ABSTRACT
Background
Poor management of postoperative pain results in physiological and psychological side effects with higher morbidity. Erector spinae plane block (ESPB) has shown efficacy in controlling pain in many surgeries. Dexmedetomidine has improved the quality of analgesia in many regional techniques. This study aimed to assess the analgesic outcome of adding dexmedetomidine to bupivacaine in ultrasound (US) guided ESPB for perioperative analgesia for thoracic cancer surgeries.
Patients and Methods
In this randomized controlled, double-blind study, 42 patients aged 18–65 years, ASA (American Society of Anesthesiologists) physical status II, scheduled for thoracotomy for cancer surgeries under general anesthesia were included. Patients were allocated into two equal groups: group 1 (ESPB by 28 ml bupivacaine 0.25% + 2 mL saline) and group 2 (ESPB 28 ml bupivacaine 0.25% + 2 mL dexmedetomidine 0.5 µg/kg). Blocks were performed before anesthesia induction.
Results
Group 2 consumed lower intraoperative fentanyl and postoperative morphine and had a lower pain score at rest and cough compared to group 1. Group 2 had prolonged time to first request of rescue analgesia compared to group 1. Postoperative nausea and vomiting, and sedation were comparable between both groups. No block-related complications were observed.
Conclusions
Adding dexmedetomidine to bupivacaine in US-guided ESPB provided more effective and safe analgesia in thoracotomy.
1. Introduction
The surgical incision produces post-thoracotomy pain (PTP), damage to the ribs and intercostal nerves, inflammation of the chest wall, pleura or pulmonary parenchyma cutting, and placement of the thoracotomy drainage tube. Acute PTP inhibits the ability to breathe and cough normally [Citation1]. Numerous analgesic techniques are used to relieve PTP, including systemic opioids, regional techniques (such as paravertebral nerve blockade, intercostal nerve blockade, intrapleural analgesia, and epidural opioids with or without local analgesia), cryo-analgesia, and transcutaneous electrical nerve stimulation (TENS). Apart from these approaches, patient-controlled analgesia (PCA) has been utilized extensively, either systemically or epidural [Citation2].
The Erector Spinae Plane block (ESPB) is a recently developed technique for PTP reduction. ESPB was initially reported to treat persistent thoracic neuropathic pain by injecting a local anesthetic (LA) deep into the erector spinae muscle at the level of T5 [Citation3]. Moreover, ESPB is a reasonable method to administer, with clearly identifiable sonographic landmarks and LA needle insertion and injection locations [Citation3]. ESPB has been applied in thoracic [Citation3] and abdominal surgeries [Citation4], with high success rates providing both visceral and somatic analgesia.
The LA drugs have a limited duration of action, so we need to add adjuvants such as opioids, alpha two agonists, neostigmine, or magnesium [Citation5]. Dexmedetomidine is a very selective α2 adrenoceptor agonist that is tenfold more selective than clonidine. It is a highly flexible medication in anesthetic practice, finding use in an expanding range of clinical circumstances, and is no longer restricted to intensive care unit (ICU) sedation [Citation6].
It induces dose-dependent drowsiness, anxiety reduction, and analgesia (at spinal and supraspinal locations) without causing respiratory depression [Citation7]. By activating central α2 receptors, dexmedetomidine increases the anesthesia-induced by other anesthetics, induces perioperative sympatholytic, and reduces blood pressure [Citation8,Citation9].
Despite known benefits of dexmedetomidine in pain control, there are very few studies about its use as an adjuvant to LA in ESPB, so we have decided to take up this randomized study to evaluate the effects of adding 0.5 μg/kg dexmedetomidine to 0.25% bupivacaine in ESPB in patients undergoing thoracic cancer surgeries.
The aim of this study was to evaluate the effect of adding dexmedetomidine as an adjuvant to bupivacaine in ultrasound (US) guided ESPB as perioperative analgesia for thoracic cancer surgeries and its impact on decreasing perioperative opioid consumption.
2. Material and methods
Our prospective randomized controlled, double-blind study was conducted on 42 patients aged 18–65 years, body mass index (BMI) ranged between 20 and 40 kg/m2, ASA (American Society of Anesthesiologists) physical status II scheduled for thoracic cancer surgeries under general anesthesia (GA). The study was done from July 2020 to April 2021 at National Cancer Institute and Al Kasr Alainy – Cairo University after approval from Ethical Committee (MS-100-2020) and obtaining informed written consent.
The exclusion criteria were patient refusal, known sensitivity or contraindication to LAs or dexmedetomidine, history of psychological disorders and/or chronic pain, localized infection at the site of block, coagulopathies, and significant liver or renal insufficiency.
Patients were allocated in a parallel manner into two equal groups: Group 1 (ESPB without Dexmedetomidine group) (control group): (n = 21) patients received preoperative US guided ESPB on the operated side by 28 ml bupivacaine 0.25% + 2 mL saline. Group 2 (ESPB with Dexmedetomidine group): (n = 21) patients received US guided ESPB on the operated side by 28 ml bupivacaine 0.25% + 2 mL dexmedetomidine 0.5 µg/kg.
In the anaesthesia clinic, randomization was accomplished through the use of computer-generated random numbers and closed opaque envelopes. Another anesthesiologist who was not involved in the other parts of the study opened the envelopes to enroll patients. A pharmacist prepared drugs, and both patients and outcome assessors were blind to the assignment of groups.
2.1. Preoperative management
Preoperative assessment of all patients comprised history taking, clinical examination, laboratory testing (complete blood count, kidney function tests, liver function tests, prothrombin time, and partial thromboplastin time), electrocardiogram, and chest X-ray. The study protocol was explained to the patients, and their consent was taken. All patients were made familiar with the use of the pain score; visual analog scale score (VAS) identifying 0 as no pain and ten as the worst possible pain.
The patients were continuously monitored in the holding room for a pulse, blood pressure, and oxygen saturation (baseline values). An intravenous (IV) 18-gauge cannula was inserted. Midazolam 0.02 mg/Kg IV was administered for each patient. Patients received US-guided ESPB before induction of GA.
2.2. The US guided ESPB
Blocks were performed with a US machine (Sonosite Edge; Sonosite Inc., USA) equipped with an HFL38X high-frequency linear transducer (13–16 MHz). With patients in sitting position, the transducer was positioned longitudinally 3 cm lateral to the T5 spinous process. The trapezius, rhomboid major, and erector spinae muscles were revealed superficial to the shadow of the hyperechoic transverse process. Then, 3 ml lidocaine 2% was used to anesthetize the skin. Using a 20-gauge block needle put in-plane in a cephalad-to-caudad orientation to position the tip into the fascial plane on the deep (anterior) side of the erector spinae muscle, 28 ml bupivacaine 0.25% + 2 mL saline or 28 ml bupivacaine 0.25% + 2 mL dexmedetomidine 0.5 µg/kg were injected. The needle tip’s placement was confirmed by observable fluid spread lifting the erector spinae muscle away from the transverse process’s bony shadow.
2.3. Anesthesia management
GA was induced for both groups using IV fentanyl 2 μg/kg and propofol 2 mg/kg. Tracheal intubation was facilitated by rocuronium 0.5 mg/kg and done by a left-sided double-lumen endobronchial tube (Mallinckrodt’s 37 or 39 Fr) and a fiberoptic bronchoscope was used to ensure the correct position of the tube. Tidal volume was adjusted to be 6–8 ml/kg, and the respiratory rate was adjusted to keep the end-tidal CO2 between 30 and 40 mmHg.
Anesthesia was maintained with inhaled sevoflurane with MAC 2–2.5% in oxygen-enriched air (FiO2:50%) and top-up doses of rocuronium (0.1 mg/kg) IV administered as required. Ringer acetate was infused to replace their fluid deficit, maintenance, and losses.
All patients received 1 g of IV paracetamol. Additional bolus doses of fentanyl one µg/kg IV were given if the mean arterial blood pressure (MBP) or heart rate (HR) rises above 20% of baseline levels.
One reading of MBP and HR were taken before induction of GA to be defined as a baseline reading and then recorded immediately before surgical incision and at 30 min intervals intraoperatively. Hypotension was treated with 100 ml 0.9% normal saline bolus and 5 mg ephedrine IV in incremental doses to maintain MBP above 70 mmHg. Bradycardia (HR <60) was treated with 0.1 mg/Kg atropine IV.
The residual neuromuscular blockade was reversed using 2 mg/kg sugammadex IV, and extubation was performed after complete recovery of the airway reflexes.
Then, patients were transferred to the post-anesthesia care unit (PACU) and were observed for two hours. A modified Aldrete score >9 was required for discharge from the PACU. Following that, the patients were transferred to a ward and given 1 g of acetaminophen IV every 8 hours.
VAS (at rest and cough), MBP, and HR were noted immediately on arrival to PACU (0 h) and at 2, 4, 6, 12,18, and 24 h postoperatively. Rescue analgesia was provided in the form of IV morphine 3 mg boluses if VAS >3. The time to first request rescue analgesia and the total morphine administered during the first 24-hour postoperative were recorded.
Patient satisfaction (satisfied or not satisfied) was recorded at 24 hours postoperative. Adverse events were reported (PONV, hypotension, bradycardia, excessive sedation, and hematoma). Postoperative sedation was recorded according to Ramsey sedation score [Citation10] (1: anxious or restless or both; 2: cooperative, orientated, and tranquil; 3: responding to commands; 4: brisk response to a stimulus; 5: sluggish response to a stimulus; and 6: no response to stimulus).
The primary outcome was the time first to request rescue analgesia, and the secondary outcomes were the total amount of morphine in the first 24-hour postoperative, pain score, and safety.
2.4. Sample size
PASS 13 was used for sample size calculation. Based on data from a previous study [Citation11], the mean (± SD) time to first request of rescue analgesia was 15 (± 8) hours in the dexmedetomidine group and 9 (± 5 hours) in the control group. Using a two-sided two-sample t-test, the sample size of 21 patients per group was needed to achieve 80% power with a significant level (alpha) of 0.05 to detect a difference of 6.0 hours between both groups.
2.5. Statistical analysis
The statistical analysis was performed using SPSS version 23 (IBM, Chicago, IL, USA). Categorical variables were represented as a number (percent) and analyzed using the chi-square or Fisher’s Exact tests. Normally distributed numerical variables were presented as mean ± SD and compared using the independent Student’s t-test. Not normally distributed numerical variables were presented as median (IQR) and compared using Mann–Whitney test. A two-tailed P-value of less than 0.05 is considered statistically significant.
3. Results
CONSORT flowchart of the enrolled patients is shown in . There were no significant differences in demographic data, duration of surgery, type of surgery, need for intraoperative blood transfusion and occurrence of intraoperative hypothermia between both groups.
Table 1. Demographic data and surgical data of the studied groups
Intraoperative fentanyl consumption and postoperative morphine consumption were significantly decreased in ESPB with Dexmedetomidine group compared to ESPB without Dexmedetomidine group. Patients who required rescue analgesia in the first 24-hour postoperative were insignificantly higher in ESPB without Dexmedetomidine group than ESPB with Dexmedetomidine group. Time to first rescue analgesia (in patients required analgesia) was significantly delayed in ESPB with Dexmedetomidine group compared to ESPB without Dexmedetomidine group.
Table 2. Intraoperative and postoperative analgesia of the studied groups
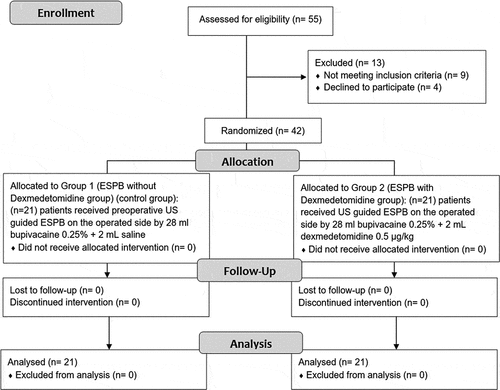
ESPB with Dexmedetomidine group showed significantly lower VAS at rest and during cough. (A, B)
Intraoperative and postoperative HR and MBP were insignificantly different between both groups. (A-D)
Figure 3. Intraoperative heart rate (A) and mean arterial blood pressure (B) and postoperative heart rate (C) and mean arterial blood pressure (D)
Satisfied patients, PONV, and postoperative sedation (Ramsey sedation score >2) were comparable in both groups. No block-related complications were observed in both groups.
Table 3. Satisfied patients and adverse events of the studied groups
4. Discussion
In this study, adding dexmedetomidine to bupivacaine in US-guided ESPB for patients undergoing open thoracotomy was associated with better analgesia, which was apparent by a longer duration of analgesia and reduction of VAS at rest and cough and consumption of both intraoperative fentanyl and postoperative morphine in 24 hours. But hemodynamic parameters, complications related to opioid use, or block technique were insignificantly different in both groups.
Perineural dexmedetomidine blocks the hyperpolarization-activated cation current affecting the activity of peripheral nerve. Also, it can lead to vasoconstriction at injection site resulting in effect prolongation by delaying LA absorption. Moreover, it has analgesic properties [Citation11].
Gao et al. [Citation11] assessed the impact of dexmedetomidine or dexamethasone as adjuvants to ropivacaine in US-guided ESPB for video-assisted thoracoscopic lobectomy surgery (VATLS). They were in line with our results as they demonstrated that postoperative VAS was significantly decreased in the ropivacaine with dexmedetomidine group more than those in the ropivacaine alone group. Also, ropivacaine with dexmedetomidine group had prolonged median durations of sensory block compared to ropivacaine alone group. The longer block may be due to the different types of operation (as video-assisted thoracic surgery is less invasive), different types of LA, and different intraoperative and postoperative opioids (propofol and remifentanil infusion intraoperative, PCA with sufentanil and flurbiprofen postoperative).
Rao et al. [Citation12] assessed the role of adding nalbuphine or dexmedetomidine to ropivacaine in ESPB in VATLS. Their results were in line with us as they showed that VAS, postoperative analgesic consumption was significantly decreased with dexmedetomidine group than control group without dexmedetomidine. Also, the first use was delayed with dexmedetomidine group. PONV was insignificantly different between both groups.
Wang et al. [Citation13] assessed the role of adding dexmedetomidine 1 μg/kg to ESPB compared to ESPB without dexmedetomidine after modified radical mastectomy. Their results agreed ours as they demonstrated that VAS at rest and an active state was significantly lower with dexmedetomidine group at most times of measurement. Also, the intraoperative and postoperative analgesics were significantly lower with dexmedetomidine group. PONV was insignificantly different between both groups. There was no postoperative bradycardia nor hypotension occurred in both groups.
Also, Gad and El-Metwally [Citation14] evaluated the effectiveness of adding dexmedetomidine to levobupivacaine in a US-guided serratus plane block for modified radical mastectomy. In accordance with their results, adding dexmedetomidine to levobupivacaine significantly decreased VAS at 8 and 12 hours postoperatively and total postoperative pethidine consumption. Also, it significantly prolonged the duration of adequate analgesia (617 ± 125 vs. 443 ± 71 min; P < 0.001). But in disagreement with their results, the total intraoperative fentanyl requirement was insignificantly different between levobupivacaine alone and levobupivacaine-dexmedetomidine groups. This difference may be due to the difference in the type of surgery or LA used.
Mohta et al. [Citation15] assessed the impact of the use of dexmedetomidine as an additive to bupivacaine in the paravertebral block during breast cancer surgery. In agreement with our results, the mean intraoperative fentanyl requirements were lower in bupivacaine with dexmedetomidine group (54.6 µg) than bupivacaine alone group (58 µg). Additionally, bupivacaine with dexmedetomidine group required lower mean morphine postoperatively (2.4 mg) compared with bupivacaine alone group (18.3 mg) and had lower pain scores. Moreover, they revealed a more significant duration of paravertebral block and sustained postoperative analgesia when dexmedetomidine was added to bupivacaine. This wide variation in the duration of analgesia provided by dexmedetomidine could be attributed to the difference in the block’s location, different doses of LA used, the differences in the nature of surgeries performed, and differences in the methods of pain evaluation.
In contrast to our trial, Packiasabapathy et al. [Citation16] demonstrated that adding 1 μg/kg of dexmedetomidine to bupivacaine in femoral nerve block did not reduce morphine consumption significantly after total knee arthroplasty.
The incidence of adverse effects related to dexmedetomidine was no difference between the groups. There are mixed reports on the incidence of systemic adverse effects with perineural dexmedetomidine. Studies [Citation17–19] have shown no significant increase with the use of dexmedetomidine. Other studies [Citation14,Citation20] have observed significant bradycardia and hypotension after dexmedetomidine use, which we did not notice in our trial. Considering the low incidence of systemic adverse effects associated with perineural dexmedetomidine, our study did not find any significant difference in the incidence of side effects between the groups. However, a study with a larger sample is needed to confirm this.
Study limitations included 1) relatively small sample size as larger sample size may give different results according to secondary outcome 2) We did not include a control group that used systemic analgesia or one of the gold standard regional techniques in thoracic surgery (thoracic epidural analgesia) 3) We did not use a catheter insertion for intermittent boluses or continuous infusions of LA 4) The follow-up of acute pain was for 24 hours only 5) We did not follow the effect on chronic pain.
5. Conclusion
US-guided ESPB with dexmedetomidine as adjuvant provides more effective and safe analgesia in open thoracotomy in the form of lower consumption intraoperative fentanyl and postoperative morphine and lower VAS.
Sponsors and funding sources
Nil
Acknowledgments
Nil.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Casati A, Alessandrini P, Nuzzi M, et al. A prospective, randomized, blinded comparison between continuous thoracic paravertebral and epidural infusion of 0.2% ropivacaine after lung resection surgery. Eur J Anaesthesiol. 2006;23(12):999–1004.
- Momeni M, Crucitti M, Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66(18):2321–2337.
- Forero M, Rajarathinam M, Adhikary S, et al. Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: a case report. A A Case Rep. 2017;8(10):254–256.
- Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. 2017;42:372–376.
- Swain A, Nag DS, Sahu S, et al. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases. 2017;5(8):307–323.
- Naaz S, Ozair E. Dexmedetomidine in current anaesthesia practice – a review. J Clin Diagn Res. 2014;8:1–4.
- Zhao L-H, Shi Z-H, Yin -N-N, et al. Use of dexmedetomidine for prophylactic analgesia and sedation in delayed extubation patients after craniotomy: a study protocol and statistical analysis plan for a randomized controlled trial. Trials. 2013;14(1):251.
- Bekker AY, Kaufman B, Samir H, et al. The use of dexmedetomidine infusion for awake craniotomy. Anesth Analg. 2001;92:1251–1253.
- Mack PF, Perrine K, Kobylarz E, et al. Dexmedetomidine and neurocognitive testing in awake craniotomy. J Neurosurg Anesthesiol. 2004;16(1):20–25.
- Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659.
- Gao Z, Xiao Y, Wang Q, et al. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019;7(22):668.
- Rao J, Gao Z, Qiu G, et al. Nalbuphine and dexmedetomidine as adjuvants to ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Medicine (Baltimore). 2021;100(32):e26962.
- Wang X, Ran G, Chen X, et al. The effect of ultrasound-guided erector spinae plane block combined with dexmedetomidine on postoperative analgesia in patients undergoing modified radical mastectomy: a randomized controlled trial. Pain Ther. 2021;10(1):475–484.
- Gad M, El-Metwally M. Efficacy of adding dexmedetomidine as adjuvant with levobupivacaine in ultrasound-guided serratus plane block for modified radical mastectomy surgery. Research and Opinion in Anesthesia and Intensive Care. 2019;6(2):234.
- Mohta M, Kalra B, Sethi AK, et al. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30(2):252–260.
- Packiasabapathy SK, Kashyap L, Arora MK, et al. Effect of dexmedetomidine as an adjuvant to bupivacaine in femoral nerve block for perioperative analgesia in patients undergoing total knee replacement arthroplasty: a dose-response study. Saudi J Anaesth. 2017;11(3):293–298.
- Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: a prospective randomized controlled trial. Saudi J Anaesth. 2012;6(2):109–114.
- Obayah GM, Refaie A, Aboushanab O, et al. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27(3):280–284.
- Swami SS, Keniya VM, Ladi SD, et al. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: a randomised double-blind prospective study. Indian J Anaesth. 2012;56(3):243–249.
- Das A, Majumdar S, Halder S, et al. Effect of dexmedetomidine as adjuvant in ropivacaine-induced supraclavicular brachial plexus block: a prospective, double-blinded and randomized controlled study. Saudi J Anaesth. 2014;8(5):S72–7.