ABSTRACT
Background: Spinal anesthesia is the recommended technique in elective cesarean sections. Usage of vasopressors is more widely accepted as an effective method for decreasing postspinal hypotension than fluid loading. However, the ideal vasopressor to prevent spinal hypotension during cesarean section has been a subject of much debate. It should maintain maternal blood pressure and placental perfusion, with minimal adverse effect on fetus and mother.
Aim: The primary aim was to compare the effect of prophylactic infusion of phenylephrine versus norepinephrine versus ephedrine in the prevention of postspinal hypotension in elective cesarean section. The secondary aim was to assess their effects on neonate.
Methods: Seventy-five patients were enrolled in this study and randomly divided into three groups. Group P received phenylephrine infusion and group N received norepinephrine infusion, while group E received ephedrine infusion. The changes in mean arterial blood pressure and heart rate were recorded throughout the surgery. Maternal and neonatal perioperative complications were controlled and recorded.
Results: MAP were higher in the ephedrine group than the phenylephrine and norepinephrine groups. Maternal tachycardia was significantly more common in ephedrine, and bradycardia was more common in phenylephrine group without significant difference. Nausea and vomiting were more common in ephedrine group. Neonatal acidosis was lesser in phenylephrine and norepinephrine groups than in the ephedrine group.
Conclusion: Prophylactic infusion of phenylephrine and norepinephrine can be successfully used to prevent postspinal hypotension in parturient undergoing cesarean section with less drawbacks and fetal well-being than ephedrine.
1. Introduction
For a safe elective cesarean delivery, the updated obstetric anesthesia guidelines recommend the administration of neuraxial anesthesia, whenever feasible [Citation1]
Hypotension is the most common side effect associated with spinal anesthesia in obstetrics [Citation2]. The reported incidences of hypotension varied between 1.9% and 71% [Citation3]. The most significant cause of hypotension is rapid onset of sympatholysis due to increased sensitivity of nerve fibres to local anesthetics during pregnancy [Citation4]. Aortocaval compression of the pregnant uterus aggravates incidence and severity of hypotension in pregnant women, compared to nonobstetric patients [Citation5]. Pregnant women also exhibit an increased level of sympathetic activity compared to parasympathetic activity [Citation4]. Higher sympathetic block reduces the occurrence of compensatory mechanisms and increases the risk of cardio-inhibitory reflexes [Citation5]. Hypotension leads to adverse maternal outcomes such as nausea, vomiting, and dizziness [Citation6]. Decrease in systolic pressure can compromise uterine blood flow and foetal circulation, and thus cause foetal hypoxia and acidosis. Furthermore, hypotension leads to a significant fetal ischemia and reperfusion injury [Citation7]. Prolonged hypotension may also affect the neurobehavioral outcome of the newborn [Citation8].
One of the main challenges in obstetric anesthesia is to find efficient treatment for hypotension of spinal block. Many interventions were tried like the administration of crystalloids and/or colloids before and during anesthesia [Citation9], and administration of smaller doses of local anesthetics combined with opioid analgesics but not provide a satisfactory efficiency [Citation10]. Vasopressor’s administration was proven to be essential to manage hypotension in obstetric patients [Citation11].
Ephedrine, phenylephrine, and norepinephrine are the three commonly used vasopressors for managing spinal hypotension [Citation11,Citation12]. Ephedrine is a long-established and readily available familiar drug to most anesthetists for treatment and prophylaxis against spinal anesthesia-induced hypotension. Ephedrine is sympathomimetic amine that acts directly as alpha- and beta-adrenergic agonist and indirectly through release of norepinephrine from sympathetic neurons. Recently, there are some concerns about its use due to the probability of fetal acidosis, maternal supraventricular tachycardia, and tachyphylaxis [Citation13].
Phenylephrine is a pure alpha-adrenergic receptor agonist with no beta-adrenergic receptor activity. It can effectively prevent or treat postspinal hypotension. Despite it could decrease utero-placental perfusion, recent studies have proven that it can improve neonatal outcome by maintaining maternal mean arterial blood pressure and organs perfusion pressure [Citation14].
Norepinephrine has weak beta-adrenergic receptor agonistic properties, other than alpha adrenergic receptor agonist property. It has favorable effect on maternal heart rate and CO, rendering it a promising alternative to phenylephrine in obstetric anesthesia [Citation12,Citation15]
To the best of our knowledge, there was no study comparing phenylephrine, norepinephrine, and ephedrine, to select the ideal vasopressor for prevention of postspinal hypotension with least side effects. This research was designed to investigate and compare the efficacy and safety of (phenylephrine, norepinephrine, and ephedrine) constant infusions to prevent postspinal hypotension in elective cesarean sections.
.2. Methods
This randomized prospective trial was conducted from October 2018 to October 2019 after approval from the ethics and research committee of Menoufia University Hospitals (IRB 3-2018 ANET4). The trial was registered at www.pactr.org (PACTR 201810518426098). An informed written consent was obtained from each parturient. We studied 75 parturient, ASA physical status I or II, aged 18-40 years, 37 week or more normal singleton pregnancy, scheduled for elective cesarean section under spinal anesthesia block.
We excluded patients if they refused to participate or had pre-eclampsia, eclampsia, cardiac disorders, asthmatic patients, Known fetal abnormality, coagulations defect or any contraindication to spinal anesthesia.
Simple randomization using a randomization table created by a computer software program was used to allocate patients into 3 equal parallel groups according to the type of vasopressor drug. Phenylephrine (P) group: 25 patients received phenylephrine (Phenylephrine HCL® 10mg/1ml STEROP, Belgium) infused at 0.1 ug/kg/min I.V. Norepinephrine (N) group: 25 patients received norepinephrine (Levophrine® 4mg/4ml, EgyPharma, Egypt) infused at 0.05 ug/kg/min I.V. Ephedrine group: 25 patients received ephedrine (Ephedrine® 30mg/1ml, CID, Egypt) 1 mg/min I.V infusion. All infusions started just after receiving spinal anesthesia using syringe pump.
Three measurements of noninvasive blood pressure (at 1-min interval) were recorded after the parturient were allowed a 5-min rest period in the supine position with left lateral tilt. The average of the three readings of mean arterial blood pressure (MAP) was used as a baseline.
An anesthetist, who was not involved in the case management, prepared a syringe for the drug infusions with the designated concentration. Both the parturient and the anesthetist in charge of the case were blinded to the drug in the syringe.
On arrival to operating room, patients were monitored using: electrocardiogram, noninvasive arterial blood pressure, and pulse oximeter. Intravenous cannula 16 gauge was inserted and ringer solution (10 ml/kg/hr) was started. An arterial cannula was inserted for continuous maternal blood pressure monitoring.
The parturient was adjusted in the sitting position. The head was tilted anteriorly, and the shoulders were relaxed.
The skin was prepared with povidone iodine. After 3 min, the injection area was cleared using sterile gauze. After the skin was infiltrated with 2% lidocaine. A 25 G spinal needle was inserted at the L2-3 or L3-4 interspace. Subarachnoid injection of 0.5% hyperbaric bupivacaine with 25 µg fentanyl. The injected volume was chosen according to parturient height. If she was < 155, 155–170, >and 170 cm, we gave 1.8, 2.2, and 2.6 ml of hyperbaric bupivacaine, respectively. Then, parturient were positioned supine with left lateral tilt and two pillows supporting the head and shoulders. Oxygen (4 L/min) by a clear face mask was given.
Immediately after spinal anesthesia, a vasopressor infusion pump started according to type of the group. The infusion rate was constant, and boluses of phenylephrine (0.2 ug/kg), norepinephrine (0.1ug/kg), and ephedrine (5 mg) were given if the MAP decreased to more than 20% of baseline.
The upper sensory level of anesthesia was assessed. Heart rate and mean arterial blood pressure of parturient recorded immediately from the time of receiving spinal anesthesia then every 5 min until skin closure. If bradycardia (heart rate <50 beats/min) developed, intravenous atropine (0.01 mg/kg) was used. Incidence of maternal complication as nausea and vomiting was recorded and treated with metoclopramide (10 mg IV).
After delivery, an intravenous infusion of oxytocin (20 IU) was slowly administered. Apgar score of fetuses [Citation16] (appendix- 1) was assessed at 1 and 5 min after delivery, and cord blood sample was taken for pH, PO2, PCO2, and HCO3 measurement. After the end of surgery, the mother was transported to the recovery room with routine monitoring.
2.1. Sample size calculation
During the study design, depending on our primary outcome (maternal MAP) and according to previous clinical studies [Citation17,Citation18], we assumed the differences in MAP means and SDs would be 10%. By setting level of significance (α) to 5% and a power to 80% using G Power program version 3.1.9.2, Franz Faul, Universitat Kiel, Germany, we calculated that appropriate group size would require 22 patients. We planned to include 25 patients per group to allow for potential dropouts or protocol violations.
2.2. Statistical design
Data were collected, coded, revised, and entered to the Statistical Package for Social Science (IBM SPSS) version 20. The data were presented as number and percentages for the qualitative data, mean, standard deviations, and ranges for the quantitative data with parametric distribution and median with interquartile range (IQR) for the quantitative data with nonparametric distribution. Chi-square test was used in the comparison between two groups with qualitative data and Fisher exact test was used instead of the chi-square test when the expected count in any cell found less than 5. The comparison between more than two groups with quantitative data and parametric distribution were done by using one-way analysis of variance (ANOVA) test, and Kruskal-Wallis test was used in the comparison between more than two groups with quantitative data and nonparametric distribution followed by Student–Newman Kaul’s post hoc test. P-value was considered significant as the p< 0.05.
3. Results
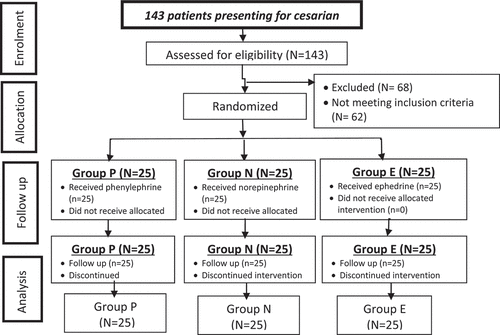
From 143 patients who were eligible for the study, 68 patients were excluded as 62 were not meeting the inclusion criteria and 6 refused to participate in the study; 75 pregnant women were enrolled in this study. There was no significant difference in the studied groups according to demographic data. In addition, variables as time of surgery and number of interventions (number of boluses of studied drugs), there was no significant difference in studied drugs according to these variables although more boluses were given in group E (4 boluses) than in other groups P and N (2 boluses each) ().
Table 1. Comparison between studied groups as regards demographic data
There was no statistically significant difference between studied drugs in baseline values of maternal pulse (p-value = 0.87). Maternal pulse increased significantly in ephedrine group (P value < 0.001). The heart rate slightly decreased in group P than in group N with frequent significance in some points of time measuring ().
Table 2. Comparison between studied groups as regards maternal heart rate
There was no significant difference among the three groups in basal mean BP. However, maternal mean blood pressure significantly increases in ephedrine in comparison with phenylephrine and norepinephrine. MAB was slightly higher in group P than in group N with frequent significance in some point of time measuring ().
There was no significant difference among groups in terms of the 1st and 5th mints Apgar score, which was better in group P and N than group E. Umbilical arterial blood gas analyses are summarized in . Based on the one-way ANOVA test, there was significant difference among groups regarding pH, PO2, HCO3 (P =0.001, 0.012, 0.016 respectively). Two neonates in group E (8.0%) reported to have neonatal acidosis, no one in other groups. Acidosis was significantly higher in group E than in the other groups (P=0.043). ()
Table 3. Comparison between studied groups as regards maternal mean blood pressure
Table 4. Comparison between studied groups as regards Neonatal outcome
As regards maternal complications, nausea and vomiting were more reported in group E and group P than in group N, with no significant difference between the groups (p = 0.348, 0.353, respectively). The same was reported for the need for anti-emetic drug (metoclopramide). Tachycardia was significantly higher in group E than others (p = 0.044). Although, four patients experienced maternal bradycardia in group P, while two patients in group N and no case in ephedrine group, there was no significant difference between the groups (p = 0.129) ().
Table 5. Comparison between studied groups as regards maternal complications
4. Discussion
The results of this study showed that mean arterial blood pressures were best maintained with a prophylactic fixed dose infusion of either phenylephrine or norepinephrine compared with those who received prophylactic ephedrine infusion with no serious or significant maternal complications except for more bradycardia in phenylephrine group. On the neonatal side, general condition was better in phenylephrine and norepinephrine groups than neonates of the ephedrine group who developed mild acidosis.
The reduction of systematic vascular resistance (SVR) is the main mechanism involved in postspinal hypotension. It is secondary to small artery vasodilatation along with a mild degree of venous vasodilatation [Citation19,Citation20].
Since then, prevailing opinion suggests use of vasopressors, to rescue spinal anesthesia-induced hypotension. The current study designed to compare between ephedrine, phenylephrine, and norepinephrine as three commonly used vasopressors in obstetric patients to detect the ideal one used in fixed rate infusion prophylactically to prevent hypotension without maternal and fetal side effects.
Much research has compared the effectiveness of phenylephrine and ephedrine or norepinephrine in different doses and routes of administration.
The traditional idea that ephedrine is the preferred choice as vasopressor to combat postspinal hypotension for cesarean sections was challenged by many researches comparing between ephedrine and phenylephrine [Citation21,Citation22]. These researches concluded that phenylephrine was associated with better fetal acid–base status, although there was no difference in the clinical outcome based on the Apgar scores between the two drugs.
Our result documented that both phenylephrine and norepinephrine are better choices than ephedrine in controlling maternal MAP, as ephedrine group needed more frequent boluses on top of already infused fixed dose to control hypotension than in phenylephrine and norepinephrine groups. That was in agree with a meta-analysis of four randomized clinical trials by Lee A et al. [Citation22], who noticed that ephedrine cannot prevent hypotension in low doses by infusion, so it failed to be used for prophylaxis against hypotension. In addition, ephedrine may produce hypertension in high doses, which is confirmed by the present study as there was two cases of hypertension and three cases of tachycardia in ephedrine group, which was not reported in the other groups. However, those treated with noradrenaline and phenylephrine needed fewer rescue boluses as compared to ephedrine, which needed more frequent boluses until stability of maternal MAP.
Also, our results were in agree with Sayasach et al. [Citation18] who found that phenylephrine infusion for prophylaxis against maternal hypotension is a better choice than ephedrine or their combination with lower doses. In addition, a combination of the drugs was better than ephedrine alone but had no additional benefit over phenylephrine.
There was moderate increase in MAB and decrease in heart rate in P group in comparison with N and E groups, this is due to increase in blood pressure with an α-agonist (phenylephrine) that may lead to reactive bradycardia (baroreceptor reflex). However, norepinephrine is a potent α-adrenergic receptor agonist and has weak β-adrenergic receptor agonist activity, and therefore, it has neutral and less negative effects on heart, so it is more suitable for maintaining blood pressure without bradycardic effect compared with phenylephrine [Citation23].
In parturient treated with ephedrine, there were decreased pH, base excess, and oxygen tension in umbilical cord arterial blood. Two babies of these patients developed neonatal acidosis in this study. The mechanism of this side effect was explained in old studies by the different action of various vasopressors on uteroplacental circulation [Citation24]. But Ngan Kee et al. [Citation25] explained the depressed fetal acid–base status due to ephedrine crossing the placenta and associated with greater fetal concentrations of lactate, glucose, and catecholamines because of the metabolic processes in the fetus caused by activation of fetal β-adrenergic receptors [Citation25]. Mercier et al [Citation26] reported that the addition of phenylephrine to ephedrine infusion improve the neonatal pH in comparison with ephedrine infusion alone.
Current study supports the use of either phenylephrine or norepinephrine as the vasopressors of choice than ephedrine because of improved fetal acid–base status [Citation27,Citation28]. With favorable effect of norepinephrine over phenylephrine, the phenylephrine’s effect of on feto-maternal physiology due to the possibility of bradycardia decreased cardiac output, which have adverse impact on placental perfusion [Citation23,Citation29]. Furthermore, norepinephrine is not proved to cross the placenta [Citation30] while ephedrine crosses the placenta more than phenylephrine [Citation24]. Ngan Kee et al. [Citation23] suggested that the use of norepinephrine may reduce catecholamine level of the baby compared to phenylephrine, by keeping out the potential stimulation of fetal metabolism and acidemia often observed with ephedrine.
By evaluation of first- and fifth-minute Apgar scores, there were no difference among groups. This agreed with previous studies that used the three vasopressors in management of postspinal hypotension in cesarean section [Citation11,Citation23,Citation31]. Therefore, prophylactic use of vasopressors is very effective for neonatal outcome, due to feasibility to control of maternal blood pressure and utero-placental perfusion [Citation31,Citation32].
Regarding of maternal complications, the most important observation was modest bradycardia related to phenylephrine infusion, which was transient and only in four cases (HR <50 per min) in comparison with two cases in norepinephrine group that managed by 0.01 mg/kg I.V. atropine. These findings were in accordance with other previous studies [Citation2,Citation11,Citation15].
Nausea and vomiting are common complications in obstetric anesthesia. They were higher in ephedrine and phenylephrine groups than norepinephrine in group, which responded rapidly to metoclopramide 10 mg IV. The possible cause of nausea and vomiting may be the increase of vagal tone following the reduction of preload as documented by Cooper et al. [Citation33]. None of the observed complications were severe.
5. Limitation of the study
Our current study included a relatively small number of patients and was done in a single centre, so larger and multi-center studies will be needed to verify the safe and efficient replacement of norepinephrine instead of phenylephrine and ephedrine in the management of postspinal hypotension during cesarean section.
6. Conclusion
In summary, the current study results showed that both norepinephrine and phenylephrine are more effective than ephedrine in preventing postspinal hypotension during cesarean section through their more maternal hemodynamic stability and fetal outcome with a more favorable effect of norepinephrine over phenylephrine on maternal heart rate, nausea, and vomiting.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgments
none.
Additional information
Funding
References
- Gandhi KA, Jain K. Management of anaesthesia for elective, low-risk (Category 4) caesarean section. Indian J Anaesth. 2018;62(9):667.
- Ngan Kee WD. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anesthesiol. 2010;23(3):304–309.
- Klöhr S, Roth R, Hofmann T, et al. Definitions of hypotension after spinal anaesthesia for caesarean section: literature search and application to parturients. Acta Anaesthesiol Scand. 2010;54(8):909–921.
- Salinas FV, Sueda LA, Liu SS, et al. Physiology of spinal anaesthesia and practical suggestions for successful spinal anaesthesia. Best Pract Res Clin Anaesthesiol. 2003;17(3):289–303.
- Neal JM. Hypotension and bradycardia during spinal anesthesia: significance, prevention, and treatment. Tech Reg Anesth Pain Manage. 2000;4(4):148–154.
- Macarthur A, Riley ET. Obstetric anesthesia controversies: vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin. 2007;45(1):115–132.
- Okudaira S, Suzuki S. Influence of spinal hypotension on fetal oxidative status during elective cesarean section in uncomplicated pregnancies. Arch Gynecol Obstet. 2005;271(4):292–295.
- Maayan-Metzger A, Schushan-Eisen I, Todris L, et al. Maternal hypotension during elective cesarean section and short-term neonatal outcome. Am J Obstet Gynecol. 2010;202(1):56. e1-5.
- Vercauteren M, Hoffmann V, Coppejans H, et al. Hydroxyethylstarch compared with modified gelatin as volume preload before spinal anaesthesia for caesarean section. Br J Anaesth. 1996;76(5):731–733.
- Ben-David B, Miller G, Gavriel R, et al. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25(3):235–239.
- Ngan Kee W, Khaw K, Lau T, et al. Randomised double-blinded comparison of phenylephrine vs ephedrine for maintaining blood pressure during spinal anaesthesia for non-elective caesarean section. Anaesthesia. 2008;63(12):1319–1326.
- Wang X, Shen X, Liu S, et al. The efficacy and safety of norepinephrine and its feasibility as a replacement for phenylephrine to manage maternal hypotension during elective cesarean delivery under spinal anesthesia. BioMed Res Int. 2018;2018.
- Moslemi F, Rasooli S. Comparison of prophylactic infusion of phenylephrine with ephedrine for prevention of hypotension in elective cesarean section under spinal anesthesi: a randomized clinical trial. Iran J Med Sci. 2015;40(1):19.
- Saravanan S, Kocarev M, Wilson R, et al. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in caesarean section. Br J Anaesth. 2006;96(1):95–99.
- Stewart A, Fernando R, McDonald S, et al. The dose-dependent effects of Phenylephrine for elective cesarean delivery under spinal anesthesia. Anaesth Analg. 2010;111(5):1230–1237.
- Persson M, Razaz N, Tedroff K, et al. Five and 10 minute apgar scores and risks of cerebral palsy and epilepsy: population based cohort study in Sweden. BMJ. 2018;360:k207.
- Feng K, Wang X, Feng X, et al. Effects of continuous infusion of phenylephrine vs. norepinephrine on parturients and fetuses under LiDCOrapid monitoring: a randomized, double-blind, placebo-controlled study. BMC Anesthesiol. 2020;20(1):229.
- Das S, Mukhopadhyay S, Mandal M, et al. A comparative study of infusions of phenylephrine, ephedrine and phenylephrine plus ephedrine on maternal haemodynamics in elective caesarean section. Indian J Anaesth. 2011;55(6):578.
- Sharwood-Smith G, Drummond G. Hypotension in obstetric spinal anaesthesia: a lesson from pre-eclampsia. Br J Anaesth. 2009;102(3):291–294.
- Dyer RA, Reed AR, Van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiol. 2009;111(4):753–765.
- Lee A, Kee WDN, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94(4):920–926.
- Lee A, Kee WDN, Gin T. A dose-response meta-analysis of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for elective cesarean delivery. Anesth Analg. 2004;98(2):483–490.
- Kee WDN, Lee SW, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiol. 2015;122(4):736–745.
- Kee WDN. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anesthesiol. 2010;23(3):304–309.
- Kee WDN, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiol. 2009;111(3):506–512.
- Mercier FJ, Riley ET, Frederickson WL, et al. Phenylephrine added to prophylactic ephedrine infusion during spinal anesthesia for elective cesarean section. Anesthesiol. 2001;95(3):668–674.
- Jl A, Jl H, Agarkar M, et al. Practice guidelines for obstetric anesthesia: an updated report by the American society of anesthesiologists task force on obstetric anesthesia and the society for obstetric anesthesia and perinatology. Anesthesiol. 2016;124(2):270–300.
- Loubert C. Fluid and vasopressor management for cesarean delivery under spinal anesthesia: continuing professional development. Can J Anesth. 2012;59(6):604–619.
- Habib AS. A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114(2):377–390.
- Puolakka J, Kauppila A, Tuimala R, et al. The effect of parturition on umbilical blood plasma levels of norepinephrine. Obstet Gynecol. 1983;61(1):19–21.
- Arago FF, Arago PW, Martins CA, et al. Comparison of metaraminol, phenylephrine and ephedrine in prophylaxis and treatment of hypotension in cesarean section under spinal anesthesia. Rev Bras Anestesiol. 2014;64(5):299–306.
- Veeser M, Hofmann T, Roth R, et al. Vasopressors for the management of hypotension after spinal anesthesia for elective caesarean section. Systematic review and cumulative meta-analysis. Acta Anaesthesiol Scand. 2012;56(7):810–816.
- Cooper DW, Carpenter M, Mowbray P, et al. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiol. 2002;97(6):1582–1590.
Appendix
Table A1 Apgar Score