ABSTRACT
Background
Evaluation of the impact of at-admission hyperglycemia and its management on the outcome of non-diabetic COVID-19 patients concerning development of critical illness.
Methods
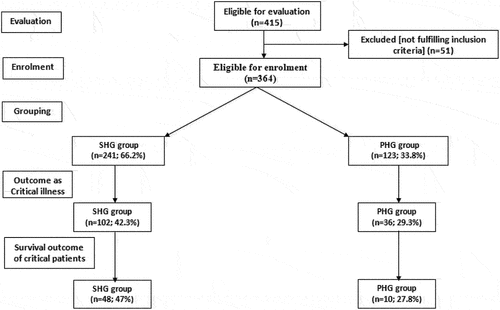
In total, 364 patients were categorized according to at-admission random blood glucose (RBG) as persistent hyperglycemic (PHG) if at-admission and 6-hr RBG was >140 mg/dl or as stress hyperglycemic (SHG) if at-admission RBG was >140 mg/dl but decreased 6 hr later. Only PHG patients received intensive insulin therapy (IIT) using dose titration schedule according to Leuven titration protocol. All patients were evaluated for critical illness risk using the COVID-GRAM Critical Illness Risk (CG-CIR) score. Study outcomes are the incidence of critical illness and mortality rate (MR) and its relation with the levels of at-admission RBG and inflammatory markers.
Results
One hundred and twenty-three patients had PHG and received IIT, and 241 had SHG. Unfortunately, 138 patients developed critical illness and 58 of them deceased with significantly lower incidences among patients who received IIT. Progress to critical disease and mortality were significantly correlated with high CG-CIS scores, RBG and serum levels of CRP and IL-6 but showed negative significant correlation with the application of IIT. The automatic linear modeling analysis defined high at-admission RBG as the most important predictor for progress to critical illness (57%) and mortality (60%), and the Mentel-Haenszel statistic defined IIT as a significant independent predictor for survival of critical COVID patients with at-admission hyperglycemia.
Conclusion
Admission hyperglycemia worsens the outcomes of non-diabetic COVID patients, and this effect is positively correlated with RBG. IIT is a safe and effective management and improves outcomes.
1. Background
Hyperglycemia is a common phenomenon in critically ill patients and is associated with poor clinical outcomes, especially those detected at admission [Citation1]. Hyperglycemia promotes the release of reactive oxygen species and inhibition of T cell activation, and excess glucose promotes the formation of the advanced glycation end-product that plays a role in perpetuating pneumonia and acute respiratory distress syndrome (ARDS) [Citation2]. Insulin administration using insulin infusion protocols is the preferred strategy to control hyperglycemia in critically ill patients [Citation3].
The coronavirus disease (COVID-19) caused by SARS-CoV-2 infection has invaded the entire world as a global pandemic, targeting lung alveolar epithelial cells [Citation4]. However, with the wide spread of the disease for about two years, it was manifested by multiple extra-pulmonary manifestations with long-term devastating consequences of heterogeneous symptoms [Citation5].
The SARS-CoV-2 virus has direct and indirect impacts on the liver; around 15–30% of COVID-19 patients have underlying liver disease, and 20–35% of COVID-19 patients had altered liver enzymes at admission [Citation6]. Moreover, severe COVID-19 induces significant transcriptional shift that may lead to tissue remodeling, mitochondrial dysfunction, and disturbed secretory and excretory hepatic function, resulting in liver dysfunction and metabolic derangement [Citation7].
Angiotensin-converting enzyme-2 (ACE2) is the cell-surface receptor that enables cellular entry of SARS-CoV-2 and is highly expressed in adipose tissue [Citation8], rendering it as a potential reservoir contributing to massive viral spread in obese COVID-19 patients [Citation9]. The increased visceral fat mass that constitutes a source of cytokines induces a state of low-grade inflammation leading to multiple metabolic disorders characterized by insulin resistance [Citation10], and cytokine storm induced by SARS-CoV-2 infection superimposes these metabolic disorders, thus forming a trap for obese COVID patients leading to increased disease severity [Citation11]. Moreover, ACE2, which is highly expressed in both endocrine and exocrine pancreatic tissues, allows SARS-CoV-2 to infect the pancreas leading to pancreatic damage with subsequent impairment of insulin secretion [Citation12].
1.1. Objectives
The current study aimed to evaluate the impact of at-admission hyperglycemia and its management on the outcome of COVID-19 patients admitted to quarantine hospitals.
1.2. Design
This is a prospective comparative multicenter study.
1.3. Setting
Anesthesia and ICU Departments, Faculty of Medicine, Tanta University.
1.4. Methods
All patients admitted to the quarantine hospitals at Al-Gharbia governorate with COVID-19 diagnosis that was confirmed by having positive nasopharyngeal swab for SAR-CoV-19 virus using PCR were vulnerable to the evaluation for enrolment criteria. The study protocol was approved by the Local Ethical Committee, according to instructions of the Ministry of Health, and written informed consents were signed by all enrolled patients to undergo the assigned assessment and treatment protocols.
Using the strict personal protective equipment, patients were clinically examined for the collection of the demographic data, age, sex, weight, and height for the calculation of body mass index (BMI in kg/m2) as weight (kg)/height (m2) [Citation13], history, especially taking past and/or present history of diabetes mellitus (DM), hypertension, cardiac diseases, hypercoagulability events, chest, liver or kidney diseases or endocrinopathy. The chest was examined clinically and CT imaging was performed to assess the disease severity. Blood samples were obtained for random blood glucose (RBG) estimation, other routine lab works, and special COVID-related investigations.
1.5. Clinical parameters
1.5.1. COVID-19 disease severity grading
Patients were categorized according to the guidelines of the National Institutes of Health (NIH) into the following COVID-severity categories [Citation14]:
The asymptomatic or pre-symptomatic infection category included individuals who had no symptoms that are consistent with COVID-19.
The mild illness category included individuals who had any of the signs and symptoms of COVID-19 but were free of shortness of breath, dyspnea, or abnormal chest imaging.
Moderate illness category included individuals who had evidence of lower respiratory disease during clinical assessment or imaging, but their oxygen saturation of oxygen (SpO2) of ≥94% on room air at sea level.
Severe illness category included individuals who had SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, respiratory frequency >30 breaths/min, or lung infiltrates >50%.
The critical Illness category included individuals who had or developed respiratory failure, septic shock, and/or multiple organ dysfunction.
1.5.2. Diagnosis of in-hospital hyperglycemia
According to the American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control, hyperglycemia that occurs during acute illness in previously normoglycemic patients was termed as “stress hyperglycemia” and was defined as any BG value >140 mg/dl, and if it was persistently >140 mg/dl, treatment was indicated [Citation15]. Blood samples were obtained at admission and 6 hr thereafter and put in a tube containing sodium fluoride (2 mg sodium fluoride/ml blood) to prevent glycolysis until the estimation of RBG levels using the glucose oxidase method [Citation16].
1.5.3. COVID-GRAM Critical Illness Risk score
The risk for progression to critical COVID-19 illness that was defined by Liang et al. [Citation17] as admission to the ICU, a need for invasive mechanical ventilation (IMV), or death was evaluated by the calculation of the COVID-GRAM Critical Illness Risk (CG-CIR) score that can be computed using the following formula: CG-CIR score = (X-ray abnormality × 27.1464) + (age × 0.6139) + (hemoptysis × 33.6210) + (dyspnea × 14.0569) + (unconsciousness × 34.4617) + (number of comorbidities × 10.3826) + (cancer history × 31.2211) + (neutrophil/lymphocyte ratio [N/L] × 1.25)+ (lactate dehydrogenase [LDH] × 0.0534) + (direct bilirubin × 3.0605). Age, number of comorbidities, N/L, LDH, and direct bilirubin are continuous variables, and the others are categorical variables and were scored by 1 if positive and 0 if negative. COVID-GRAM score provided both the numerical risk score and the percentage of probability for progress to critical illness.
1.6. Exclusion criteria
The exclusion criteria include patients of critical COVID-19 disease severity, admission to ICU at the time of hospital attendance for any indication, presence of a history of DM, endocrinopathy causing disturbed glucose homeostasis, preexisting autoimmune diseases, current maintenance on immunosuppressive drugs, malignancy elsewhere in body organs, refusal to sign the written consent to receive the study protocol, or death just on admission or before completion of the diagnostic protocol.
1.7. Inclusion criteria
Patients with confirmed COVID-19 disease of mild-to-severe NIH disease severity grade and at-admission RBG > 140 mg/dl, in patients who documented that they were previously normoglycemic and free of exclusion criteria, were included in the study.
1.8. Study protocol
All COVID-19 patients with at-admission RBG > 140 mg/dl were admitted to the intermediate care unit and started their appropriate therapy for manifestations other than hyperglycemia and were assured psychologically. After 6 hr, RBG was re-estimated, and patients who had blood glucose >140 mg/dl on two estimations 6 hr apart without glucose infusion or hypoglycemic therapy were considered as persistent hyperglycemic (PHG group) and patients who had subsidence of their stress hyperglycemia with 6-hr RBG < 140 mg/dl were considered as stress hyperglycemic (SHG group). Patients who were included in the SHG group received COVID treatment protocol without hypoglycemic therapy, while patients in the PHG group received intensive insulin therapy (IIT) in addition to the COVID treatment protocol. For all patients, fluid therapy was provided as Lactated Ringer’s solution 500 ml/4–6 hr to maintain a urine output of 0.1 ml/min.
1.9. Intensive insulin therapy protocol
Preparation: 50 IU of Actrapid (HM, Novo Nordisk) in 50 ml of 0.9% saline solution was delivered through a 50-ml syringe-driven pump.
Monitoring: The insulin dose was adjusted to whole-blood glucose levels, which were measured hourly or 2-hourly using capillary blood samples and a glucometer. After reaching a BG level in a range of 80–110 mg, BG measurements were conducted every 4 hr.
Dose titration schedule: According to Leuven titration protocol [Citation18], if BG was >110–140 mg/dl, the starting dose was 2 IU/hr, but if it was >140 mg/dl, the starting dose was 4 IU/hr. If the next BG was still in a range of 110–140 mg/dl, the infusion rate was increased by 1 IU/hr, while if BG approached the normal range, the infusion rate was reduced by 0.1–0.5 IU/hr, and the rate that adjusted BG within the normal range was maintained. If BG falls by >50% of baseline, the infusion rate was reduced by 50%, but if BG was in a range of 40–60 mg/dl, infusion was stopped, and if BG dropped to <40 mg/dl glucose, 10 gm boluses were given.
1.10. Study outcomes
The primary outcome for both groups is the incidence of progress to critical illness as judged by the CG-CIR score and percentage of probability of critical illness
Secondary outcomes include:
The relation between outcomes and at-admission RBG levels.
Evaluation of the relation between at-admission RBG and COVID-induced inflammatory markers.
Evaluation of the predictability of at-admission variables for the incidence of critical illness and survival outcome.
Evaluation of the prognostic value of the application of IIT protocol for such patients.
1.11. Statistical analysis
Obtained data were presented as mean, standard deviation, numbers, and percentages. Results were analyzed using one-way ANOVA for analysis of variance between groups and Chi-square test (X2 test) for analysis of non-numeric data. Spearman’s correlation analysis was applied to evaluate correlations between at-admission variables and outcomes. The receiver operating characteristic (ROC) curve was used to determine the predictors of outcomes among the correlated at-admission variables. The automatic linear modeling analysis was used to determine the importance of the variables for the prediction of outcomes. Statistical analysis was conducted using IBM® SPSS® Statistics (Version 22, 2015; Armonk, USA) for Windows statistical package. p-Value <0.05 was considered statistically significant.
2. Results
During the study duration from June 2020 to March 2021, 415 patients with confirmed SAR-CoV-19 virus infection and had at-admission RBG >140 mg/dl were admitted to the quarantine hospitals. Fifty-one patients were excluded for not fulfilling the inclusion criteria, and 364 patients were enrolled in the study. At 6 hr after admission, RBG was persistent >140 mg/dl in 123 patients (33.8%) who were collected as PHG group, while 241 patients (66.2%) had decreased BG levels and were collected as SHG group (). Based on the guidelines of NIH, 176 patients (48.3%) had mild, 109 patients had moderate, and 79 patients had severe COVID disease. Patients’ distribution according to NIH severity grades showed a non-significant (p = 0.743) difference between the two groups. There were non-significant differences between patients’ data collected at the time of admission as shown in .
Table 1. At-admission demographic, clinical, and laboratory data of patients of both groups
During the hospital stay, 138 patients (37.9%) had progressed to critical grade; 102 and 36 patients in the SHG and PHG groups, respectively, with significantly (p = 0.015) lower incidence of critical illness among patients in the PHG group. The incidence of critical illness was significantly lower among patients in the PHG group who had at-admission moderate (p = 0.01) and severe (p = 0.0026) disease but was non-significantly (p = 0.832) lower among patients who had at-admission mild disease grade in comparison to corresponding patients in the SHG group. Unfortunately, 58 patients deceased among those who progressed to critical disease (42%) for a total mortality rate (MR) of 15.9%. MR among critical patients in the PHG group was significantly (p = 0.043) lower in comparison to that of critical patients in the SHG group. MR among patients who had at-admission severe COVID and progressed to critical grade was significantly (p = 0.033) lower in the PHG group than in the SHG group, while the difference was non-significant between patients with mild (p = 0.287) and moderate (p = 0.469) at-admission disease severity and progressed to critical illness (, ).
Table 2. Outcome data of patients of both groups
Spearman’s correlation analysis showed that at-admission RBG was positively correlated with BMI, at-admission CRP and IL-6 serum levels, and incidence of critical illness and MR. Patients’ outcomes as the incidence of progress to critical disease and mortality were significantly correlated with high at-admission CG-CIS scoring system and serum levels of CRP and IL-6 but showed negative significant correlation with the application of IIT protocol ().
Table 3. Spearman’s correlation analysis of patients’ outcomes and at-admission data and insulin therapy for the management of at-admission hyperglycemia
ROC curve analysis for at-admission variables as predictors for patients’ outcomes defined high CG-CIS risk percentage, at-admission RBG, and CRP as significant predictors with high positive predictive value, while application of IIT is a significant negative predictor with high negative predictive value for progress to critical disease grade () and/or mortality (, ).
Figure 2. ROC curve analysis of at-admission variables as predictors for progress to critical illness.
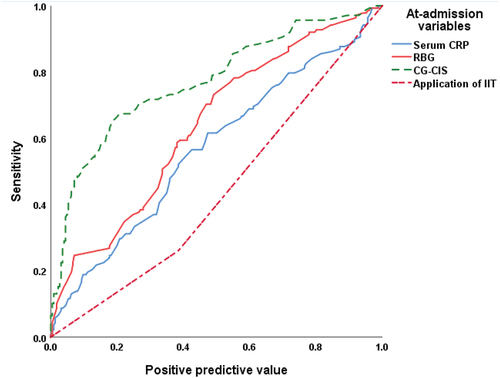
Figure 3. ROC curve analysis of at-admission variables as predictors for mortality of patients who had progressed to critical illness.
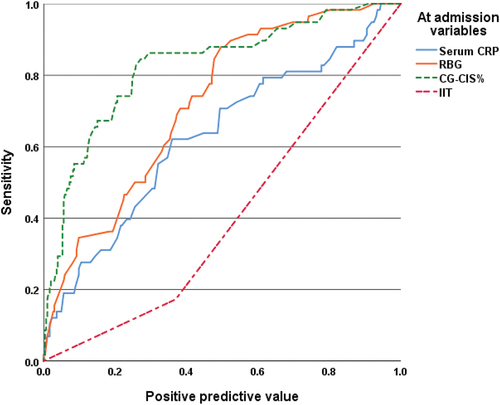
Table 4. ROC curve analysis of at-admission data and insulin therapy for the management of at-admission hyperglycemia for prediction of patients’ outcomes
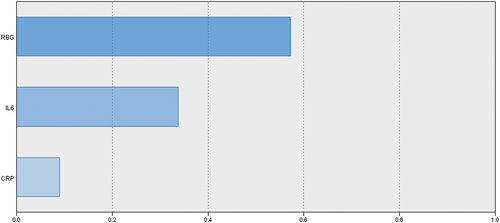
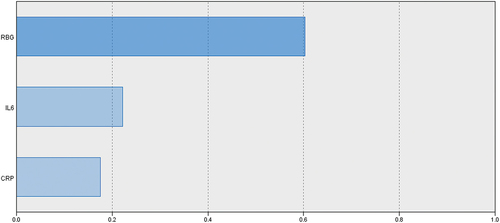
Evaluation of the ability of at-admission RBG and COVID-induced inflammatory markers to predict oncoming progress to critical illness or mortality using the automatic linear modeling analysis defined at-admission hyperglycemia as manifested by RBG as the most important predictor for progress to critical illness and mortality by 57% and 60%, respectively, IL-6 by 34% and 22%, respectively, and lastly, CRP by 9% and 18%, respectively (). The Mentel-Haenszel statistic defined the application of IIT as a significant (p = 0.005) independent predictor for survival outcome of critical COVID patients with at-admission hyperglycemia, with an odds ratio range of 0.173–0.731.
3. Discussion
At-admission RBG estimated levels detected hyperglycemia (RBG > 140 mg/dl) in 364 COVID-19 non-diabetic patients; 123 patients (33.8%) had PHG up to 6 hr after admission and received IIT. Similarly, Montefusco et al. [Citation19] found that about 46% of 551 hospitalized non-diabetic COVID-19 patients were hyperglycemic, and Gómez et al. [Citation20], in a small series of COVID-19 patients, detected an incidence of at-admission hyperglycemia of 26.7%. Also, Ilias et al. [Citation21] documented that a substantial number of patients with and without diabetes had admission hyperglycemia, and critically patients had compromised insulin secretion and tissue sensitivity to insulin.
Estimation of blood glucose is not included in the routine investigations for evaluation of COVID disease severity unless the patient was diabetic; thus, these 123 patients were to be overlooked and may be exposed to disease progression or to the development of complications secondary to the uncontrolled hyperglycemia without noticeable explanation for deterioration, which is mostly secondary to a missed diagnosis of their hyperglycemia. In support of this, irrespective of being persistent or not, 138 patients with at-admission hyperglycemia progressed to critical illness and 58 patients had deceased. In the line of the association between hyperglycemia and worsening of the outcome of COVID-19 patients, Lopez-Huamanrayme et al. [Citation22] detected an association between diabetic control and reduced mortality of diabetic COVID patients, and Lazzeri et al. [Citation23] documented that mortality of COVID-related ARDS patients admitted to ICU with hyperglycemia is more common, especially in patients who showed a significantly higher glucose variability in the first 48 hr since ICU admission.
The applied protocol of IIT for patients who had PHG significantly decreased both the incidence of critical illness and the mortality in comparison to those who had decreased BG levels at 6hr after admission, but were hyperglycemic at admission. In support of the efficacy of ITT, Gómez et al. [Citation20] found that basal-bolus insulin regimen was safe and effective in achieving inpatient glycemic control in most patients with COVID-19 with significantly lower mortality between patients who achieved the targeted BG level and those who did not properly respond. Also, Rajpal et al. [Citation24] recommended safe but stringent control of BG with the use of insulin and frequent monitoring of BG levels once COVID-19 infection occurs, and this policy could potentially serve to decrease the disease severity, and Lazzeri et al. [Citation23] assured the clinical significance of in-ICU glucose strict control to improve the outcome of severe COVID patients. Moreover, Lopez-Huamanrayme et al. [Citation22] found that early and continuous use of the sliding scale insulin therapy was associated with higher mortality in comparison to the fixed-dose insulin regimens.
The obtained results indicated that at-admission hyperglycemia, even if BG had decreased, is an alarm for the possibility of progression and must be managed or even screened for and observed. Similarly, Carrasco-Sánchez et al. [Citation25] recommended screening and early treatment of hyperglycemia in non-diabetic patients who were hospitalized with COVID-19.
In support of this finding, the current study also detected a positive significant correlation between at-admission RBG levels and COVID severity markers, especially inflammatory markers, and regression analyses defined at-admission RBG as an independent predictor for risk of progression to a critical stage and mortality of these critical patients. These results coincided with Fadini et al. [Citation26], who documented that newly diagnosed diabetes and admission hyperglycemia are powerful predictors of COVID-19 severity due to rapid respiratory deterioration. Also, Zhang et al. [Citation27] found that hyperglycemia was positively correlated with higher inflammation levels and more severe illness, and it is a risk factor for the increased severity of COVID-19 disease, and Carrasco-Sánchez et al. [Citation25] documented that admission hyperglycemia is a stronger and independent risk factor for mortality in COVID-19. Moreover, Montefusco et al. [Citation19] detected altered glycometabolic control, with insulin resistance and an abnormal cytokine profile in hyperglycemic, as well as in normoglycemic, COVID-19 patients and found that these glycemic abnormalities extended for at least 2 months after recovery of these patients.
These data spotlighted on the association between SARS-CoV-19 infection and the development of hyperglycemia with varying severity and indicated that the SARS-CoV-19 virus is diabetogenic. In support of this assumption, Lee et al. [Citation28] documented that severe hyperglycemia, including diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome, and new-onset diabetes mellitus may be triggered by COVID-19 vaccination, despite being either attenuated or dead virus.
Three mechanisms were supposed for the diabetogenic effect of SARS-CoV-19 infection: direct viral entry through several receptors in the β-cells leads to direct β-cell dysfunction and apoptosis or initiation of β-cell autoimmunity [Citation29]; viral entry through infecting viral receptor-expressing pancreatic cells leads to their structural and functional transformation with local inflammation and generation of a pro-diabetic milieu that can perturb the integrity of neighboring non-infected β-cells in a paracrine fashion and potentially leads to β-cell loss or dysfunction [Citation5]; and induction of insulin resistance through targeting putative viral receptor-expressing cells in metabolic organs such as liver and adipose tissue causes loss of disease-tolerance mechanisms, metabolic derangement, and maladaptive functions with induction of systemic inflammation and accumulation of prediabetic metabolites [Citation10].
As another explanation for the relation between COVID-19 disease and hyperglycemia, Lima-Martínez et al. [Citation30] supposed a bidirectional relation, where chronic hyperglycemia can compromise innate and humoral immunity inducing a low-grade chronic inflammatory state that favors the development of an exaggerated inflammatory response with subsequently increased liability to get infection and appearance of ARDS; on the other side, SARS-CoV-2 was found to cause direct damage to the pancreas with subsequent induction of, or worsening of, already-present hyperglycemia up to induction of new-onset diabetes in previously non-diabetic subjects.
4. Conclusion
Admission hyperglycemia worsens the outcomes of non-diabetic COVID patients admitted to quarantine hospitals. The deleterious effect of hyperglycemia is positively correlated with at-admission RBG. IIT is a safe and effective management policy and is associated with improved outcomes.
4.1. Recommendations
The inclusion of RBG estimation in the basic severity evaluation investigations is mandatory. Insulin therapy must be applied for all patients admitted with hyperglycemia, even if blood glucose concentration decreased spontaneously. Considering the diabetogenic effect of the SARS-CoV-19 virus, regular estimation of blood glucose is advocated for all COVID-19 patients, irrespective of its severity.
4.2. Limitation
Regular estimations of blood glucose were, unfortunately, not routine in the quarantine hospitals, so the diabetogenic effect of the virus could not be documented. Also, follow-up blood glucose estimation was not applied for follow-up of COVID patients, despite its importance.
List of Abbreviation
Acknowledgments
The authors thank the effort provided by the staff members of the quarantine hospitals included in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Liu D, Tang Y, Zhang Q. Admission Hyperglycemia Predicts Long-Term Mortality in Critically Ill Patients With Subarachnoid Hemorrhage: a Retrospective Analysis of the MIMIC-III Database. Front Neurol. 2021 Oct 5;12: 678998.
- Viurcos-Sanabria R, Escobedo G. Immunometabolic bases of type 2 diabetes in the severity of COVID-19. World J Diabetes. 2021 Jul 15;12(7):1026–1041.
- Zeitoun M, Abdel-Rahim A, Hasanin M, et al. A prospective randomized trial comparing computerized columnar insulin dosing chart (the Atlanta protocol) versus the joint British diabetes societies for inpatient care protocol in management of hyperglycemia in patients with acute coronary syndrome admitted to cardiac care unit in Alexandria. Egypt. Diabetes Metab Syndr. 2021 May-Jun;15(3):711–718.
- Azab M, Hasaneen S, Hanifa H, et al. Optic neuritis post-COVID-19 infection. A case report with meta-analysis. Interdiscip Neurosurg. Dec 2021;26:101320.
- Geravandi S, Mahmoudi-aznaveh A, Azizi Z, et al. SARS-CoV-2 and pancreas: a potential pathological interaction? Trends Endocrinol Metab. 2021 Nov;32(11):842–845.
- Vranić L, Radovan A, Poropat G, et al. Non-Alcoholic Fatty Liver Disease and COVID-19-Two Pandemics Hitting at the Same Time. Medicina (Kaunas). 2021 Oct 3;57(10):1057.
- Hammoudeh S, Hammoudeh A, Bhamidimarri P, et al. Insight into molecular mechanisms underlying hepatic dysfunction in severe COVID-19 patients using systems biology. World J Gastroenterol. 2021 Jun 7;27(21):2850–2870.
- Gómez-Zorita S, Milton-Laskibar I, García-Arellano L, et al. An Overview of Adipose Tissue ACE2 Modulation by Diet and Obesity. Potential Implications in COVID-19 Infection and Severity. Int J Mol Sci. 2021 Jul 26;22(15):7975.
- De Ligt M, Hesselink M, Jorgensen J, et al. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte. 2021 Dec;10(1):408–411.
- Reiterer M, Rajan M, Gómez-Banoy N, et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021 Sep 16;33(11):2174–2188.e5.
- Gammone MA, D’Orazio N. COVID-19 and Obesity: overlapping of Two Pandemics. Obes Facts. 2021 Sep 24; 1–7. 10.1159/000518386.
- Al-Kuraishy H, Al-Gareeb A, Alblihed M, et al. COVID-19 in Relation to Hyperglycemia and Diabetes Mellitus. Front Cardiovasc Med. 2021 May 20;8: 644095.
- Bray GA. Pathophysiology of obesity. Am J Clin Nutr. 1992;55(2):488S–94S.
- https://www.google.com/search?q=severity+of+covid+19&oq=severit&aqs=chrome.3.0i457j0j69i57j0l2.5858j0j4&client=ms-android-samsung-gn-rev1&sourceid=chrome-mobile&ie=UTF-8
- Moghissi E, Korytkowski M, DiNardo M, et al. American Association of Clinical Endocrinologists, American Diabetes Association: american Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009 Jun;32(6):1119–1131.
- Tinder P. Determination of blood glucose. Ann Clin Biochem. 1969;6:24.
- Liang W, Liang H, Ou L, et al. China Medical Treatment Expert Group for COVID-19: development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern Med. 2020 Aug 1;180(8):1081–1089.
- Van den Berghe G, Wouters P, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003 Feb;31(2):359–366.
- Montefusco L, Nasr M, D’Addio F, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021 Jun;3(6):774–785.
- Gómez A, Henao D, Muñoz O, et al. Glycemic control metrics using flash glucose monitoring and hospital complications in patients with COVID-19. Diabetes Metab Syndr. 2021 Mar-Apr;15(2):499–503.
- Ilias I, Diamantopoulos A, Pratikaki M, et al. Dimopoulou I: glycemia, Beta-Cell Function and Sensitivity to Insulin in Mildly to Critically Ill Covid-19 Patients. Medicina (Kaunas). 2021 Jan 14;57(1):68.
- Lopez-Huamanrayme E, Garate-Chirinos D, Espinoza-Morales F, et al. Association between hyperglycemia treatment and mortality in patients with diabetes and COVID-19 in a Peruvian hospital: a retrospective cohort study. J Clin Transl Endocrinol. 2021Dec;26:100265.
- Lazzeri C, Bonizzoli M, Batacchi S, et al. The prognostic role of hyperglycemia and glucose variability in covid-related acute respiratory distress Syndrome. Diabetes Res Clin Pract. 2021May;175:108789.
- Rajpal A, Rahimi L, Ismail-Beigi F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J Diabetes. 2020 Dec;12(12):895–908.
- Carrasco-Sánchez F, López-Carmona M, Martínez-Marcos F, et al. SEMI-COVID-19 Network: admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med. 2021 Dec;53(1):103–116.
- Fadini G, Morieri M, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020Oct;168:108374.
- Zhang W, Li C, Xu Y, et al. Hyperglycemia and Correlated High Levels of Inflammation Have a Positive Relationship with the Severity of Coronavirus Disease 2019. Mediators Inflamm. 2021 Mar 18;2021: 8812304.
- Lee H, Sajan A, Tomer Y. Hyperglycemic Emergencies Associated With COVID-19 Vaccination: a Case Series and Discussion. J Endocr Soc. 2021 Sep 25;5(11):bvab141.
- Steenblock C, Richter S, Berger I, et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat Commun. 2021 Jun 10;12(1):3534.
- Lima-Martínez M, Boada C, Madera-Silva M, et al. COVID-19 and diabetes: a bidirectional relationship. Clin Investig Arterioscler. 2020 May-Jun;33(3):151–157.