ABSTRACT
Background: N-methyl-D-aspartate (NMDA) receptors are thought to be for pain memory, and blocking them can help to reduce pain. We aimed to assess the preventive effects of small doses of ketamine administered prior to the skin incision in abdominal surgery, to assess analgesic efficacy and intra-operative and post-operative side effects.
Methods: 60 adult patients with ASA I and II who were scheduled for abdominal surgery were randomly divided into two groups in a prospective, double-blind study. Before the skin incision, group K received 1 mg/kg ketamine intravenously, followed by a 0.12 mg/kg/h ketamine infusion till the skin was closed. Instead of ketamine, group C received normal saline. After extubation, both groups received morphine 5 mg IV. The visual analogue scale (VAS) was used to measure analgesic effectiveness at rest and after exercise, as well as the duration to the first analgesic and morphine intake in 24 h, and analyze changes in serum CRP and IL6 levels as indicators of its impact. Also, side effects of opioid or ketamine have been recorded.
Results: Within 24 h, patients in the ketamine group had a significantly lower VAS score, a longer time to the first analgesic (326.3 ± 49.5 minutes), and lower morphine consumption (6.9 ± 2.91 mg). Also, postoperative interleukin 6 and CRP showed significant difference between the two groups (p ≤ 0.05). In group K, no side effects were detectable.
Conclusion: Ketamine may be a promising drug in low doses that can limit and even prevent worsened inflammation. It also resulted in decreased postoperative opioid use, decreased pain rating, earlier retains of bowel motility, and decreased incidence of side effects.
1. Introduction
Colorectal surgery is often associated with pain, complications, high costs, and long hospital stay [Citation1]. High ileus rates, anastomosis, surgical site infection, and readmission are factors that may lead to these associations [Citation2].
The inflammatory response to surgery and trauma is aided by cytokine secretion, which is triggered by the stress response to surgery [Citation3]. Furthermore, surgery-induced peritoneal injury has been related to the development of adhesions since it causes an inflammatory response [Citation4].
For a variety of reasons, the IL-6 concentration in the first six postoperative hours was chosen as a representative result for the inflammatory response. First, in several studies that provided data in the early postoperative period, IL-6 was the most widely identified inflammatory biomarker. Second, it has anti-inflammatory properties, and it has been suggested that ketamine may be used as an anti-inflammatory drug [Citation4].
Ketamine is the most widely researched general anesthetic in the quest for strategies to modulate the systemic peri-operative cytokine response. Ketamine is an anesthetic and analgesic with a high potency. In adults, ketamine given intravenously during anesthesia decreased postoperative pain severity for up to 48 h, delayed the time to first request analgesia, and reduced total 24-h morphine consumption [Citation5]. There is evidence of a level I opioid-sparing benefit, as well as evidence of level II anti-hyperalgesia and opioid tolerance safety effects, as well as a reduction in persistent postoperative pain, based on current ketamine recommendations [Citation6].
In patients undergoing cardiac surgery with cardiopulmonary bypass (CPB), off-pump heart surgery, hysterectomy, and abdominal surgery, the impact of ketamine on peri-operative inflammatory responses has been examined. Doses ranged from a single additional small dose to full narcotic doses of ketamine with racemic drug or S – (+) – the more pharmacologically active ketamine. Ketamine has been discovered to have immune-modulating properties. Ketamine is also claimed to be a unique, anti-inflammatory drug that inhibits a systemic reaction without interfering with local healing processes [Citation7].
This study was showed to investigate the use of small bolus dose of ketamine followed by continuous infusion to prevent postoperative pain, reduce opioid consumption after intestinal surgery and examine the changes in serum CRP and IL6 levels as indicator of its effect.
2. Patients and methods
2.1. Patients
This prospective randomized-controlled double-blind study was accepted by the Medical Research Ethics Committee, Faculty of Medicine, Assiut University, Assiut, Egypt, (approval no: 17,200,147). It was registered at ClinicalTrials.gov (NCT03344393) and followed Helsinki Declaration. This study was conducted at General Surgery department from December 2017 to March 2019. We enrolled a total of 60 patients aged 18 to 60 years old with ASA status I or II who were undergoing elective intestinal surgery with operation times ranging from 60 min to 180 min after obtaining written informed consent from patients scheduled for elective adult intestinal procedures under general anesthesia.
Patients with known history of significant hepatic, renal, heart disease, autoimmune disease and any known convulsive disorder, any psychiatric disorders, chronic pain, pregnant females, regular use analgesics, anti-depressants or opioids in previous 2 months, corticosteroid therapy, history of NMDA receptor antagonist treatment, morbid obesity (BMI ≥ 95th percentile for age), allergy to ketamine or morphine were excluded from the study.
Randomization and blinding: According to a computer‐generated randomized number table, the patients were randomly assigned to two groups (each group number was 30). Group K (ketamine): 30 patients received intra-operative intravenous ketamine infusion. Group C (control): 30 patients received intra-operative intravenous normal saline infusion.
Loading drugs: Two 10-ml syringes, one filled with 1 mg/kg of ketamine and the other one filled with 10 ml 0.9% normal saline.
Infusion drugs: Two another 50-ml syringes, one filled with ketamine in a concentration of 1 mg/ml and the another one filled with 50 ml 0.9% normal saline [Citation8].
The preparation of syringes with drugs was carried out by well-trained investigators who were not involved in the data collection. The surgeon, patients, the anesthesiologist, and the investigators who collected data and interpreted the results were unaware of the intervention assignments. The study drug infusions were prepared by an anesthesiologist based on the weight of the patient pre-operatively with random drug selection. All syringes were stored in opaque envelopes numbered from 1 to 60. Accesses to the envelope’s codes were only available to one anesthesiologist who packed the envelopes.
2.2. Anesthesia and monitoring
Preoperative assessment and evaluation of patients participating in the study was done in the preoperative anesthesia clinic. All patients included in the study were informed about the procedure and were instructed to report their pain through using the Visual Analogue Score from 0 to 100; 0 representing no pain and 100 for the worst pain.
Study protocol was explained to the patients. Patients were informed that they can stop participating in the study at any time without any loss of medical service. Standard preoperative fasting strategies were followed prior to the elective anesthesia procedure.
All patients were premedicated with IV midazolam (1 mg) 45 min before surgery plus ondansetron (4 mg). Routine monitoring including electrocardiography (ECG), pulse oximetry (SpO2), and noninvasive blood pressure (NIBP), end tidal CO2 and temperature were applied. General anesthesia was induced using fentanyl (1 ug/kg) and propofol (2.5 mg/kg) and tracheal intubation was facilitated using 0.5 mg/kg of atracurium. Anesthesia was maintained with isoflurane in 50% oxygen/air mixture, and the depth of anesthesia was adjusted accordingly, aiming for a non-invasive mean baseline arterial pressure of 80–120%. Controlled mechanical ventilation was adjusted to maintain end-tidal PCO2 around 35 mmHg.
Before skin incision, both the groups received the loading drug. A constant infusion rate of the drug was started prior to skin incision which delivered at the rate of 0.12 mg /kg/hour for all patients.
At the end of surgery, infusion drug was stopped, anesthesia was discontinued, and residual neuromuscular blockade was antagonized with neostigmine (0.05 mg/kg) and atropine (0.02 mg/kg).
If there was no postoperative airway compromise or hemodynamic instability, the patient was transported to the Post-Anesthetic Care Unit (PACU). Pain intensity was assessed by VAS scores as none, mild, moderate, or severe, the following cut points on the pain VAS have been recommended: no pain (0–4 mm), mild pain(5–44 mm), moderate pain (45–74 mm), and severe pain (75–100 mm) [Citation9] and Aldrete–Krolik recovery score [Citation10] were recorded every 10 min until an Aldrete score > 9 was achieved. Then, the patients were transported to the ward.
Five mg morphine was given after extubation to both groups. The patients were transferred to recovery room where IV paracetamol 1 g was administrated every 6 h till the end of the study.
3. Assessment parameters
The following data were collected:
Patients’ demographic and clinical data including age, sex, weight, height, BMI, ASA, and duration of anesthesia and surgery.
Hemodynamic data were recorded intra-operative every 15 min including (mean arterial blood pressure, heart rate, arteria oxygen saturation, end tidal carbon dioxide and cutaneous body temperature). Also, postoperatively at 0, 2, 4, 6, 8, 12, 24, 36, and 48 h.
Post-operative pain scores in recovery room were recorded at rest and movement at 2, 4, 6, 12, 24, 36, and 48 h after surgery. IV morphine 0.05 mg/kg was given as top-up boluses for rescue analgesia, if VAS scores were >40.
Serum CRP and IL6 was measured preoperatively and 6 h postoperatively. Serum CRP was measured using nephelometry done on Advia 1800 fully automated analyzer of Siemens. Serum IL6 was measured using enzyme-linked immune-sorbent assay (ELISA) done by Sino-Gene-Clon Biotech kits.
The degree of sedation was evaluated using Modified Ramsay Sedation Scale at 2, 4, 6, 8, 12, 24, 36, and 48 h after the surgery.
The time at which the patient was given the first dose of rescue analgesia was recorded.
Number of doses of rescue analgesia needed in the postoperative period were also recorded.
Time of return of gastrointestinal motility using bowel sounds.
Satisfaction score was recorded using Likert score: 1 = Strongly un-satisfied, 2 = moderate un-satisfied, 3 = neutral, 4 = moderate satisfied, 5 = strongly satisfied [Citation11].
Patients were observed for any complications or side effects like tachycardia, increase blood pressure, confusion, hallucination, nausea and vomiting were recorded.
4. Outcomes
Primary outcome was the analgesic effect of intra-operative ketamine intravenous infusion presented by total dose of morphine was consumed postoperative. Secondary outcomes were the effect of ketamine infusion on the level of postoperative serum IL6 and CRP, pain score (during rest and movement) in the first 48 h using visual analogue score (VAS), time to the first request for rescue analgesics, return of intestinal motility, sedation score, satisfaction of the patients and any side effects happened.
5. Sample size
The primary endpoint of this study was the total amount of morphine consumption. Based on previous studies [Citation8], a target sample size was calculated. A power analysis estimated that a sample size of 25 patients in each group would have an 80% power at 0.05 level of significance to detect a difference of 1 h in the total amount of morphine consumption between the two groups. To compensate for patients’ dropout, a total of 60 subjects were enrolled.
6. Statistical analysis
Distribution of baseline variables was assessed by the Shapiro-Wilk test. Continuous variables were described as mean (±SD) and were analyzed using the student t-test and one-way analysis of variance (ANOVA) test with post-hoc multiple comparisons. Nonparametric data were presented as median (range) and were analyzed between the two groups using the Mann–Whitney U‐test. Categorical data were reported as numbers and percentages and were analyzed using the chi-square test or Fisher exact test. A P-value <0.05 was considered statistically significant. All statistical analyses were done using IBM SPSS statistics version 20 (SPSS Inc, Chicago, IL, USA).
7. Results
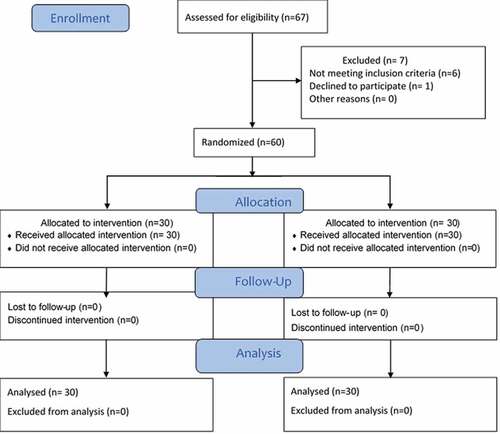
Sixty-seven patients were assessed for eligibility. Six of them did not meet the selection criteria, and one patient refused to participate in the study. Sixty patients (30 in each group) were enrolled in this study ().
The two groups were similar in age, sex, BMI, height, weight, type, and duration of both surgery and anaesthesia; there were no significant intergroup differences in these demographics ().
Table 1. Demographic data in the two studied groups
Hemodynamic vitals: No significant differences were recorded among groups in the mean arterial blood pressure, the mean heart rate or SPO2in all time points (data not presented).
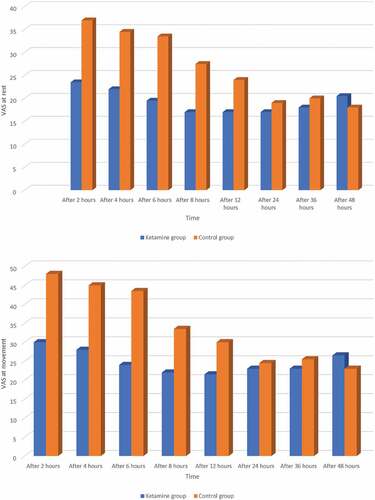
In the postoperative period: The VAS scores at (rest and movement) were significantly lower in ketamine group. The difference in VAS scores of the two groups becomes statistically significant at nearly all-time intervals before 36 h with P< 0. 05. There was no significance difference at 24 and 48 h (p˃0.05). Ketamine group had lower scores of VAS scores at nearly all-time intervals ().
Figure 2. (a) Visual analogue scale at rest between the two studied groups (b) (VAS) at movement between the two studied groups
The rescue analgesia and analgesic consumption: The total amount of postoperative morphine consumption showed significance difference between the two groups (p ≤ 0.05) where the mean morphine amount required as rescue analgesia in ketamine group was 6.9 ± 2.91 mg and in control group was 13.5 ± 7.95 mg. There was significant difference (p ≤ 0.05) between the two groups regarding time of first analgesic request 326.3 ± 49.5 min in ketamine group and 176.17 ± 40 min in control group. As regard number of opioid doses, patients in ketamine group received 1.6 ± 0.72 while patients in control group received to 2.9 ± 1.5; with significant difference between both groups (). The postoperative rescue analgesia was administered with IV morphine injection bolus 0.05 mg/kg as required or whenever the VAS score was >40 over a period of 48 h.
Table 2. Time to first request, total consumption of postoperative rescue analgesia, number of opioid doses and return of GIT motility
GIT motility: Regarding postoperative return of GIT motility showed significance difference between the two studied groups (p ≤ 0.05) ().
7.1. Patients’ satisfaction
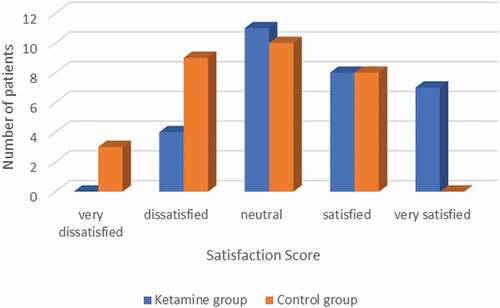
Patients’ satisfaction was assessed using Likert scale; the satisfaction score was adequate (very satisfied, satisfied, neutral) in 87% of the ketamine group as compared with 60% in the control group (P = 0.018) ().
Inflammatory responses: Perioperative inflammatory responses were comparable between the ketamine and control groups. Serum IL-6 levels before surgery were similar between the two groups (P = 0.940). After surgery, IL-6 levels were significantly higher than the baseline values in both groups. Postoperative interleukin 6 showed significance difference between the two groups (p ≤ 0.05).
Similarly, CRP levels increased after surgery in both groups compared with baseline values. Postoperative CRP showed significance difference between the two groups (p ≤ 0.05) ().
Table 3. Pre- and postoperative interleukin 6 and CRP level in the two studied groups
7.2. Sedation and side effects
There was no significant difference in postoperative sedation score between groups (p˃0.05). Only five patients in group K and three patients in group C had nausea but vomiting occurred in only one patient in each group that required treatment.
Transient tachycardia, confusion, hallucination, and drowsiness were found, but no active intervention was performed, and it was self-limiting. The difference was not statistically significant (p˃0.05). No other side effects were observed in post-operative period in both groups.
7.3. Discussion
The current study demonstrates the analgesic effect of 1 mg/kg ketamine (IV) bolus before skin incision followed by intra-operative (IV) 0.12 mg/kg infusion in patients underwent intestinal surgeries. In contrast to group C, there was a decrease in overall postoperative morphine intake, a delay in the first request for rescue analgesia, and a decrease in postoperative pain scores up to 48 h postoperatively in the K group. Furthermore, as compared to group C, group K had a lower surgical inflammatory response, less side effects, and higher patient satisfaction than the control group.
In accordance with Jouguelet-Lacoste et al., several conclusions can be drawn regarding the use of low-dose intravenous ketamine infusions, as the benefits of ketamine are primarily to reduce opioid use more than reduce pain levels, as ours lead to a reduction in pain intensity for up to 12 h after the surgery and less resulted in opioid use [Citation12].
Several studies have demonstrated the analgesic efficacy of ketamine given peri-operatively during the acute postoperative time [Citation13,Citation14]. The analgesic benefits of ketamine have been described in a systematic study, especially in operations with high levels of postoperative pain [Citation14], and when combined with morphine to lower morphine consumption [Citation15].
Due to the high affinity of ketamine in the NMDA receptor, it has been noted that the interaction of ketamine with the NMDA receptor is more selective with the low dose [Citation16].
We found that the morphine consumption regarding total doses and number of doses was less in ketamine infusion group, and the use of the low dose of ketamine infusion was associated with no side effects.
Anesthesia and surgery are associated with a weakening of the immune system expressed as an overactive pro-inflammatory immune response and suppression of cellular immunity that may affect the course of the postoperative period. The addition of anesthetic agents capable of ameliorating changes in immune function perioperative may have a positive effect on patients’ recovery. In our study, perioperative ketamine infusion attenuated the inflammatory response to anesthesia and surgery.
In our study giving ketamine infusion before skin incision and the start of surgery resulted in decreased the cytokines response to surgery by decreasing the production of CRP due to activation of nuclear factor kappa B in liver cells and decreased the postoperative increase of serum IL-6 level.
Since IL-6 plays an important role in regulating both cellular and humoral inflammatory responses, the ability of ketamine, observed in the present study, to weaken its secretion in the postoperative phase is an additional contribution to the avoidance of postoperative complications.
Ketamine can have positive effects on the postoperative immune response through a variety of mechanisms. First, it acts as an analgesic and relieves pain, which in itself stimulates pro-inflammatory cytokine production and suppresses IL-2 secretion. Therefore, it can be useful in administering preventive analgesia by suppressing inflammation [Citation17]. Second, the direct suppressive effect of ketamine on pro-inflammatory cytokine production by PBMCs was shown in the present study. In addition, ketamine has an anti-inflammatory effect by inhibiting the reactivity of leukocytes and suppressing the increased production of superoxide anions by neutrophils [Citation18].
Also, our results in accordance with that of Abbas et al., who concluded that ketamine (0.25 mg/kg) pretreatment may reduce inflammatory response (IL-6, IL-10 and CRP) to surgical trauma and can prevent the host’s auto destruction by secondary damage to tissues/organs that were not originally affected by the surgery; Reducing inflammation also reduces the post-operative pain and analgesics required [Citation19]. In our study, there was no significant statistical difference between both groups. And this is in agreement of [Citation20].
In agreement with our results, Nesher et al. reported that IV-PCA with a subanesthetic dose of ketamine and morphine following transthoracic lung and heart surgery resulted in lower pain scores, morphine consumption, and incidence of nausea-vomiting without increasing side effects [Citation21].
Also, in accordance with this study, Zakine et al. compared ketamine infusion during the intraoperative period alone with that for the perioperative period (intraoperative plus postoperative, 48 h). The authors demonstrated that low-dose ketamine improved postoperative analgesia, reduced morphine consumption and incidence of nausea [Citation22].
Remerand and colleagues demonstrated that an IV bolus at the beginning of surgery followed by a 24 h infusion decreased morphine consumption in patients undergoing total hip arthroplasty [Citation23]. Also, Akhavanakbari et al. showed that adding ketamine to morphine in IV-PCA reduced pain score and morphine consumption [Citation24]. All these studies come in agreement with our results.
In agreement with our results, many studies stated that there were no differences in the incidence of adverse events (nausea, vomiting, sedation, and psychomimetic events) when ketamine was given compared to placebo [Citation25,Citation26].
In accordance with this study, Zoumprouli et al. explained the early retain of intestinal motility in ketamine, by the decreased amount of postoperative analgesics consumption in this group compared by the other [Citation27].
7.3.1. Limitation
Limitation to our study might be the small sample size, the less frequent and type of cytokines assessed. The shorter duration of follow-up to the attenuation to the cytokines in the postoperative period.
8. Conclusion
In conclusion, ketamine, at low doses, is perhaps a promising drug that can limit and even prevent increased inflammation. It also led to reduced post-operative opioid consumption, reduced pain score, earlier retains of intestinal motility and decreased incidences of adverse effects.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
References
- Carmichael JC, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and rectal surgeons and Society of American gastrointestinal and endoscopic surgeons. Dis Colon Rectum. 2017;60(8):761–784.
- Halabi WJ, Kang CY, Nguyen VQ, et al. Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg. 2014;149(2):130–136.
- KANG SH, Kim Y-S, Hong T-H, et al. Effects of dexmedetomidine on inflammatory responses in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2013;57(4):480–487.
- Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17(41):4545.
- Elia N, Tramèr MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain. 2005;113(1–2):61–70.
- Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesthesia Analg. 2003;97(6):1730–1739.
- Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62(1):47–58.
- Parikh B, Maliwad J, Shah VRJJOA. clinical pharmacology, Preventive analgesia: effect of small dose of ketamine on morphine requirement after renal surgery. 2011;27(4):485.
- Aun C, Lam Y, Collett B. Evaluation of the use of visual analogue scale in Chinese patients. Pain. 1986;25(2):215–221.
- Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesthesia Analg. 1970;49(6):924–934.
- Likert RJAOP. A technique for the measurement of attitudes. 1932.
- Jouguelet-Lacoste J, La Colla L, Schilling D, et al. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med. 2015;16(2):383–403.
- Hadi BA, Daas R, Zelko R. A randomized, controlled trial of a clinical pharmacist intervention in microdiscectomy surgery–low dose intravenous ketamine as an adjunct to standard therapy. Saudi Pharm J. 2013;21(2):169–175.
- Ding X, et al. Morphine with adjuvant ketamine versus higher dose of morphine alone for acute pain: a meta-analysis. Int J Clin Exp Med. 2014;7(9):2504.
- Himmelseher S, Durieux ME, Weiskopf RB. Ketamine for perioperative pain management. J Am Soc Anesthesiologists. 2005;102(1):211–220.
- Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. JBJS. 2007;89(6):1343–1358.
- Beilin B, Shavit Y, Trabekin E, et al. The effects of postoperative pain management on immune response to surgery. Anesthesia Analg. 2003;97(3):822–827.
- Zilberstein G, et al. Ketamine attenuates neutrophil activation after cardiopulmonary bypass. Anesthesia Analg. 2002;95(3):531–536.
- Abbas H, et al. Effect of ketamine on inflammatory markers and postoperative analgesia in patients undergoing general anaesthesia. Int J Anesthesiol. 2013;32(1).
- Attalla HA, Habeeb RM, Elzwedy AI. Intravenous infusion of ketamine versus fentanyl for postoperative analgesia in spine surgeries. Menoufia Med J. 2018;31(4):1206.
- Nesher N, Serovian I, Marouani N, et al. Ketamine spares morphine consumption after transthoracic lung and heart surgery without adverse hemodynamic effects. Pharmacol Res. 2008;58(1):38–44.
- Zakine J, Samarcq D, Lorne E, et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesthesia Analg. 2008;106(6):1856–1861.
- Remérand F, Le Tendre C, Baud A, et al. The early and delayed analgesic effects of ketamine after total hip arthroplasty: a prospective, randomized, controlled, double-blind study. Anesthesia Analg. 2009;109(6):1963–1971.
- Akhavanakbari G, Mohamadian A, Entezariasl MJPICR. Evaluation the effects of adding ketamine to morphine in intravenous patient-controlled analgesia after orthopedic surgery. Perspect Clin Res. 2014;5(2):85.
- Behdad A, Hosseinpour M, Khorasani P. Preemptive use of ketamine on post operative pain of appendectomy. Korean J Pain. 2011;24(3):137.
- Safavi M, Honarmand A, Nematollahy Z. Pre-incisional analgesia with intravenous or subcutaneous infiltration of ketamine reduces postoperative pain in patients after open cholecystectomy: a randomized, double-blind, placebo-controlled study. Pain Med. 2011;12(9):1418–1426.
- Zoumprouli A, Chatzimichali A, Papadimitriou S, et al. Gastrointestinal motility following thoracic surgery: the effect of thoracic epidural analgesia. A randomised controlled trial. BMC Anesthesiol. 2017;17(1):1–10.