ABSTRACT
Background: Eliminating propofol injection pain (PIP) should be multimodal, including pharmacological and non-pharmacological interventions. Understanding risk factors – body mass index, menstrual cycle phase and age for example – leading to increased pain sensitivity will identify patients at most risk for PIP, permitting effective treatment strategies to be initiated at an early stage.
Objective: Our goal is to determine occurrence and severity of propofol injection pain among female patients, whether they are obese or non-obese, during different menstrual cycle phases.
Methods: Design: A prospective observational study.
Setting: Single university teaching hospital’s operating rooms.
Patients: 84 female undergoing surgeries under general anesthesia, classified as American Society of Anesthesiologists (ASA) physical status I–II, between 18 and 65 years of age were enrolled.
Interventions: We evaluated propofol injection pain using visual analogue scale (VAS) during induction of general anesthesia.
Main outcome measure: The relation between propofol induced peripheral venous pain and female body mass index, menstrual cycle phase and age.
Results: 71.4% of patients experienced PIP. Of those patients who experienced PIP; 40.5% (34/84 patients) had moderate to severe pain. Age <35 years was the only independent predictor of moderate/severe PIP (sensitivity 62%, specificity 78%). After adjustment for age category, there was no statistically significant association between occurrence of moderate/severe PIP and obesity or menstruation.
Conclusion: Propofol injection pain prophylactic measures should be considered in young females <35 years as they may be more prone to moderate/severe PIP.
Trial Registration: NCT04078087
1. Introduction
All phenols irritate skin and mucous membrane. Thus, propofol being an alkylphenol is expected to cause pain [Citation1]. Propofol injection pain (PIP) and its severity may differ among patient populations with an incidence of 28%–90% in adult patients, in the absence of other pretreatments [Citation1]. PIP usually is percepted as tingling, cold, or numbing or, at its worst, a severe burning pain. Despite this discomfort, the incidence of venous sequel, such as phlebitis, is less than 1% [Citation2] PIP may be immediate or delayed after 10–20 seconds. The immediate pain is due to irritation of vein endothelium by free propofol present in the aqueous phase [Citation3], whereas delayed pain is due plasma kallikrein-kinin system activation after propofol contacting with free nerve endings of vessels, thus locally liberating pain mediators [Citation4].
Obviously, the approach to eliminate PIP should be multimodal, including pharmacological and non-pharmacological interventions [Citation5]. In the last 10 years, the incidence of PIP was 0% in only three clinical trials [Citation4,Citation6,Citation7]. This highlights the importance to detect risk factors leading different pain responses among patients.
It has been suggested that obese subjects may have a different pain perception [Citation8] Additionally, several studies have suggested that the hormonal fluctuations characteristic of the menstrual cycle can have an important influence on pain perception [Citation9]. So here in this study, our goal is to determine occurrence and severity of propofol injection pain levels among female patients, whether they are obese or non-obese during different menstrual cycles phases, aiming to detect the group of patients at most risk.
2. Materials and methods
2.1. Ethics
Ethical approval for this study (FMASU R 43/2019) was provided by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University, Abbasia, Cairo, Egypt (Chairperson Prof F. Tash) on 20/8/2019. The study was prospectively registered at Clinical trial Registry ClinicalTrials.gov Identifier:NCT04078087 in accordance with WHO and ICMJE standards. Written informed consent was obtained from all subjects. This trial followed the STROBE statement.
2.2. Inclusion criteria
We enrolled ninety patients who were: 18–65 years old, American Society of Anesthesiologists (ASA) physical status I–II, scheduled to undergo elective surgery, either menopause or with regular menstrual cycle, in this prospective observational study.
A regular cycle is defined as lasting 23–35 days with no variations in the length of cycle of more than 2 days) [Citation10]. Only patients in the following time intervals were recruited:
8th – 12th day after the first day of the last menstruation (considered to be in the follicular phase)
20th – 24th day after the first day of the last menstruation (considered to be in the luteal phase)
Menopausal females who were not on hormonal replacement therapy
Gynecologists divide the menstrual cycle into phases based on physiological events [Citation11]. The patients who were on the 1st to 12th days after the first day of their menstruation bleeding were considered to be in the follicular phase of the cycle. Those on the 20th to 24th days after the first day of the last menstruation were considered to be in the luteal phase of the cycle [Citation11]. Luteinizing hormone peaks on the 13th day, and progesterone starts to increase at the 18th day of the cycle [Citation12]. For this reason, we excluded patients who were on 13th to 19th days of their menstrual cycle, for clear discrimination between the luteal and follicular phases, especially we didn’t measure hormonal levels
2.3. Exclusion criteria
Gynecological causes for exclusion: irregular menstrual cycle, hormonal therapy for any cause, oral contraceptive pills, breast feeding, pregnant, patients with surgical history of total abdominal hysterectomy and/or bilateral salpingo-opherectomy.
General causes for exclusion: Patients on antidepressants and analgesics were excluded because antidepressants and analgesics may have an effect on pain threshold [Citation13]
2.4. Patients’ recruitment
Patients were recruited after admission to the hospital, same day they were scheduled for surgery. All patients were instructed on a vertical visual analogue scale (VAS) [Citation14].
Patients were divided into 2 groups according to body mass index (BMI) levels:
1- Non-obese (whether normal weight or overweight) = Group “NO”
2- Obese = Group “O”.
Normal weight was defined as BMI 18.5–24.9, overweight as BMI 25.0–29.9 and obesity as BMI ≥ 30 [Citation15].
2.5. Anesthesia and surgical procedure
On arrival to the operating room and after standard anesthesia monitoring, a 20 gauge intravenous cannula was inserted. After adequate preoxygenation, 25% of (2 mg/kg) total propofol dose was injected over 3–5 seconds (Propofol Lipuro 1%, B. Braun Melsungen AG, Germany). The patients were observed and asked immediately whether they had pain in the arm, its VAS score, type of pain (burning, parethesia or other) and its onset was documented (to differentiate early or delayed type of pain). An anesthesiologist, who was unaware of the study groups, assessed the intensity of pain after propofol injections and other related collected data.
Afterwards, all patients were administered the remaining propofol dose over 10 seconds. After loss of verbal contact, mask ventilation was initiated. Atracurium besylate 0.5 mg/kg was given to facilitate tracheal intubation. Three minutes later, tracheal intubation was attempted.
2.6. Patient reported outcomes
The primary outcome was the relation between propofol induced peripheral venous pain and female body mass index, menstrual cycle phase and age. No pain was defined as VAS score 0, Mild pain was defined as VAS score 1–3, Moderate as VAS score 4–6, Severe as VAS score 7–10. Pain in excess of 3 on the VAS scale was regarded as moderate to severe pain and its % was calculated.
Secondary aims:
Type of Propofol injection pain, early or delayed (after 10 seconds),
Within 24 hours after operation, a researcher blinded to this study checked the injection site for pain, edema, wheal, flare response or any other adverse effect.
2.7. Sample size
Sample size was calculated using STATA program, setting the type-1 error (α) at 0.05 and the power (1-β) at 0.9. Result from previous study [Citation16] showed that moderate/severe pain was found in 55% of non-obese patients after receiving 1% propofol, while moderate/severe pain was found among 25% of obese patients based on a result of a pilot study. Calculation according to these values produced a sample size of 45 cases per group after taking in account 10% drop out rate. 10% drop out rate, allowed “81 patients” to be the minimum allowed number of patients.
2.8. Statistical methods
Data were analyzed using IBM© SPSS© Statistics version 26 (IBM© Corp., Armonk, NY). Categorical variables are presented as numbers or proportions and percentages and intergroup differences were compared using the Pearson chi-squared test or Fisher’s exact test. Ordinal data were compared using the chi-squared test for trend. Continuous numerical data are presented as mean and standard error and between-group differences were compared using the independent-samples t-test.
We conducted two-way analysis of variance (ANOVA) to examine the influence of obesity and menstrual cycle phase on the VAS score for propofol-injection pain (PIP). The Levene test was used to examine the assumption of homoscedasticity (homogeneity of variance) across levels of the independent variables.
Receiver-operating characteristics (ROC) curve analysis was used to identify a cut-off age for the occurrence of moderate/severe propofol-injection pain (PIP). Multivariable binary logistic regression analysis was used to examine the independent effect of obesity or menstrual cycle phase on the occurrence of moderate/severe PIP as adjusted for the age category.
Two-sided P-values <0.05 were considered statistically significant. P-values for all pair-wise comparisons were corrected using the Bonferroni methods.
3. Results
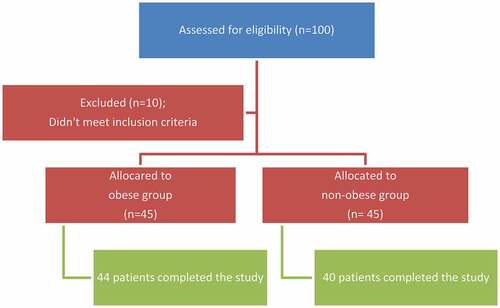
A total of 100 patients were assessed for eligibility. 10 patients didn’t meet the inclusion criteria and 6 patients were excluded from the study analysis due to either of: difficult cannulation requiring a smaller size intravenous (IV) catheter or inability to score PIP inspite of preoperative demonstration and instructions (). Perioperative characteristics of patients are shown in .
Table 1. Characteristics of the study population
3.1. Propofol injection pain incidence and severity
In our study, 60 patients out of 84 (71.4%) experienced PIP, with mean VAS of 3.05 ± 0.29 (). Number of patients with VAS score >3 were 34 patients out of 84 (40.5%). PIP was almost burning in character (in 80% of patients) and had an early onset (in 73.3% of patients)
Table 2. Percentage and characteristics of propofol-injection pain (PIP)
3.2. Effect of obesity and menstrual cycle phase on PIP
We conducted two-way analysis of variance (ANOVA) to examine the effect of obesity and menstrual cycle phase on the VAS score for PIP. The Levene test was not statistically significant, F (5, 78) = 1.836, P-value = 0.116, denoting homoscedasticity (homogeneity of variance) across levels of the independent variables. Test of between-subjects effects showed no statistically significant interaction between obesity and menstrual cycle phase, F (2, 78) = 0.092, P-value = 0.912. So, we examined the main effects rather than the simple effects of the independent variables. There was no statistically significant effect of obesity, F (1, 78) = 0.494, P-value = 0.484. However, the menstrual cycle phase did show a statistically significant effect on the VAS score, F (2, 78) = 4.345, P-value = 0.016 (). Pairwise comparisons showed a statistically significant difference between females in the follicular phase compared to menopausal females (mean difference = 1.87, SE = 0.69, 95% CI = 0.18 to 3.56, P-value = 0.025). Otherwise, Differences between females in the follicular phase and those in the luteal phase or between menopausal females and those in the luteal phase were not statistically significant (P-value = 1.000 and P-value = 0.066, respectively).
Table 3. Interaction between obesity and menstrual cycle phase
3.3. Risk factors of moderate/severe PIP
Bivariate analysis for “risk factors of moderate/severe PIP” are shown in . Based on the results of bivariate analysis (), we conducted receiver-operating characteristic (ROC) curve analysis to identify age cut-off for occurrence of moderate/severe PIP. Age had a fair predictive value with an area under the ROC curve (AUC) of 0.7 (SE = 0.057, 95% CI = 0.587 to 0.812, P-value <0.001). The best cut-off criterion was age <35 years (sensitivity 62%, specificity 78%).
Table 4. Bivariate analysis for risk factors of moderate/severe PIP
So, we re-classified patients according to this cut-off criterion and conducted multivariable binary logistic regression analysis to adjust for age category. The results of multivariable analysis are shown in . After adjustment for age category, there was no statistically significant association between occurrence of moderate/severe PIP and obesity (odds ratio = 0.725, SE = 0.501, 95% CI = 0.272 to 1.937, P-value = 0.522) or menstrual cycle phase (luteal phase odds ratio = 1.582, SE = 0.691, 95% CI = 0.409 to 6.125, P-value = 0.507; follicular-phase odds ratio = 0.740, SE = 0.774, 95% CI = 0.162 to 3.375, P-value = 0.697). Age <35 years was the only independent predictor of moderate/severe PIP (odds ratio = 5.947, SE = 0.654, 95% CI = 1.649 to 21.441, P-value = 0.006).
Table 5. Multivariable binary logistic regression analysis for determinants of PIP
4. Discussion
We studied “propofol injection pain in relation to the phases of the menstrual cycle and menopause in obese and non-obese female patients” at induction of general anesthesia (GA) time point. We found that there was no statistically significant association between occurrence of moderate/severe PIP and obesity or menstrual cycle phase. And that age < 35 years could be an independent fair predictor of moderate/severe PIP, with sensitivity 62%, specificity 78%. These findings thus indicate the need to provide more preventative pain management in younger females with age < 35 years.
4.1. BMI in relation to PIP
Recently, the number of patients with obesity who present for surgery is increasing. It has been suggested that obese subjects may have a different pain perception with conflicting results [Citation8]. These conflicting results are likely due to: 1- Differences in methodologies with associated confounding variables such as gender, age, socio-economic factors [Citation17]. 2- The use of BMI solely to assess obesity, which cannot differentiate between fat mass and lean muscle mass. 3- Associated co-morbidities that may influence pain sensitivity such as diabetes, anxiety and depression [Citation8].
In our study, there was no statistically significant association between occurrence of moderate/severe PIP and obesity. Supporting our results, in a retrospective cohort study done by Grodofsky and Sinha [Citation18], they demonstrated absence of significant differences in immediate postoperative pain scores or analgesic requirements between obese and non-obese patients. Also, in a unique prospective cross-sectional study done by Motaghedi & their colleagues [Citation19], they concluded that although obesity was associated with a proinflammatory state after total hip arthroplasty; through enhanced cytokine reactivity, it was not associated with increased postoperative pain. Also, same finding was found in a retrospective study done by Narain and their colleagues [Citation20], who studied the impact of BMI on narcotic consumption after cervical discectomy in 277 patients. They revealed no differences in immediate postoperative VAS scores or in patients’ narcotic consumption among different BMI groups. Another study done by Armaghani and their colleagues [Citation21], determined that BMI was not among risk factors leading to increased opioid demand in the postanesthesia care unit (PACU) in spinal surgery. Finally, a unique observational study done by Thomazeau & their colleagues [Citation22] recorded demographic, clinical, psychological and genetic variables in relation to postoperative acute pain intensity and opioid requirement after knee replacement. They found that neither mean post-operative pain intensity nor, mean post-operative opioid requirements, were correlated with body weight or BMI.
In discordance with our results, three studies observed more experienced pain in obese when compared to non-obese patients perioperatively. Majchrzak and their colleagues [Citation23] for example, observed that VAS scores were more severe in obese than in non-obese patients after thoracic surgery whether thoracotomy or video-assisted. Also, in a retrospective study done by Campbell and their colleagues [Citation24], VAS pain scores were observed to be consistently higher at almost all time points in obese patients, together with higher narcotic requirement. Noteworthy, they investigated the effects of body mass index on pain control after joint replacement. Finally, in a retrospective study done by Liu & their colleagues [Citation25], they identified increased BMI (among other factors), as a preoperative risk factor for moderate to severe pain after total hip and knee replacements.
4.2. Menstrual cycle phase in relation to PIP
We usually encounter female patients perioperatively in different phases of the menstrual cycle. Several studies have suggested that the hormonal fluctuations characteristic of the menstrual cycle can have an important influence on the central nervous system and pain perception [Citation9], especially after clinical manifestations worsening in several painful chronic conditions during certain menstrual cycle phases [Citation26]. Also, progesterone and its metabolites show functions related to sedation, anxiolysis and analgesia through their direct action on the γ-amino butyric acid (GABA) type A receptor, while estrogen is thought to have the opposite effect by suppressing GABA-A receptor [Citation27].
Most studies have used experimental pain when evaluating the effect of menstrual cycle on pain perception, but there are limited data for clinical pain. Even within the context of studies that have considered clinical pain, the results were ambiguous. Conflicting findings are mostly due to: 1- methodological variations across studies, such as definition of menstrual cycle phases. 2- no hormonal level 3- ignoring the premenstrual syndrome (PMS) that has a prevalence of 70%, in female patients and itself might has an effect on pain perception [Citation28]
In our study, we found that there was no statistically significant association between occurrence of moderate/severe PIP and menstrual cycle phase. Supporting our results, Islamoglu and their colleagues [Citation29] investigated the effect of the menstrual cycle and menopause on extracorporeal shock wave lithotripsy (ESWL)-related pain outcome and concluded that menstrual cycle phase has no effect on pain perception during the ESWL session. Also, in correspondence to our results, three studies [Citation27,Citation30,Citation31] did not observe any differences in postoperative pain perception and analgesic requirements in females undergoing elective surgery under general anesthesia irrespective of their menstrual cycles.
On the contrary, two studies have reported that pain sensitivity was increased in the follicular phase of menstruation: Arab and their colleagues [Citation28] for example, observed in 140 patients who underwent gynecological surgery that patients who were in follicular phase, whether with a history of PMS or not, suffered from high postoperative pain sensation and analgesia request. Also, reza Moradhakhani and their colleagues [Citation32] noticed higher incidence of postdural puncture headache in patients undergoing spinal anesthesia during their follicular phase
Besides, other studies have reported that pain sensitivity is increased in the luteal phase of menstruation. Three studies [Citation12,Citation33,Citation34], have studied the effect of the menstrual cycle on anesthetic drug injection pain. Propofol injection pain was examined by 10-point numeric rating scale and rocuronium injection pain was examined by withdrawal movement under GA. All of the three studies, showed higher pain perception in luteal-phase groups. Furthermore, 2 studies [Citation9,Citation35] have shown that females exhibited higher postoperative pain scores by undergoing surgery in the luteal phase compared to those operated in the follicular phase
4.3. Age in relation to PIP
In our study, we observed that age <35 years was the only independent predictor of moderate/severe PIP. Supporting our results; Campbell and their colleagues [Citation24], observed that younger age was associated with increased narcotic requirement postoperatively after total joint arthroplasty in a retrospective analysis of 2629 patients. Also, perioperative risk factors for the development of severe postoperative pain – after different type of surgeries – were investigated in several studies [Citation22,Citation25,Citation36–38]. All of these studies, concluded that younger age (among other predictors), was associated with higher incidence of postoperative pain.
4.4. Limitation
First of all, in our study, the menstruation phases were defined based on the calendar method. Although, numerous studies published in the medical literature were based also on the calendar method, but we confirm that, hormone level measurement is very important and it is better to evaluate PIP based on hormonal levels. Secondly, we didn’t ask about PMS, which itself might has an effect on pain perception. Thirdly, BMI may be an inaccurate index of obesity. Additional anthropometric parameters of central adiposity, and percent body fat should be examined. Fourthly, we didn’t ask about presence of chronic pain history. Previous studies [Citation15] observed that chronic moderate/severe pain worsens the ability of pain scoring, which was related to pain-induced neuroplastic changes in the central nervous system rather than to the presence of pain itself. Finally, VAS for pain may not be the best scale used during propofol injection for induction of anesthesia. Instead, verbal rating scale (VRS) is a better scale. It divides pain into minimal moderate and severe. It has the advantage of being reliable and easier method. Additionally. VRS is a verbal response to auditory order while VAS is visual and motor coordination to auditory order.
5. Conclusion
Our results showed that younger age patients < 35 years are more sensitive to pain following IV injection of propofol, independent to their menstrual cycle phase and BMI. Therefore clinicians need to consider preventative aggressive strategies to reduce or prevent that pain for this specific age category.
Ethics approval and consent to participate
Ethical approval for this study (FMASU R 43/2019) was provided by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University, Abbasia, Cairo, Egypt (Chairperson Prof F. Tash) on 20/8/2019. The study was prospectively registered at Clinical trial Registry ClinicalTrials.gov Identifier:NCT04078087 in accordance with WHO and ICMJE standards. This trial followed the STROBE statement.
Acknowledgments
1. Assistance with the article: None
2. Financial support and sponsorship: None
3. Conflicts of interest: None
4. Presentation: None
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Macario A, Weinger M, Truong P, et al. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999 May 1;88(5):1085–1091. PMID: 10320175. .
- Gupta M, Mishra S, Gupta D, et al. Prevention of pain on propofol injection: a comparative, randomized, double blind study between lignocaine, pethidine, dexamethasone and placebo. Internet J Anesthesiol. 2006;11:1–6.
- Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998 May;53(5):468–476. PMID: 9659020.
- Aouad MT, Siddik-Sayyid SM, Al-Alami AA, et al. Multimodal analgesia to prevent propofol-induced pain: pretreatment with remifentanil and lidocaine versus remifentanil or lidocaine alone. Anesth Analg. 2007 Jun 1;104(6):1540–1544. PMID: 17513655.
- Li X, Chen CJ, Tan F, et al. Effect of dexmedetomidine for attenuation of propofol injection pain in electroconvulsive therapy: a randomized controlled study. J Anesth. 2018 Feb 1;32(1):70–76. PMID: 29127492. .
- Iwata M, Inoue S, Kawaguchi M, et al. Ketamine eliminates propofol pain but does not affect hemodynamics during induction with double-lumen tubes. J Anesth. 2010 Feb 1;24(1):31–37. PMID: 20039078.
- DeSousa K, Ali MS. Sevoflurane to alleviate pain on propofol injection. J Anesth. 2011 Dec 1;25(6):879–883. PMID: 21881932.
- Torensma B, Thomassen I, van Velzen M, et al. Pain experience and perception in the obese subject systematic review (revised version). Obes Surg. 2016 Mar 1;26(3):631–639. PMID: 26661107.
- Piroli A, Mattei A, Carta G, et al. Influence of the menstrual cycle phase on pain perception and analgesic requirements in young women undergoing gynecological laparoscopy. Pain Pract. 2019 Feb;19(2):140–148. PMID: 30269411.
- Šimurina T, Mraovic B, Skitarelić N, et al. Influence of the menstrual cycle on the incidence of nausea and vomiting after laparoscopic gynecological surgery: a pilot study. J Clin Anesth. 2012 May 1;24(3):185–192. PMID: 22459340.
- Fatima N, Babu PR, Sisinty VS, et al. Pain perception and anxiety levels during menstrual cycle associated with periodontal therapy. Int J Dent. 2014;2014:472926. PMID: 25371677. PMCID: PMC4209788.
- Honca M, Purtuloglu T, Honca T, et al. Effects of the menstrual cycle on injection pain due to rocuronium. J Clin Anesth. 2013 Aug 1;25(5):399–402. PMID: 23965205.
- Chung DT, Bogle G, Bernardini M, et al. Pain experienced by patients during periodontal maintenance. J Periodontol. 2003 Sep 1;74(9):1293–1301. PMID: 14584861.
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (vas pain), numeric rating scale for pain (nrs pain), mcgill pain questionnaire (mpq), short‐form mcgill pain questionnaire (sf‐mpq), chronic pain grade scale (cpgs), short form‐36 bodily pain scale (sf‐36 bps), and measure of intermittent and constant osteoarthritis pain (icoap). Arthritis Care Res (Hoboken). 2011 Nov;63(S11):S240–52. PMID: 22588748.
- Torensma B, Oudejans L, van Velzen M, et al. Pain sensitivity and pain scoring in patients with morbid obesity. Surg Obes Relat Dis. 2017 May 1;13(5):788–795. PMID: 28216116.
- Aggarwal S, Kumar M, Sharma V. A single-centre randomized-controlled trial to study effect of dilution on propofol-induced injection pain at injection site. Saudi J Anaesth. 2011 Jul;5(3):282–285. PMID: 21957407. PMCID: PMC3168345.
- Emerson NM, Nahman-Averbuch H, Coghill RC. Pain sensitivity does not differ between obese and healthy weight individuals. bioRxiv. 2020 Jun 05;136598. DOI: 10.1101/2020.06.05.136598.
- Grodofsky SR, Sinha AC. The association of gender and body mass index with postoperative pain scores when undergoing ankle fracture surgery. J Anaesthesiol Clin Pharmacol. 2014 Apr;30(2):248–252. PMID: 24803767. PMCID: PMC4009649.
- Motaghedi R, Bae JJ, Memtsoudis SG, et al. Association of obesity with inflammation and pain after total hip arthroplasty. Clin Orthop Relat Res. 2014 May;472(5):1442–1448. PMID: 24096457. PMCID: PMC3971228.
- Narain AS, Hijji FY, Haws BE, et al. Impact of body mass index on surgical outcomes, narcotics consumption, and hospital costs following anterior cervical discectomy and fusion. J Neurosurg Spine. 2018 Feb 1;28(2):160–166. PMID: 29192877.
- Armaghani SJ, Lee DS, Bible JE, et al. Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine (Phila Pa 1976). 2014 Dec 1;39(25):E1524–30. PMID: 25417827.
- Thomazeau J, Rouquette A, Martinez V, et al. Acute pain factors predictive of post‐operative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain. 2016 May;20(5):822–832. PMID: 26517014.
- Majchrzak M, Brzecka A, Daroszewski C, et al. Increased pain sensitivity in obese patients after lung cancer surgery. Front Pharmacol. 2019 Jun 14;10:626. PMID: 31258474. PMCID: PMC6586739.
- Campbell AL, Yu S, Karia R, et al. The effects of body mass index on pain control with liposomal bupivacaine in hip and knee arthroplasty. J Arthroplasty. 2018 Apr 1;33(4):1033–1039. PMID: 29208329.
- Liu SS, Buvanendran A, Rathmell JP, et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop. 2012 Nov;36(11):2261–2267. PMID: 22842653. PMCID: PMC3479283.
- Wang JV, Hattier G, Jhawar N, et al. Variations in pain perception during the menstrual cycle: implications for esthetic procedures. Clin Dermatol. 2019 Nov 1;37(6):689–691. PMID: 31864450.
- Fu F, Chen X, Feng Y, et al. Propofol EC50 for inducing loss of consciousness is lower in the luteal phase of the menstrual cycle. Br J Anaesth. 2013 Nov 26;112(3):506–513. PMID: 24285693.
- Arab M, Mirkheshti A, Noghabaei G, et al. The effect of premenstrual syndrome and menstrual phase on postoperative pain. Anesth Pain Med. 2015 Apr 20;5(2):e19333. PMID: 25893183. PMCID: PMC4377162.
- Islamoglu E, Tas S, Karamik K, et al. Does extracorporeal shock wave lithotripsy-related pain get affected by menstrual cycle and menopause? Urolithiasis. 2019 Dec;47(6):575–581. PMID: 30362030.
- Ahmed A, Khan F, Ali M, et al. Effect of the menstrual cycle phase on post‐operative pain perception and analgesic requirements. Acta Anaesthesiol Scand. 2012 May;56(5):629–635. PMID: 22404180.
- Sari S, Kozanhan B, Egilmez AI, et al. The influence of the menstrual cycle on acute and persistent pain after laparoscopic cholecystectomy. Rev Bras Anestesiol. 2018 Jun;68(3):231–237. PMID: 29373141.
- Reza Moradkhani M, Karimi A, Zarei Z, et al. The relationship between the phases of the menstrual cycle on the incidence and severity of headache after spinal anesthesia. Surg J (N Y). 2019 Sep 24;5(3):e126–e130. PMID: 31555745. PMCID: PMC6759416.
- Hanci V, Ayoglu H, Yilmaz M, et al. Effect of menstrual cycle on the injection pain due to propofol. Eur J Anaesthesiol. 2010 May 1;27(5):425–427. PMID: 20216071.
- Yilmaz S, Sezer SD. Effects of the menstrual cycle on rocuronium injection-related withdrawal movement. Tepecik Eğit ve Araşt Hast Dergisi. 2018;28(3):175–180. .
- Sener EB, Kocamanoglu S, Cetinkaya MB, et al. Effects of menstrual cycle on postoperative analgesic requirements, agitation, incidence of nausea and vomiting after gynecological laparoscopy. Gynecol Obstet Invest. 2005;59(1):49–53. PMID: 15467297.
- Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014 May;120(5):1237–1245. PMID: 24356102.
- Hartwig M, Allvin R, Bäckström R, et al. Factors associated with increased experience of postoperative pain after laparoscopic gastric bypass surgery. Obes Surg. 2017 Jul;27(7):1854–1858. PMID: 28144798. PMCID: PMC5489569.
- Mei W, Seeling M, Franck M, et al. Independent risk factors for postoperative pain in need of intervention early after awakening from general anaesthesia. Eur J Pain. 2010 Feb;14(2):149.e1–7. PMID: 19423369.