ABSTRACT
Objectives: To compare the safety and efficacy of oral theophylline versus oral sumatriptan in the treatment of post-dural puncture headache (PDPH).Background: PDPH is the most frequent complication of procedures associated with a dural puncture for spinal anaesthesia, cerebrospinal fluid (CSF) sampling, or following inadvertent dural puncture during epidural anaesthesia. Since invasive treatments have known complications, pharmacologic management may be preferable.Patients and Methods: This was a prospective, randomized, double-blind, phase four, comparative clinical trial; carried out on 60 patients presented with PDPH at Damanhour Teaching Hospital, El Beheira, Egypt; between February 2020 and May 2021. Patients were randomly allocated into two equal groups; group T, which received oral theophylline, and group S, which received oral sumatriptan.Results: There were no statistically significant differences between both groups as regards: demographic data, American Society of Anesthesiologists (ASA) physical status, type of operation, type, and size of the spinal needle. PDPH duration and length of hospital stay were significantly shorter in group T than in group S. Numerical pain rating scale (NPRS) scores were significantly lower in group T than in group S. Palpitation, dizziness, gastric irritation, and nausea/vomiting occurred in both groups with no statistically significant differences. No patient in either group needed an epidural blood patch.Conclusion: Oral theophylline is more effective and safer than oral sumatriptan in the treatment of PDPH. It lowered NPRS scores, shortened the duration of PDPH, and length of hospital stay, and was associated with minimal side effects.
1. Introduction
Post-dural puncture headache (PDPH) is a frequent complication of spinal anaesthesia or dural puncture and is an uncomfortable situation for both the patient and the anaesthetist. It is attributed to decreased cerebrospinal fluid (CSF) pressure leading to meningeal traction and cerebral vasodilation. [Citation1] It is an orthostatic headache occurring within 5 days of a dural puncture, 66% starts within the first 48 hours, and about 90% within the first 72 hours. It is usually accompanied by neck pain, tinnitus, hearing changes, photophobia, and/or nausea and relieved spontaneously within 2 weeks, after normalization of CSF pressure, rarely may last for up to 6 weeks. [Citation2] Risk factors include: young age, female sex, pregnancy, large needle size, the direction of the cutting needle bevel when puncturing the dura, multiple dural punctures, and previous history of PDPH. [Citation3]
Management includes conservative measures such as bed rest, hydration, caffeine administration, and analgesics. [Citation4] Pharmacological treatments as; gabapentin, pregabalin, [Citation3] neostigmine/atropine, [Citation5] methylxanthines, and triptans. [Citation6] Minimally invasive procedures as bilateral greater occipital nerve block [Citation7] or sphenopalatine ganglion block. [Citation8] Invasive procedures as; epidural blood patch (EBP) and epidural injections of saline, dextran 40, or hydroxyethyl starch. Since invasive treatments have known complications, [Citation9] pharmacologic therapy may be preferred.
This study aimed to compare and evaluate the efficacy and safety of oral theophylline versus oral sumatriptan in the treatment of PDPH.
2. Patients and methods
After obtaining approval from the local ethics committee, written consent was obtained from each patient to participate in the study. This was a prospective, randomized, comparative, double-blind, phase four clinical trial carried out on 60 patients with postoperative PDPH who were candidate of various surgical procedures under spinal anaesthesia at Damanhour Teaching Hospital, El Beheira, Egypt, between February 2020 and May 2021. The trial was registered on ClinicalTrials.gov, (NCT04257851), before first participant enrolment.
2.1. Inclusion and exclusion criteria
Patients’ selection for this study was based on clinical diagnosis suggesting PDPH based on the International Headache Society criteria. [Citation10] Inclusion criteria were; patients with an NPRS score of ≥5, American Society of Anesthesiologists (ASA) physical status ≤ II, age from 21 to 50 years, and first attempt spinal anaesthesia. Exclusion criteria included patients with NPRS score <5, ASA physical status >II, age <21 years or >50 years, pregnant women, history of; chronic headache, cluster headache, migraine, convulsions, cerebrovascular accident, previous neurological diseases, signs of meningismus, dysrhythmia, hypertension, ischemic heart disease, hyperthyroidism, peripheral vascular disease (ischemic colitis), liver or renal impairment, use of other methylxanthine derivatives, use of selective serotonin reuptake inhibitors, use of ergotamine derivatives in the past 24 hours, use of monoamine oxidase inhibitors in the last 2 weeks, use of any kind of opiates, allergy to the study medications and any contraindication of oral intake.
2.2. Treatment management
Patients were randomly allocated into two equal groups (30 patients each) and received: in group T; oral 150 mg theophylline anhydrous tablet (Quibron-T/SR, 300 mg dividose tablet, SmithKline Beecham Egypt L.L.C) every 12 hours, whereas in group S; oral 25 mg sumatriptan succinate tablet (Sumigran 25, 25 mg tablet, Sigma pharmaceutical industries, Egypt) every 12 hours.
All patients in both groups received conservative management for 48 hours, after hospital admission, which consisted of nursing in the supine position, hydration with continuous infusion of 30 mL/kg/day Ringer’s acetate solution, 1 g paracetamol (Perfalgan, Bristol-Myers Squibb Pharmaceuticals) IV every 6 hours. 75 mg diclofenac sodium (Voltaren, Novartis) IM every 12 hours. The intervention was continued until achieving an NPRS score ≤3 or for a maximum of 48 hours after treatment.
The time point 48 hours was selected as a priority endpoint, at which EBP is deemed necessary to manage PDPH if the NPRS score was ≥7 after the failure of the medical treatment in the study groups, following patient approval and consent, or if requested by the patient at any time during the study and will be recorded.
2.3. Assessment
Demographic data [age, weight, height, body mass index (BMI), sex], ASA physical status, PDPH onset (time interval between dural puncture and occurrence of PDPH), PDPH duration (time interval from occurrence of PDPH till NPRS score ≤3), length of hospital stay (time interval from hospital admission till discharge), type/size of spinal needle and type of surgery were recorded. Participants were asked to report the severity of their headache after sitting upright for 15 min, using a Numeric Pain Rating Scale (NPRS), which is a psychometric response scale for measuring subjective characteristics; baseline, before drug treatment (T0), 2 hours (T2), 6 hours (T6), 12 hours (T12), 18 hours (T18), 24 hours (T24), then every 12 hours till 48 hours (T48) after drug treatment, where 0 = no pain, and 10 = worst possible pain.
Treatment-related side effects were defined and recorded throughout the study period by YES/NO questionnaire as palpitation, dizziness, gastric irritation, nausea/vomiting, diarrhea, warm sensations in the body, tingling sensation, and tightness in the chest, throat, neck, or jaws.
2.4. Sample size calculation
The primary outcome variable of this study was NPRS score at 24 hours after drug treatment, the secondary outcome variables were PDPH duration, length of hospital stay, and treatment-related side effects. In this randomized clinical trial, after reviewing the literature, we found that with a confidence level of 95%, significance level 5% (i.e., α = 0.05, Zα = 1.96) and with a power of 95% (i.e., β = 0.05, Zβ = 1.64) to detect a difference of one (i.e., δ = 1) in the mean NPRS score of PDPH at 24 hours after drug treatment (primary outcome variable) between groups using 2-tailed t-test, with a standard deviation of one (i.e., σ = 1), and effect size of one (d = 1), the sample size was calculated using G*Power program, version 3.1.9.6, Institute für Experimentelle Psychologie, Heinrich-Heine-Universität, Düsseldorf, Germany and determined that at least 27 patients were required per group. We included 30 patients in each group to allow for dropouts and protocol violations.
2.5. Randomization
Randomization was performed by the online application (https://www.randomizer.org/) and concealed using sealed, opaque envelopes. The study was double-blinded; participants and data collectors were blinded to the group assignment, the medications were prepared and given by a physician who was not involved in the trial.
2.6. Statistical analysis
Charts were drawn using Microsoft® Excel® 2016 MSO, 64-bit, USA. Data were statistically analyzed using statistical package for social science program (IBM® SPSS® Statistics for Windows 2011, Version 20.0: IBM Corp., Armonk, NY, USA). Data were tested with Student’s t-test; for comparison between parametric means and expressed as mean ± standard deviation (SD), Chi-square tests (x2); for comparison between the incidences and expressed as the number of patients (percentage) and Mann–Whitney U-test; for comparison between non-parametric values and expressed as median (range). A P-value <0.05 was considered statistically significant and P-value <0.001 was considered highly significant.
3. Results
All 60 patients recruited and randomized completed the study and were included in the analysis (). There were no statistically significant differences between both studied groups regarding age, weight, height, BMI, sex, ASA physical status, and the onset of PDPH. The duration of PDPH was shorter in group T (18.40 ± 1.52 hours) than in group S (33.20 ± 5.62 hours), with a highly statistically significant difference between both groups, P < 0.001, and consequently the length of hospital stay was shorter in group T (1.083 ± 0.189 days) than in group S (1.717 ± 0.252 days), with highly statistically significant difference between both groups, P < 0.001 (). There were no statistically significant differences between both studied groups regarding types of operations, and types/sizes of the spinal needles ().
Table 1. Demographic data, PDPH onset (h), PDPH duration (h), and duration of hospital stay (days) of the two studied groups
Table 2. Types of operations and type/size of spinal needle (G) of the two studied groups
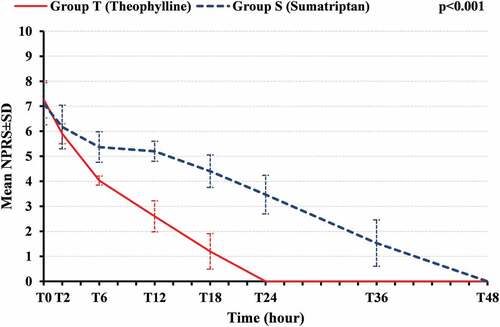
Analysis of PDPH assessment by NPRS score showed no statistically significant differences between both studied groups at T0. Improvements in NPRS scores occurred in group T than in group S, 2 hours after drug treatment (T2), but with no statistically significant difference. Pain scores were more decreased in group T at T6, T12, and T18 than in group S, and there was a highly statistically significant difference between both groups, P < 0.001. At T24, all patients in group T were resolved of PDPH, and at T48, all patients in group S were resolved of PDPH (). The mean sum of NPRS scores at T0, T2, T6, T12, T18, T24, T36, and T48 was lower in group T (21.00 ± 2.364) than group S (33.23 ± 4.725), with a statistically highly significant difference between both groups, P < 0.001 ().
Table 3. Numeric pain rating scale (NPRS) score at different points of time of the two studied groups
Regarding treatment-related side effects, there were no statistically significant differences between both groups concerning incidences of palpitation, dizziness, gastric irritation, nausea, and vomiting. No patient in either group complained of diarrhea, warm sensations in the body, tingling sensation, or tightness in the chest, throat, neck, or jaws, with no statistically significant difference between both groups. These side effects were clinically transient, self-limited, well tolerated, and none required any medical intervention. EBP was not performed on any patient in both studied groups ().
Table 4. Treatment-related side effects of the two studied groups
4. Discussion
PDPH after surgical operations is a disabling condition that limits the ability of the patient to resume walking and usual activity, in addition to delayed hospital discharge. Patients may require prolonged use of analgesics or an EBP, which are not free of side effects and complications. PDPH is thought to result from CSF leakage and volume depletion causing intracranial hypotension, which leads to traction of anchoring pain-sensitive structures in the brain, dilatation of the cerebral veins and venous sinuses, and increase in cerebral blood flow. [Citation11] Vascular expansion can be the main cause of PDPH. [Citation12]
Theophylline, a methylxanthine derivative, can reduce intracranial blood flow and venous enlargement [Citation13] by two mechanisms: first; it interferes with calcium uptake by the sarcoplasmic reticulum, inhibits phosphodiesterase enzymes, and blocks adenosine receptors, which all result in cerebral vasoconstriction, second; it stimulates sodium/potassium pump in the choroid plexus, which increases CSF production. [Citation14] Also, it may decrease the headache by blocking the transmission of pain perception. [Citation15]
Sumatriptan is the first generation of the triptans family, which is used mainly in the management of migraine headaches. It applies its effects by intracranial extracerebral vasoconstriction, and inhibition of neurotransmitter release at both peripheral and central trigeminal nociceptive terminals, via 5-hydroxytryptamine (5-HT1B/1D) receptor stimulation (trigeminovascular afferents and trigeminal nucleus caudalis). [Citation16]
In the current study, our results showed that oral theophylline significantly shortened the duration of PDPH, and the length of hospital stay in comparison to oral sumatriptan, and to our knowledge, there were no previous trials that studied the effect of either oral theophylline or sumatriptan on the duration of PDPH or length of hospital stay.
The results of the present study showed that oral theophylline improved pain scores at 2 h and significantly decreased pain scores at 6 h, 12 h, and 18 h after treatment in comparison to oral sumatriptan, and resolved PDPH completely at 24 h, in comparison to 48 h after treatment with oral sumatriptan.
In agreement with these results, a randomized clinical trial conducted by Mahoori et al., [Citation17] on 60 patients with PDPH, reported a significant decrease in pain scores in the oral theophylline than in the oral acetaminophen group; at 2 h. (5 ± 1.57 vs. 5.97 ± 1.27), at 6 h (3.43 ± 1.73 vs.4.33 ± 1.49), and at 12 h (2.67 ± 2.35 vs. 4.24 ± 1.97), P < 0.05. Also, a randomized clinical study by Sen and Sen, [Citation18] on 40 patients with PDPH, found a decrease in pain scores at 8 h, 16 h, and 24 h intervals in the oral theophylline than in the conservative group, which were in line with our results, despite giving caffeine-containing beverages and injectable opioids in their conservative group.
In the current study, the mean sum of pain scores at T0, T2, T6, T12, T18, T24, T36, and T48 was lower in the oral theophylline than in the sumatriptan group, with a statistically highly significant difference between both groups, P < 0.001.
These results concede with that of Feuerstein and Zeides, [Citation19] who conducted the first pilot study of 11 patients with severe headache following diagnostic lumbar puncture, and reported that the mean sum of pain scores in the oral theophylline group was significantly lower than in the placebo group (16 ± 3.91 versus 28 ± 4.73), P = 0.0398. Also, Sen and Sen, [Citation18] found that the combined pain scores were lower in the oral theophylline than in the conservative group (9.3 ± 5.7 vs. 56.7 ± 10.2), P < 0.001.
On the other side, a case report by Sprigge, [Citation20] of the first case treated with oral sumatriptan from PDPH, after inadvertent dural puncture by Tuohy needle, found that PDPH was relieved within 12 hours after treatment, which disagreed with our results. This can be explained by the usage of a large dose of oral sumatriptan (100 mg, q8h) that was preceded by subcutaneous sumatriptan (6 mg, q12h).
A recent randomized clinical trial conducted by Botros and Sayed, [Citation21] on 189 parturients with PDPH, reported significant decreases in the pain scores in the oral sumatriptan group than in the oral naratriptan and placebo groups at 6 h.; 1 (1–2) vs 2 (1–2) P < 0.007 and 3 (2–3) P < 0.001, at 12 h.; 1 (0–2) vs 2 (1–2) P < 0.005 and 2 (2–3) P < 0.001, at 24 h.; 0 (0–1) vs 1 (0–2) P < 0.019 and 2 (1–2) P < 0.001, at 48 h.; 0 (0–0) vs 0 (0–1) P = 0.137 and 2 (1–2) P < 0.001, respectively. These results were lower than the present study, which can be explained by the usage of a large dose of oral sumatriptan (50 mg, q12h in the 1st day, then q24h) and giving oral caffeine-containing drinks like coffee and tea, to all their studied groups.
The safety of oral theophylline or sumatriptan treatments of PDPH was evaluated based on the occurrence of treatment-related side effects, in the present study, there were insignificant incidences of palpitation, dizziness, gastric irritation, nausea, and vomiting in both studied groups. No patient of either group complained of diarrhea, warm sensations in the body, tingling sensation, or tightness in the chest, throat, neck, or jaws.
Theophylline-related side effects in this study were similar to that of two previous studies, which reported minimal side effects (cardiac dysrhythmias, gastric irritation, or central nervous system stimulation) in their studied groups. [Citation18,Citation19]
On the contrary, one prior study reported no adverse events in their studied groups, [Citation17] which disagreed with our results. This can be explained by the higher percentage of males in their studied group, who can tolerate side effects.
In contrast, sumatriptan-related side effects in the current study were comparable to a recent study that demonstrated insignificant incidences of nausea, vomiting, and dizziness, and the incidences of tingling and tightness were significantly higher in the naratriptan group than in the other two groups. [Citation21]
To the best of our knowledge, this was the first randomized clinical trial that compared oral theophylline versus oral sumatriptan and using lower doses of each drug in the treatment of PDPH.
4.1. Limitations
The limitation of this study was the use of NPRS for pain assessment, which is a subjective method that differs from one patient to another depending on their cultures and backgrounds.
5. Conclusion
From the current study, we declare that oral theophylline is more effective and safe than oral sumatriptan in the treatment of PDPH. It accelerated the recovery from PDPH, lowered the pain scores, shortened the duration of PDPH and length of hospital stay, associated with minimal side effects, and avoided the need for EBP. Oral theophylline is a practical, non-invasive, rapidly effective, and low-cost way to treat PDPH.
5.1. Recommendations
Since we have enrolled only patients with class I and II ASA physical status to our clinical trial, we recommend further studies to evaluate theophylline or sumatriptan in the treatment of PDPH in other ASA physical status classes.
Previous publications
-Abdalgaleil MM, Shaat AM, Elbalky OS, Ibrahim MM, Elnagaar MS. Is it safe to do laparoscopic cholecystectomy for acute cholecystitis up to 7 days? MMJ 2019, 32: 1267–1271. https://doi.org/10.4103/mmj.mmj_206_19.
-Abdalgaleil MM, Shaat AM, Elbalky OS, Elnagaar MS, Kamoun AM. Early versus delayed feeding after placement of percutaneous endoscopic gastrostomy tube with safe anesthetic techniques. MMJ 2018, 31: 1058–1063. https://doi.org/10.4103/mmj.mmj_164_18.
Contribution details
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kwak KH Postdural puncture headache. Korean J Anesthesiol. 2017;70(2):136–143. https://doi.org/10.4097/kjae.2017.70.2.136
- Buddeberg BS, Bandschapp O, Girard T. Post-dural puncture headache. Minerva Anestesiol. 2019;85(5):543–553.
- Mahoori A, Noroozinia H, Hasani E, et al. Comparing the effect of pregabalin, gabapentin, and acetaminophen on post-dural puncture headache. Saudi J Anaesth. 2014;8(3):374–377.
- Bakshi SG, Gehdoo RP. Incidence and management of post‑dural puncture headache following spinal anaesthesia and accidental dural puncture from a non‑obstetric hospital: a retrospective analysis. Indian J Anaesth. 2018;62(11):881–886.
- Mahmoud AA, Mansour AZ, Yassin HM, et al. Addition of Neostigmine and Atropine to conventional management of postdural puncture headache: a randomized controlled trial. Anesth Analg. 2018;127(6):1434–1439.
- Fawaz AA, El-Gendy HA, Saleh AN, et al. Aminophylline versus Acetaminophen in the treatment of post-dural puncture headache. Ain Shams Med J. 2021;72(1):49–58.
- Stohy EM, El-Sayed MM, Bastawesy MS. The effectiveness of bilateral greater occipital nerve block by ultrasound for treatment of post-dural puncture headache in comparison with other conventional treatment. Al-Azhar Med J. 2019;48(4):479–486.
- Jespersen MS, Jaeger P, Ægidius KL, et al. Sphenopalatine ganglion block for the treatment of postdural puncture headache: a randomised, blinded, clinical trial. BJA. 2020;124(6):739–747.
- Patel R, Urits I, Orhurhu V, et al. A comprehensive update on the treatment and management of postdural puncture headache. Curr Pain Headache Rep. 2020;24(6):24.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):102–103. https://doi.org/10.1177/0333102417738202
- Sachs A, Smiley R. Post-dural puncture headache: the worst common complication in obstetric anesthesia. Semin Perinatol. 2014;38(6):386–394.
- Sadeghi SE, Abdollahifard G, Nasabi NA, et al. Effectiveness of single dose intravenous aminophylline administration on prevention of post dural puncture headache in patients who received spinal Anesthesia for elective cesarean section. World J Med Sci. 2012;7(1):13–16.
- Ergün U, Ünal-Artık HA, İnan LE, et al. Intravenous theophylline rapidly decreases post-lumbar puncture headaches. Acta Neurol Belg. 2016;116(3):337–339.
- Katz D, Beilin Y. Review of the alternatives to epidural blood patch for treatment of postdural puncture headache in the parturient. Anesth Analg. 2017;124(4):1219–1228.
- Yang CJ, Chen T, Ni X, et al. Effect of pre-administration with aminophylline on the occurrence of post-dural puncture headache in women undergoing caesarean section by combined spinal-epidural anaesthesia. J Int Med Res. 2019;47(1):420–426.
- Antonaci F, Ghiotto N, Wu S, et al. Recent advances in migraine therapy. SpringerPlus. 2016;5(1):637.
- Mahoori A, Hassani E, Noroozinia H, et al. Theophylline versus acetaminophen in the treatment of post-dural puncture headache (PDPH). Middle East J Anaesthesiol. 2013;22(3):289–292.
- Sen J, Sen B. Non invasive management of post dural puncture headache –A comparison. Bangladesh J Med Sci. 2014;13(2):114–118.
- Feuerstein TJ, Zeides A. Theophylline relieves headache following lumbar puncture. Klinische Wochenschrift. 1986;64(5):216–218.
- Sprigge JS. The use of sumatriptan in the treatment of postdural puncture headache after accidental lumbar puncture complicated a blood patch procedure. Anaesthesia. 1999;54(1):95–96.
- Botros JM, Sayed AM. Comparison between the effects of sumatriptan versus naratriptan in the treatment of postdural puncture headache in obstetric patients: a randomized controlled trial. Anesth Essays Res. 2019;13(2):376–382.