ABSTRACT
Background
The aim of this study was to evaluate the role of donor-to-recipient gender match in the setting of living donor liver transplant as a potential predictor of graft and recipient survivals.
Methods
In this retrospective, single-center study, the data of 342 adult primary liver transplants were analyzed. The donor and recipient’s characters, intraoperative data, and postoperative outcomes were recorded.
Results
The donor-recipient gender matched patients had significantly better graft survival outcomes than the gender mismatched ones. The 2-year graft survival probability was 95.7% in the matched group compared to 89.1% in the mismatched one (p = 0.026). A female donor-male recipient combination (87.3%) showed worse 2-years graft survival than a male-to-male transplant (94.8%), while it gave better graft survival than male to female (61.3%). The estimated relative risk of graft rejection was 5.91 times significantly higher in a male–female combination than in a male-to-male one (Hazard ratio = 5.91, 95% CI = 1.34–26.11).
Conclusion
This study suggests that donor-recipient gender mismatch is associated with poor liver graft survival outcomes, with higher risk of graft rejection in male-female transplants than in male-to-male ones. Though, further larger studies including multiple datasets are needed, with adjustments for various graft, donor, and recipient factors to reach solid evidence.
1. Introduction
Liver transplantation (LT) represents the gold-standard treatment for end-stage liver diseases and fulminant hepatic failure. Living donor liver transplantation (LDLT) has decreased the waiting time and helped to optimize the timing of the surgery. However, it is associated with high risk of postoperative complications [Citation1].
It is essential to improve the donors’ selection criteria to ensure the success of this surgical procedure [Citation2]. The effect of the donor-recipient gender match on the post-transplant outcomes in terms of graft and patient survival is largely controversial. Earlier mono-centric studies reported a significant association between donor gender and the surgery outcomes, with lower rates of patient and graft survival following liver transplantation in male recipients from female donors [Citation3–5]. Further, a systemic review and meta-analysis on the prognostic role of gender mismatch on the postoperative graft loss has concluded a detrimental effect of gender mismatch, with the poorer outcomes in the female-to-male combination [Citation6]. Alternatively, a large study used data of the European Transplant Registry and based on 16,410 LT subjects did not find any significant differences in the survival outcomes of the gender discordant versus concordant transplants [Citation7]. Moreover, the developed quantitative donor risk scores that identify the best donor-to-recipient matching outcomes did not show donor gender as a risk factor for poor graft survival [Citation8,Citation9].
The observed inconsistency in the literature concerning the prognostic role of the donor-recipient gender matches necessitate more observational cohort studies. Therefore, the aim of this study was to evaluate the role of donor-to-recipient gender match in the setting of living donor liver transplant as a potential predictor of graft and recipient survivals.
2. Methods
2.1. Study design, settings, and ethical considerations
This retrospective cohort study was conducted at Ain Shams University Specialized Hospital, Cairo, Egypt. The study was conducted according to the World Medical Association Declaration of Helsinki, after the approval of the local Research Ethics This study was approved by the Ethics Committee of the Faculty of Medicine, Ain Shams University, Egypt (approval number: IRB/0006379). Confidentiality of patients’ data was maintained by assigning a code number to each patient. Patients were not required to give informed consent for this study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
2.2. Eligibility criteria
We included adult Egyptian patients, aged 18-year-old or older who underwent LDLT for the first time. Patients who underwent re-transplantation before discharge from the intensive care unit (ICU) after the first liver transplant, those who developed cardiac arrest either intraoperative or within the first 6 hours postoperative, massive blood loss, or have pre-existing renal failure requiring hemodialysis or continuous hemofiltration were excluded.
2.3. Data collection
Data of eligible patients presenting between 2008 and 2017 were collected from the hospital patients’ medical records. Intraoperatively, both standard anesthetic and transplantation were performed by the same anesthesia and surgical team. At the end of surgery, patients were transferred to the ICU where they were monitored and received the standard protocol for postoperative management after liver transplantation.
Demographic data of the donors and recipients, clinical and intraoperative data of the recipients including medical history, Model for End-Stage Liver Disease (MELD) score, intraoperative packed red blood cells transfusion, duration of cold and warm ischemia, graft weight to recipient weight ratio, the duration of ICU and hospital stay were recorded. The primary outcome was the graft survival at 2 years post-liver transplant according to gender mismatches (Female-Male and Male-Female) versus matches (Male–Male and Female–Female). The secondary outcome were the recipient death rate and survival analysis according to the recipient-donor gender matching.
The MELD score was calculated using serum bilirubin, serum creatinine, and International Normalized Ratio (INR) and was given by the formula: 9.57 × loge (creatinine) + 3.78 × loge (total bilirubin) + 11.2 × loge (INR) + 6.43 [Citation10].
Recipients were divided into two groups; donor-to-recipient gender matched (Male–Male and Female–Female), and mismatched (Female-Male and Male-Female). Further, they were sub-divided into 4 gender match and mismatch subgroups as follows: Female to Male (F-M), Female to Female (F-F), Male to Female (M-F), and Male to Male (M-M).
2.4. Statistical analysis
Statistical analysis was conducted using the Statistical Package for Social Sciences (IBM SPSS Statistics) for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). Categorical data were represented as numbers and frequencies. A chi–square test or Fishers’ Exact Test as appropriate was performed to investigate the association between the categorical variables. For continuous data, they were tested for normality by the Shapiro–Wilk test. Normally distributed data were expressed as mean± SD and were analyzed by the Independent T-test. On the other hand, not normally distributed data were presented as the median and range or inter quartile ratio and the Mann–Whitney U test was applied. Patient and graft survival curves were generated using Kaplan–Meier survival analysis method and comparison was done by Log-rank test. P-value less than 0.05 was considered statistically significant.
3. Results
This study included 342 adult Egyptian patients who underwent living donor liver transplant for the first time. The gender matched (male–male or female–female) group constituted 62.6%, while the gender mismatched (male-female or female-male) group represented 37.4% (). The highest percent of the recipients in both groups were males (92.1% and 73.4%, respectively), with a significant difference (p < 0.001). The mean age of the recipients in the matched group was 50.0 ± 8.5 years, compared with 49.0 ± 9.5 years in the mismatched group, with no significant difference (p = 0.317). The mean BMI of the recipients was comparable in both groups (p = 0.355). Concerning data of the donors, the percent of males (92.5%) was significantly higher among the matched group, whereas females represented the highest percent (73.4%) of the mismatched group (p < 0.001). The mean ages and BMI of the donors were homogenously distributed among the studied groups (p > 0.05) as shown in .
Table 1. Baseline demographic characteristics of the recipients and donors
Clinical and operative characteristics of the gender matched, and mismatched groups were illustrated in . Diabetes mellitus alone or in combination with hypertension and ischemic heart disease were the most frequent comorbidities in both groups, with no significant difference (p = 0.858). The most commonly recorded diagnosis included Hepatitis C virus, Hepatitis B virus, Hepatocellular carcinoma, cryptogenic, and portal vein thrombosis. The frequency of hepatocellular carcinoma was significantly higher in the matched (43.5%) than the mismatched (31.3%) groups, and the same was observed for cryptogenic (14.5% versus 4.7%, p = 0.005). The median MELD score was 15.0 (13.0–18.0) in the matched compared with 16.0 (12.5–18.0) in the mismatched, with no significant difference. The need for intraoperative packed red blood cells and the median of the administered amount were non-significantly different among both groups (p > 0.05). Furthermore, the medians of the cold and warm ischemia times as well as the graft weight to recipient weight ratio were similar in both groups, with no significant differences (p > 0.05).
Table 2. Clinical and operative characteristics of the gender matched and mismatched groups
demonstrates that medical causes of graft failure was significantly lower in the matched (7.9%) than the mismatched (15.6%) groups, p = 0.027. Alternatively, the rate of surgical/vascular graft failure was more frequent in the matched (22.0%) compared to the mismatched (10.9%) groups, with a significant difference (p = 0.010). Hepatic artery thrombosis was the most frequent cause in both groups (11.7% and 5.5%, respectively), followed by portal vein thrombosis (3.3% and 1.6%respectively). The death rate was non-significantly different between the matched and mismatched groups (36.4% versus 41.4%, p = 0.361). The median length of both the ICU and the hospital stays was non-significantly different (p > 0.05).
Table 3. Outcomes of the gender matched and mismatched groups
and illustrate medical and surgical graft survival analysis according to the recipient-donor gender matching. The mean graft survival time (time from transplantation till acute or chronic graft rejection was significantly (p = 0.026) longer in the matched group than the mismatched group (119.21 vs105.40 months, respectively). The 2-year graft survival probability was 95.7 ± 0.017% in the matched group compared to 89.1 ± 0.034% in the mismatched one. The estimated relative risk of graft rejection was 2.14 (95%CI = 1.09–4.18) times significantly greater in the mismatched group compared to the matched one. The mean time from transplantation till occurrence of surgical/vascular graft failure was 101.39 months (95% CI = 92.94–109.85) in the matched group compared to 115.60 months (95% CI = 106.72–124.48) in the mismatched one, p = 0.023. The estimated relative risk of surgical/vascular graft failure was non-significantly lower in the mismatched group (hazard ratio = 0.547, 95% CI = 0.324–0.922).
Figure 2. Kaplan-Meier survival analysis curves for graft rejection according to the recipient-donor gender matching.
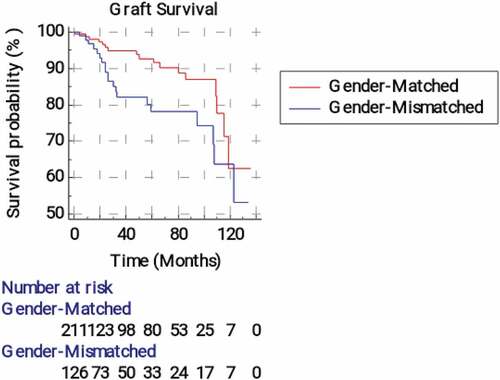
Figure 3. Kaplan-Meier survival analysis curves for surgical graft failure according to the recipient-donor gender matching.
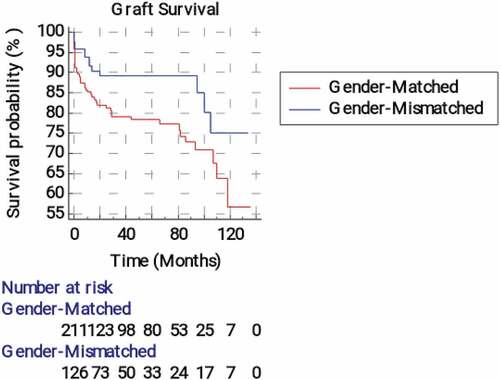
Table 4. Medical and surgical graft survival analysis according to the recipient-donor gender matching
and show significant differences between survival curves in the four donor-recipient gender matched and mismatched groups (p > 0.001). A male donor-male recipient combination showed the best 2-year graft survival (94.8%), followed by a female donor-male recipient one (87.3%), whereas male-female combination showed the worst (61.3%) outcomes. The estimated relative risk of graft rejection was 5.91 times significantly higher in male-female combination in comparison to a male–male one (Hazard ratio = 5.91, 95% CI = 1.34–26.11). Alternatively, there were no significant differences of the survival curves between the 4 groups regarding graft failure due to surgical causes (p = 0.123).
Figure 4. Kaplan-Meier survival analysis curves for medical graft failure according to the four recipient-donor gender matches.
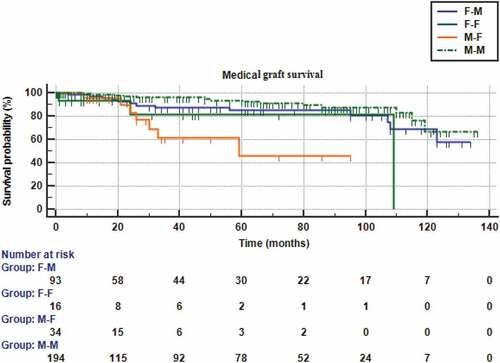
Figure 5. Kaplan-Meier survival analysis curves for surgical graft failure according to the four recipient-donor gender matches.
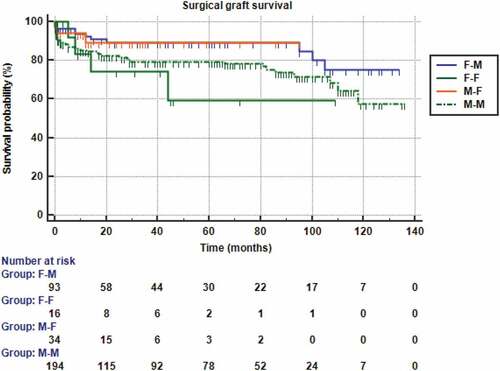
Table 5. Medical and surgical graft survival analysis according to the four subgroups of recipient-donor gender matches
and show that the mean recipient survival time and the survival probability was non-significantly higher in the matched than the mismatched groups (p = 0.374). The estimated relative risk of the patients’ death was non-significantly 1.17 (95% CI = 0.822–1.68) higher in the mismatched group than the matched one. Further, and also revealed non-significant differences between F-M, F-F, M-F, and M-M groups regarding the recipient survival time and the survival probability (p = 0.387).
Figure 6. Kaplan-Meier survival analysis for overall patients’ survival according to the recipient-donor gender matching.
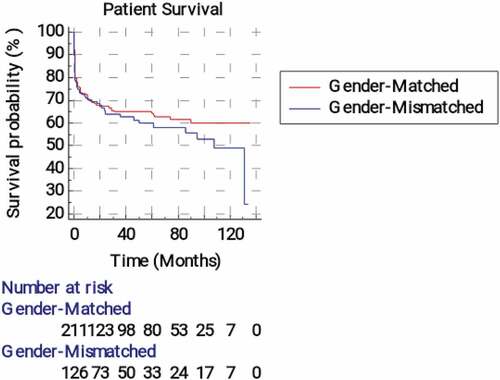
Figure 7. Kaplan-Meier survival analysis for overall patients’ survival according to the four recipient-donor gender matches.
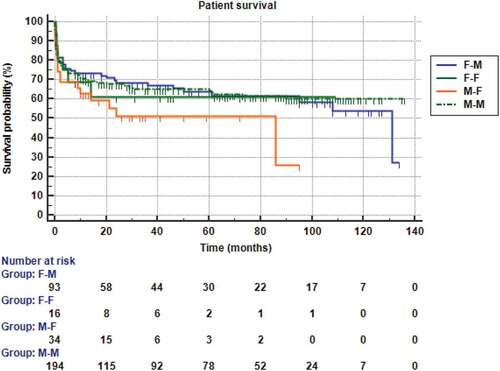
Table 6. Kaplan-Meier recipient survival analysis according to the recipient-donor gender matching
Table 7. Kaplan-Meierrecipient survival analysis according to the four subgroups of recipient-donor gender matches
4. Discussion
A growing literature has highlighted the influence of donor-recipient gender mismatching in LDLT on the postoperative outcomes of liver transplantation [Citation11]. However, there is no solid evidence regarding this aspect [Citation6]. Therefore, the present study evaluated the prognostic role of donor-to-recipient gender match as a potential predictor of graft and recipient survivals.
In the current study, survival analysis demonstrated significantly better graft survival outcomes in the donor-recipient gender matched patients compared to the gender mismatched ones. The 2-year graft survival probability was 95.7% in the matched group compared to 89.1% in the mismatched one. The mean graft survival time was significantly longer in the matched group than the mismatched group (119.21 vs 105.40 months, respectively). The estimated relative risk of graft rejection was 2.14 (95% CI = 1.09–4.18) times significantly greater in the mismatched group compared to the matched one.
Moreover, gender mismatch showed a significant influence on the graft survival times in the four donor-recipient matched and mismatched subgroups. A female donor-male recipient combination (87.3%) showed worse 2-years graft survival than male-to-male transplant (94.8%), while it gave better graft survival than male to female (61.3%). The estimated relative risk of graft rejection was 5.91 times significantly higher in male-female combination than in male to male one.
Our findings indicate poor 2-years graft survival outcomes for the mismatched transplants than the matched ones, with the best results in male to male combination. Interestingly, female to male transplants showed better outcomes than male to female ones. A comparable retrospective study that assessed the 15-year graft survival in 2144 adult primary liver transplant patients reported significantly different survival times in the 4 matched and mismatched gender groups. They found that a male donor-female recipient combination showed the best 15-year graft survival (61.1%), and a female donor-male recipient combination showed the worst graft survival (48.6%) [Citation12]. The contradictory findings of the previous study with ours might be attributed to their assessment of long-term survival (15 years), where the differences in survival curves are not related to the early effects of donor quality, but mainly because of the primary indication for the liver transplantation as stated by Schoening et al. [Citation12]. A recent study that evaluated 144, 212 first deceased donor liver transplant recipients has highlighted differences in liver graft survival in relation to the donor-recipient sex and age. When donors were females, female recipients ≥45 years had significantly better graft outcomes than males of the same age, while recipients <45 years from male donors displayed higher failure rates in females than males [Citation13].
Croome et al. conducted a single center retrospective cohort study that included 1,042 subjects who underwent primary liver transplantation to evaluate the effect of donor and recipient gender discordance on graft survival. They reported a significantly increased hazard (hazard ratio = 2.09) of graft failure in the female donor to male recipient combination; this combination showed the worst graft survival of all combinations even after adjustment for the donor and recipient characters. Further, they demonstrated a higher frequency of primary graft non-function and vascular thrombosis in the F-M combination compared to the gender matched groups [Citation14].
In agreement with our findings, some earlier studies demonstrated a similar impact of the donor-recipient gender mismatch on the graft survival, but they implicated female-to-male transplants as the worst donor-recipient combination. Brooks et al. [Citation4] reviewed the medical records of 994 liver transplant patients, and they found that F-M transplants had a significantly lower (55.9%) 2-year survival than other gender groups (75%). As well, Rustgi et al. [Citation5] demonstrated a statistically significant difference in the rate of graft failure for F-M transplants versus gender matched transplants (12.2% vs 11.3%, p = 0.013) after assessment of the United Network for Organ Sharing data from 1992 to 2000.
The observed higher risk of graft rejection in in M-F transplants than in a M-M one agrees with Tan et al. [Citation15] who detected more frequent development of H‐Y antibodies in female recipients of male kidney grafts than all other gender matching groups. They also linked the presence of H‐Y antibodies to acute graft rejection.
Nevertheless, Zeier et al. [Citation7] analyzed a large database of a collaborative transplant registry, and they demonstrated non-significant differences in liver graft survival in relation to the donor to recipient gender matching, whereas the kidney transplants demonstrated worse survival of both the recipient and the renal graft with female donor, especially with a male recipient.
Several explanations have been proposed to explain the observed worse outcomes with donor recipient mismatch. Hormonal differences between females and males might play a role [Citation16]. Wittnich et al. [Citation17] have argued that poor outcomes of liver grafts from females to males are attributed to the increased ischemic stress response in the female livers. However, several researchers [Citation18,Citation19] suggested that estrogens may have a protective role against ischemic injury and favored post-ischemic biliary repair. Size differences between females and males are another possible explanation. The smaller sizes of livers in females than males carry a greater risk of a small-for-size syndrome, with consequent higher rate of complex vascular and biliary reconstruction and, eventually, longer warm ischemia times during the operation. These possibly affect early graft function and patient survival [Citation20]. Additionally, Gasbarrini et al. [Citation21] suggested the greater susceptibility of female liver grafts to oxidative reperfusion injury at the time of the transplant as a cause of poorer outcomes with female donors.
In the current study, the frequency of surgical/vascular graft failure was significantly higher in the matched (22.0%) than the mismatched (10.9%) groups. Hepatic artery thrombosis was the most frequent cause followed by portal vein thrombosis (11.7% vs 5.5% and 3.3% vs 1.6%, respectively). However, there were no significant differences in the survival curves of the 4 subgroups.
In the present study, donor-to-recipient gender mismatches did not reveal a significant influence on the death rates and survival times of the patients. The recipient death rate was non-significantly different between the matched and mismatched groups (36.4% versus 41.4%, p = 0.361). This finding is in line with Messner et al. [Citation22] who concluded that sex matching lowers the risk of severe postoperative complications; however, it does not increase the risk of pancreas graft or patient survival. An earlier study did not identify donor gender mismatch as a risk factor for higher post-transplant mortality [Citation23].
The recipient’s death rate was 36.4% in the matched group compared with 41.4% in the mismatched group with no significant difference. Furthermore, the total death rate was much higher than the rates of graft rejection due to medical or surgical causes because the causes of death in the studied cohort were diverse and included septic shock, heart failure, chest infection, and pulmonary embolism in addition to the graft rejection.
Limitations: This study was a single-center experience with a retrospective design that carries a potential selection bias and limits generalizability of the results. However, it was not feasible to prospectively allocate the donors and recipients into matched and mismatched groups.
5. Conclusions
This is the first research done on Egyptian patients studying effect of sex on outcome of LDLT. This study suggests that donor-recipient gender mismatch is associated with poor liver graft survival outcomes with a higher risk of graft rejection in the male-to-female transplants than in the male-to-male ones. Though, further larger studies including multiple datasets are needed with adjustments for various graft, donor, and recipient factors to reach solid evidence.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Amer KE, Marwan I. Living donor liver transplantation in Egypt. Hepatobiliary Surg Nutr. 2016;5(2):98–106.
- Hackl C, Schmidt KM, Süsal C, et al. Split liver transplantation: current developments. World J Gastroenterol. 2018;24(47):5312–5321.
- Marino IR, Doyle HR, Aldrighetti L, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22(6):1754–1762.
- Brooks BK, Levy MF, Jennings LW, et al. Influence of donor and recipient gender on the outcome of liver transplantation. Transplantation. 1996;62(12):1784–1787.
- Rustgi VK. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 2002;8(6):514–518.
- Lai Q, Giovanardi F, Melandro F, et al. Donor-to-recipient gender match in liver transplantation: a systematic review and meta-analysis. World J Gastroenterol. 2018;24(20):2203–2210.
- Zeier M, Döhler B, Opelz G, et al. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13(10):2570–2576.
- Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790.
- Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12(10):2789–2796.
- Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50–60.
- Sarkar M, Watt KD, Terrault N, et al. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62(4):946–955.
- Schoening WN, Helbig M, Buescher N, et al. Gender Matches in Liver Transplant Allocation: matched and Mismatched Male-Female Donor-Recipient Combinations; Long-term Follow-up of More Than 2000 Patients at a Single Center. Exp Clin Transplant. 2016;14(2):184–190.
- De Simone AI, Zhang X, Dahhou M, et al. Differences in Liver Graft Survival by Recipient Sex. Transplant Direct. 2020;6(12):e629.
- Croome KP, Segal D, Hernandez-Alejandro R, et al. Female donor to male recipient gender discordance results in inferior graft survival: a prospective study of 1,042 liver transplants. J Hepatobiliary Pancreat Sci. 2014;21(4):269–274.
- Tan JC, Kim JP, Chertow GM, et al. Donor-recipient sex mismatch in kidney transplantation. Gend Med. 2012;9(5):335–47.e2.
- Lehner F, Becker T, Klempnauer J, et al. Gender-incompatible liver transplantation is not a risk factor for patient survival. Liver Int. 2009;29(2):196–202.
- Wittnich C, Belanger MP, Askin N, et al. Lower liver transplant success in females: gender differences in metabolic response to global ischemia. Transplant Proc. 2004;36(5):1485–1488.
- Alvaro D, Mancino MG, Onori P, et al. Estrogens and the pathophysiology of the biliary tree. World J Gastroenterol. 2006;12(22):3537–3545.
- Soria-Jasso LE, Cariño-Cortés R, Muñoz-Pérez VM, et al. Beneficial and Deleterious Effects of Female Sex Hormones, Oral Contraceptives, and Phytoestrogens by Immunomodulation on the Liver. Int J Mol Sci. 2019; 20 (19):4694.
- Yoshizumi T, Taketomi A, Uchiyama H, et al. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14(7):1007–1013.
- Gasbarrini A, Addolorato G, Di Campli C, et al. Gender Affects Reperfusion Injury in Rat Liver. Dig Dis Sci. 2001;46(6):1305–1312.
- Messner F, Etra JW, Haugen CE, et al. Sex matching does not impact the outcome after simultaneous pancreas-kidney transplantation. Clin Transplant. 2019;33(11):e13717.
- Bruns H, Lozanovski VJ, Schultze D, et al. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: a multivariate analysis. PLoS One. 2014;9(6):e98782.