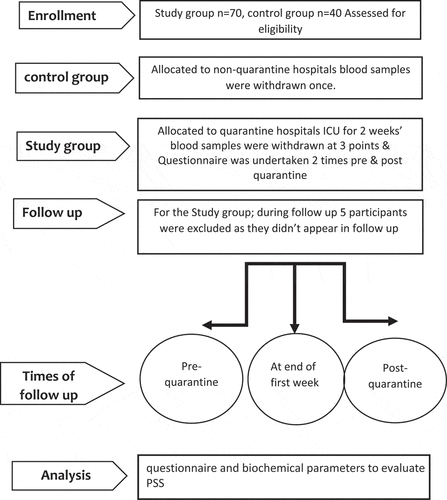
All blood samples were withdrawn after 8 hours fasting at 9:00 am; the baseline testing was the day pre-quarantine; start of duty shifts at ICU, second sample was at end of first week of isolation and third sample was two weeks after leaving quarantine (post- quarantine period). Since, exposed HCP themselves carried out active isolation at home, as recommended by the infection control unit in University hospitals. The questionnaire was conducted to all participants to assess stress (PSS) before taking blood samples at the pre- and post-quarantine periods . Study flow chart is depicted in ().
In addition, a control group was taken of 40 HCP not working in quarantine hospitals (20 physicians and 20 nurses) of matched age and BMI. Qualitative test for the COVID-19 IgG antibody from Abbott® (Abbott ARCHITECT SARS-CoV-2 IgG test, Illinois, USA) were performed for all controls. Nevertheless, questionnaire wasn’t administered to control group for two reasons; first they weren’t exposed to stress compared to study group. Secondly, the control group was enrolled to establish normal value for studied stress biomarkers, as we hypothesized that both copeptin and cortisol levels would increase upon psychological stress.
Blood was drawn into EDTA tubes (one; 1 ml for complete blood count and second;1 ml for plasma copeptin) and plain tubes (first; 2 ml for cortisol and insulin, second; 2 ml for other chemistry parameters). Afterwards serum samples were allowed to clot at room temperature for 30 minutes, then centrifuged at 4,000 × g. Then Sodium, urea, creatinine, fasting glucose were quantified immediately; by automated Hitachi autoanalyzer 704 (Hoffman-La Roche Ltd., Basel, Switzerland). For other biochemical parameters plasma and serum samples were stored at −80°C until analysis.
Cortisol was determined by a chemiluminescence immunoassay (Siemens, Erlangen, Germany) with a reference range for morning cortisol levels of 171–536 nmol/l. Copeptin levels were measured with a new sandwich immunoassay by using Human copeptin ELISA kit in certain steps recommended by the manufacturer Glory Science Co. Ltd (Del Rio, Texas, USA). Fasting insulin level was measured by enzyme linked immunosorbent assay (Monobind, Inc. LakeForest, CA, USA).
3. Questionnaire
One trained young physician administered the questionnaire to all participants. A pilot study was conducted, in which the questionnaire was pretested on a sample of six medical personnel (three physicians and three experienced nurses) to assess data-gathering mechanism, and to appraise practicality, as well as reliability of the questionnaire
PSS was analyzed as an independent variable in the current study. The PSS is a 20-question form. The participants rated their emotional and cognitive responses to specific incidents in questionnaire on a 5-point Likert scale, ranging from 0 to 5 (0 = never, 1 = almost never, 2 = sometimes, 3 = fairly often, and 4 = very often). The maximum score of the scale is 80, higher scores are indicative of greater symptom dominance [Citation6] through a computer-assisted methodology to determine stress; The Stress and Adversity Inventory [STRAIN] [Citation13]. STRAIN has displayed excellent test reliability and predictive validity in relation to a variety of health-related outcomes, including memory and decision making, working memory capacity, insomnia and sleep problems, hypertension and self-reported physical and mental health complaints
4. Sample size calculation
A minimum required sample size of 60 hHCP in study group (total 110 HCP). Sample size of study required can be calculated according to the following formula.
Where n = required sample size; t = confidence level at 95% (standard value of 1.96); p = estimated probability measurements; m = margin of error at 5% (standard value of 0.05) [Citation14].
5. Data analysis
All data were analyzed with Statistical Package for the Social Sciences version 20 software (SPSS, Inc., Chicago, IL). Results were displayed as mean ± SD. Paired Student’s t-test was used to compare the data pre-operative and nine months postoperative. The chi-squared test was used for category variables. Spearman correlation coefficient was used to detect the correlation between different variables. Statistical correlations were calculated by Pearson’s correlation test. P < 0.05 was considered significant.
6. Results
Out of the 70 originally committed participants, none of them proved positive by the COVID-19 PCR test performed after exit ICU quarantine. However, five participants didn’t come back for third samples (two male physicians and three female nurses). Years of experience in ICU was more than 3 years among all study participants. 12.3% of participants were smokers. 65 participants completed the 4 weeks follow up and constituted the material of this study. Age ranged from 26 to 38 years with a median of 31 years. Moreover, male to female (M/F) ratio among participants was (37/28) where 40 were married. On the other hand, M/F ratio among control group was (26/14) and only 27 were married ().
Table 1. Baseline clinical characteristics and metabolic variables of studied groups
Table 2. Studied parameters for quarantine HCP study group at the three measurements
The baseline mean PSS score assessment was 66.9 ± 18.3, which was calculated by questions dispersed in three dimensions incorporating; Anxiety symptoms presented main object among 53% of participants described in terms of having a lot of worried thoughts, feeling afraid that something awful might happen, awareness of heart beats in absence of physical exertion, as well as other autonomic signs as muscle pains, dry mouth. Some participants conveyed feeling of physical exhaustion, they stated excess intake of caffeinated beverages during working hours’ rest to withstand fatigue. Stress symptoms among 52.31% of participants mentioned tendency to over react to situations, difficulty to calm down after being agitated, sometimes often feeling touchy. They reasoned some belief of uncertainty with respect of compelling disease control. Those complained of insomnia were 44.29% described as poor sleep quality existed, they deduced feeling apprehensive about acquiring infection or would become carrier and take the source of infection home to their family. However, only 10.77% of them reported using hypnotics. Conversely 29.23% reported depression symptoms described by feeling down, lack of interest to work up initiatives to do things, sometimes feeling hopeless. On the other hand, post-quarantine period with a mean PSS score assessment was 23.0 ± 7.95 ()
Table 3. Comparison between stress score pre- and post-quarantine period of HCP
Illustrating correlation between plasma copeptin and other variables. It was found that there was a positive correlation between plasma copeptin and PSS score, systolic blood pressure and serum insulin ().
Table 4. Correlation of copeptin with PSS, blood pressure, cortisol, and insulin
7. Discussion
All through COVID-19 pandemic, the general public all over the world were counting on their healthcare workers to face medical challenges imparted by illness. Consequently, frontline healthcare workers were obliged to work under intense pressure [Citation15]. They worked hard to optimize the treatment of COVID-19 patients and took difficult clinical and ethical decisions concerned with patients’ mortality [Citation16,Citation17]. Hence, the role they accomplished was crucial and vital, rendering them more vulnerable to stress and anxiety [Citation17].
In the current study, the ICU- HCP baseline mean PSS score was 66.9 ± 18.3; highest pre-quarantine in ICU. Our results proposed that 53% of HCP suffered anxiety manifestations. While Studies from various regions of the world implied prevalence of anxiety among HCP to range from 11.3% to 50% [Citation15,Citation18,Citation19]. This noticeably high incidence could be explained by inclusion of HCP working in ICU, which impose greater responsibilities and require intense patients care. In addition, 44.29% of participants reported insomnia. Similarly, Lai et al. conveyed 34% insomnia among HCP [Citation17]. Insomnia described by variations in sleep criterion such as sleep latency, interrupted sleep pattern and sleep deprivation, and daytime dysfunctions ascribed to loads of worried thoughts about work in crude settings established to handle overflow of critically ill patients, need to cover additional periods due to gush of patients [Citation20]. Afterwards, PSS score became significantly low post-quarantine; which was evaluated two weeks after leave-taking, with a mean 23.0 ± 7.95 explained by relief of strain.
Theoretically psychological stress contributes to upset the body’s metabolic equilibrium. Conceivably, the body responds to acute stress by immediate AVP release, together with copeptin. AVP being an essential part of the endocrine stress response, stimulating ACTH and cortisol expression [Citation21]. In the present work, pre-quarantine; baseline copeptin concentration was significantly high 15.76 ± 8.6 pmol/l, then gradually declined in the subsequent evaluations respectively (8.45 ± 3.54 pmol/l and 3.98 ± 1.28 pmol/l). In contrast, serum cortisol level didn’t reveal any significant difference in the three measurements. This is in agreement with Nickel et al., who reported elevated copeptin level in patients admitted to emergency department presented with various acute illness [Citation22]. Similarly, Katan et al. compared copeptin in different stress settings; healthy controls, hospitalized patients and hospitalized patients exposed to major surgery. They also reported highest level in third setting [Citation11]. Furthermore, copeptin is supposed to be more sensitive than cortisol in evaluating acuity of psychological stress owing to absence of cross-reactivity with other steroid hormones [Citation23]. Moreover, in contrast to cortisol, no consistent circadian rhythm was detected for serum copeptin [Citation23]. Also, coherently serum cortisol represents peripheral endocrine response of the adrenals to stress, nevertheless, AVP plays a crucial role in stress perception at the central hypothalamic level [Citation24].
Interestingly serum insulin level was significantly elevated pre-quarantine 85.75 ± 21.82 mIU/ml, as compared to post- quarantine 77.34 ± 23.98 mIU/ml. This was explained by psycho-neuroendocrine system influence of neural intermediates resulting in increased insulin secretion together with observed rise in systolic blood pressure observed in our results [Citation25]. Similar results were obtained by Alvarez et al. who perceived lack of variation of blood glucose levels in spite of enhanced insulin sensitivity in conjunction with stress conditions [Citation25]. Another elucidation during acute stress conditions involves cortisol influences on glucose metabolism, it reduces glucose uptake in peripheral tissues as fat and muscle, through impaired translocation to plasma membrane of glucose transporter-4 [Citation26]. Also, cortisol augments gluconeogenesis in hepatic tissue, resulting in elevated blood glucose [Citation27]. Furthermore, Murakami et al. reported that increased copeptin may potentiate insulin release from β-cells of the pancreatic islets. However, if persistent may result in insulin resistance and exhaustion of the β-cells and consequently development of diabetes mellitus [Citation28]. This could explain positive correlation between copeptin and insulin obtained in our results.
In addition, plasma copeptin was positively associated with systolic blood pressure. Comparable results were obtained by Mucci et al. [Citation29]. Considering that both AVP and copeptin are released into circulation in equimolar amounts. Afsar, implied that copeptin influence blood pressure through several mechanisms [Citation30]. Primary mechanism is through local tissue Renin Angiotensin Aldosterone System activation which provokes the production of AVP. Another mechanism comprise vasoconstriction due to both direct stimuli on smooth muscle cells and indirectly by exciting renin secretion. Additionally, copeptin impels increased tubular sodium retention [Citation31]. Moreover, stress induced blood pressure reactivity may merely be due to stress hormones release [Citation30].
8. Conclusion
The finding of present study indicated that HCP working in the ICUs experienced more psychological stress than others. Therefore, psychological amendments are urgently needed to reduce stress and enhance conceptual well-being among HCP exposed to COVID-19. Additionally, copeptin might represent a sensitive substitute to cortisol or even adrenocorticotrophic hormone to evaluate perception of work related psychological stress.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Cawcutt KA, Starlin R, Rupp ME. Fighting fear in healthcare workers during the COVID-19 pandemic. Infect Control Hosp Epidemiol. 2020 Oct;41(10):1192–1193; Epub 2020 Jun 25. PMID: 32580790; PMCID: PMC7338429. DOI:10.1017/ice.2020.315.
- Nakao M. Work-related stress and psychosomatic medicine. Biopsychosoc Med. 2010 May 26;4(1):4. DOI:10.1186/1751-0759-4-4.. PMID: 20504368; PMCID: PMC2882896.
- Kumar A, Pore P, Gupta S, et al. Level of stress and its determinants among intensive care unit staff. Indian J Occup Environ Med. 2016 Sep-Dec;20(3):129–132. DOI:10.4103/0019-5278.203137.. PMID: 28446837; PMCID: PMC5384390
- Cronqvist A, Lützén K, Nyström M. Nurses lived experiences of moral stress support in the intensive care context. J Nurs Manag.. 2006;14(5):405–413. DOI:10.1111/j.1365-2934.2006.00631.x
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):367–381. DOI:10.31887/DCNS.2006.8.4/bmcewen.. PMID: 17290796; PMCID: PMC3181832
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–396. PMID: 6668417
- Sestili C, Scalingi S, Cianfanelli S, et al. Reliability and use of copenhagen burnout inventory in italian sample of university professors. Int J Environ Res Public Health. 2018 Aug 9;15(8):1708. DOI:10.3390/ijerph15081708.. PMID: 30096954; PMCID: PMC6121611.
- Bégat I, Ellefsen B, Severinsson E. Nurses’ satisfaction with their work environment and the outcomes of clinical nursing supervision on nurses’ experiences of well-being – a Norwegian study. J Nurs Manag. 2005 May;13(3):221–230. DOI:10.1111/j.1365-2834.2004.00527.x.. PMID: 15819834
- Belda X, Nadal R, Armario A. Critical features of acute stress-induced cross-sensitization identified through the hypothalamic-pituitary-adrenal axis output. Sci Rep. 2016;6(1):31244.
- Oitzl MS, Champagne DL, Van Der Veen R, et al. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010 May;34(6):853–866. DOI:10.1016/j.neubiorev.2009.07.006.. PMID 1963168
- Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010 Sep;24:140:w13101. PMID: 20872295.
- Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016 Mar;12(3):168–176; Epub 2016 Jan 22. PMID: 26794439. DOI:10.1038/nrendo.2015.224.
- Slavich GM, Shields GS. Assessing lifetime stress exposure using the stress and adversity inventory for adults (adult strain): an overview and initial validation. Psychosom Med. 2018 Jan;80(1):17–27. DOI:10.1097/PSY.0000000000000534.. PMID: 29016550; PMCID: PMC5757659
- Akinlade KS, Atere AD, Rahamon SK, et al. Serum levels of copeptin, C-reactive protein and cortisol in different severity groups of sickle cell anaemia. Niger J Physiol Sci. 2013 [28( December2013)]; 2: 159–164[www.njps.com.ng] 2
- Gupta AK, Mehra A, Niraula A, et al. Prevalence of anxiety and depression among the healthcare workers in Nepal during the COVID-19 pandemic. Asian J Psychiatr. 2020 Dec;54:102260. PMID: 32599546; PMCID: PMC7313505. Epub 2020 Jun 24. DOI:10.1016/j.ajp.2020.102260.
- Wilson N, Baker M, Crampton P, et al. The potential impact of the next influenza pandemic on a national primary care medical workforce. Hum Resour Health. 2005 Aug 11;3(1):7. DOI:10.1186/1478-4491-3-7.. PMID: 16092972; PMCID: PMC1215505.
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Network Open. 2020 Mar 2;3(3):e203976. DOI:10.1001/jamanetworkopen.2020.3976.. PMID: 32202646; PMCID: PMC7090843.
- Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020 Aug;88:901–907. PMID: 32437915; PMCID: PMC7206431. Epub 2020 May 8. Erratum in: Brain Behav Immun. 2021 Feb; 92:245. DOI:10.1016/j.bbi.2020.05.026.
- Zhu J, Sun L, Zhang L, et al. Prevalence and influencing factors of anxiety and depression symptoms in the first-line medical staff fighting against COVID-19 in Gansu. Front Psychiatry. 2020 Apr 29;11:386. DOI:10.3389/fpsyt.2020.00386.. PMID: 32411034; PMCID: PMC7202136.
- Belingheri M, Pellegrini A, Facchetti R, et al. Self-reported prevalence of sleep disorders among medical and nursing students. Occup Med (Lond). 2020 Apr 20;70(2):127–130. DOI:10.1093/occmed/kqaa011.. PMID: 31974578.
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007 Jul;87(3):873–904. DOI:10.1152/physrev.00041.2006.. PMID: 17615391
- Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012 Jan 20;10(1):7. DOI:10.1186/1741-7015-10-7.. PMID: 22264220; PMCID: PMC3275505.
- Beglinger S, Drewe J, Christ-Crain M. The circadian rhythm of copeptin, the c-terminal portion of arginine vasopressin. J Biomark. 2017;2017:4737082. Epub 2017 Jun 1. PMID: 28656120; PMCID: PMC5471567. DOI:10.1155/2017/4737082.
- Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006 Jan;52(1):112–119; Epub 2005 Nov 3. PMID: 16269513. DOI:10.1373/clinchem.2005.060038..
- Alvarez MA, Portilla L, Gonzalez R, et al. Insulin response to a short stress period. Psychoneuroendocrinology. 1989;14(3):241–244. DOI:10.1016/0306-4530(89)90022-x.. PMID: 2667017
- Ware WR. Psychological stress, insulin resistance, inflammation and the assessment of heart disease risk. Time for a paradigm shift? Med Hypotheses. 2008;71(1):45–52. DOI:10.1016/j.mehy.2008.02.008. PMID: 18406066
- Lundgren M, Burén J, Ruge T, et al. Glucocorticoids down-regulate glucose uptake capacity and insulin-signaling proteins in omental but not subcutaneous human adipocytes. J Clin Endocrinol Metab. 2004 Jun;89(6):2989–2997. DOI:10.1210/jc.2003-031157.. PMID: 15181089.
- Murakami T, Horibata Y, Tateno S, et al. Relationship between non-osmotic arginine vasopressin secretion and hemoglobin A1c levels in adult patients with congenital heart disease. Heart and Vessels. 2019 May;34(5):809–814; Epub 2018 Nov 20. PMID: 30460574. DOI:10.1007/s00380-018-1309-z.
- Mucci N, Giorgi G, De Pasquale Ceratti S, et al. Anxiety, stress-related factors, and blood pressure in young adults. Front Psychol. 2016 Oct 28;7:1682. DOI:10.3389/fpsyg.2016.01682.. PMID: 27840615; PMCID: PMC5083786.
- Afsar B. Pathophysiology of copeptin in kidney disease and hypertension. Clin Hypertens. 2017 Jun 13;23(1):13. DOI:10.1186/s40885-017-0068-y.. PMID: 28638629; PMCID: PMC5469179.
- Perucca J, Bichet DG, Bardoux P, et al. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008 Sep;19(9):1721–1731; Epub 2008 Jul 2. PMID: 18596120; PMCID: PMC2518442. DOI:10.1681/ASN.2008010021.