ABSTRACT
Background
Acute severe pre-eclampsia (PE) requires urgent antihypertensive therapy to reduce the incidence of adverse outcome. There is currently very limited evidence for the role of nitric oxide donors as antihypertensive therapy for acute severe PE. The aim of this study was to evaluate the efficiency and tolerability of nitroglycerin compared to labetalol in terms of acute control of blood pressure in severe PE.
Patients and methods
Two hundred patients with severe PE were admitted prepartum to the ICU to stabilize blood pressure. They were randomly assigned to one of two groups; Group N received nitroglycerin intravenous infusion (1 mg/ml) and Group L received labetalol intravenous infusion (10 mg/ml). The starting infusion rate was 5 ml/hr and was titrated to stabilize systolic blood pressure at 130–140 mmHg and diastolic blood pressure at 80–90 mmHg (study end point).
Results
Reduction of blood pressure at 90 minutes to the desired end point was achieved in 96% and 87% of the patients by nitroglycerin and labetalol infusion respectively. Nitroglycerin showed significantly faster control and lower incidence of persistent hypertension. The number of attacks of hypotension was comparable between both the groups. Headache, flushes, and tachycardia were significantly higher in N group compared to the L group, while bradycardia was significantly lower in the N group compared to the L group. Fetal side effects were comparable between the two groups.
Conclusion
Nitroglycerin can be considered an important alternative to labetalol for controlling the blood pressure in acute severe PE.
1. Introduction
Pre‐eclampsia (PE) is one of the most frequent pregnancy complications and is one of the main causes of maternal and fetal morbidity and mortality in its severe form [Citation1]. Prompt control of blood pressure is of crucial importance to avert maternal and fetal complications. There is no consensus on the most appropriate acute antihypertensive therapy. The choice of agents primarily depends on availability of an agent and the experience of the provider [Citation2].
The primary pathophysiology of pre-eclampsia is most likely inadequate placentation, leading to endothelial dysfunction and reduced nitric oxide bioavailability [Citation3]. Therapeutic modalities that can target the underlying pathophysiological changes and reverse the endothelial dysfunction could potentially help to ameliorate the systemic manifestations in patients with severe PE. Nitroglycerin, as nitric oxide donor that targets the underlying molecular processes, represents a potential alternative to conventional acute antihypertensive therapy. Intravenous hydralazine and labetalol as well as oral nifedipine have been considered first line therapy for the management of acute severe hypertension in PE [Citation4]. Previous studies demonstrated that nitroglycerin is an effective and safe alternative therapy to sublingual nifedipine [Citation5] and hydralazine [Citation6] as antihypertensive therapy in the acute management of patients with severe PE. To our knowledge, this is the first randomized controlled trial comparing nitroglycerin and labetalol for acute control of blood pressure in severe PE. The aim of this study was to evaluate the efficiency of controlling blood pressure and tolerability of nitroglycerin compared to labetalol in terms of acute control of blood pressure in severe PE before delivery.
2. Patients and methods
The study was conducted in the intensive care unit (ICU) of the Obstetrics and Gynecology Hospital of Ain Shams University as a randomized, double-blind, and controlled study on patients diagnosed with severe PE from August 2019 to June 2022. The study was approved by Ethical Research Committee at Ain Shams University (ethical number FMASU R 47/2019), clinical trial number (NCT05310929) and all participants provided written informed consent.
Women eligible for this study were aged between 18–40 years old at greater than 34 weeks of gestation having severe PE. Its diagnosis is made in patients with pre-eclampsia who have severe hypertension and/or significant end-organ dysfunction. Severe hypertension was diagnosed by Systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg. Significant end-organ dysfunction was presented by the presence of one or more of the following after exclusion of alternative diagnoses: new-onset cerebral or visual disturbance, severe headache that persists and progresses despite analgesic therapy, serum transaminase concentration >2 times the upper limit of the normal range or severe persistent right upper quadrant or epigastric pain unresponsive to medication, thrombocytopenia <100,000 platelets/microL, serum creatinine >1.1 mg/dL or doubling of the serum creatinine concentration, or pulmonary edema [Citation7]. Exclusion criteria were patients with chronic hypertension, active asthma, or congestive heart failure, or any known allergy to one of the study drugs or life-threatening fetal heartbeat changes.
Before delivery, two hundred patients with severe PE were admitted prepartum to the ICU to stabilize blood pressure. Patients were allocated into two equal groups in a randomized manner using computer-generated number lists and the allocation was concealed by sealed envelope method. Group N received nitroglycerin intravenous infusion (Nitronal, Sunny) in a concentration of 1 mg/ml, thus 1 µg/Kg/min equals to 4.8 ml/hr for an 80 Kg patient. Group L received labetalol intravenous infusion (Trandate, Gsk) in a concentration of 10 mg/ ml, thus 50 mg/ml equals to 5 ml/hr. The starting infusion rate of the antihypertensive medication was 5 ml/hr. The infusion rate was titrated to stabilize systolic blood pressure (SBP) at 130–140 mmHg and diastolic blood pressure (DBP) at 80–90 mmHg (study end point) by adjusting the infusion rate as required either by maintaining the same infusion rate or by changing its infusion rate by 1 ml/hr up or down according to the clinical condition every 10 minutes. On any abrupt reduction in blood pressure below 120 mmHg for SBP or 80 mmHg for DBP, the infusion was immediately discontinued, and a bolus of 150 ml lactate ringer was given. All study drugs were prepared and administered by an independent investigator not involved in the observation.
Patients received full intensive care treatment for PE according to the standard protocol of the ICU of the Obstetrics and Gynecology Hospital of Ain-Shams University. Treatment for PE included infusion of lactated Ringer solution, at a starting rate of 80 ml/hr and then adjusted according to the fluid balance, magnesium sulphate, as seizure prophylaxis and its dose was adjusted according to renal function and its signs of toxicity in addition to antihypertensive medications according to the assigned group. The study solution was stopped if maternal pulse reached less than 60 bpm or raised more than 110 bpm.
Vital signs were monitored at admission and until patients were discharged from the ICU using ECG, pulse oximetry, continuous invasive arterial blood pressure monitoring, and urine output assessment. Fetal heart rate monitoring was done before starting of the study and continuously during the study time by cardiotocography (CTG).
Treatment failure was expressed by severe persistent hypertension. It was considered when SBP was greater than 160 mmHg or DBP was greater than 110 mmHg after the administration of maximum dose of allocated drug treatment: 20 ml/hr. Hence, hydralazine was added as a rescue drug after 2 hours of starting treatment with assigned drugs. After stabilization of the patient and once the target BP was achieved, delivery was conducted.
The following data were recorded and included demographic characteristics, the time taken to control blood pressure (time from starting infusion till reaching study end point), and number of attacks of hypotension (BP values below the study end point) and the incidence of severe persistent hypertension. In addition, side effects of drugs such as tachycardia (HR>100 bpm), bradycardia HR<60 bpm) headache, nausea/vomiting, hypotension, flushing, dizziness were recorded. Fetal outcome as stillbirths and suspected fetal compromise by abnormal CTG were recorded. Fetal distress was manifested in the fetal heart rate tracing as decelerations, loss of beat-to-beat variability, and even bradycardia. 1 min Apgar score less than 7- and 5-minutes Apgar score less than 7 were also recorded.
2.1. Sample size determination
Sample size was calculated using PASS 11.0 sample size calculation program and according to a study carried out by Ali et al. [Citation6], in 2015. 85 patients in each group achieve 80% power in detecting a 15% reduction in systolic blood pressure when the significance level is alpha = 0.05 with two-sided testing showing normally distributed data and a standard deviation of 8 mmHg, so we included 100 patients in each group considering possible dropouts.
2.2. Statistical analysis
The statistical analysis was performed using a standard SPSS software package version 23 (Chicago, IL). Normally distributed numerical data are presented as mean ± SD and differences between groups were compared using the independent Student’s t-test, intragroup difference was compared using paired t-test and categorical variables were analyzed using the χ2 test or Fisher’s exact test and are presented as number. All P values are two-sided. P < 0.05 is considered statistically significant.
3. Results
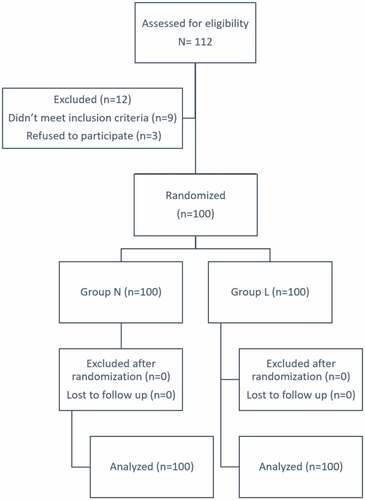
Two hundred twelve patients with severe PE were admitted prepartum to the ICU to stabilize blood pressure (). Two hundred patients were randomly assigned to one of two groups (100 in each group); Group N received nitroglycerin intravenous infusion and Group L received labetalol intravenous infusion.
Both groups were comparable regarding patients’ characteristics (). As regards blood pressure parameters, baseline values (before start of study drug infusion, = 0 minutes) of SBP and DBP were comparable in both groups. At 30 mins, 60 mins, 90 mins SBP and DBP were significantly lower when compared to baseline values in both groups and in the N group compared to the L group. The time to achieve blood pressure control was significantly shorter in the N group compared to the L group, though the number of attacks of hypotension was comparable between both groups. Severe persistent hypertension incidence was lower in N group compared to L group (). As regard maternal side effects, headache, flushes, and tachycardia were significantly higher in N group compared to the L group, while bradycardia was significantly lower in the N group compared to the L group (). Fetal side effects including fetal distress, Apgar scores and time to delivery were comparable between the two groups ().
Table 1. Patients’ characteristics.
Table 2. Blood pressure parameters.
Table 3. Maternal side effects.
Table 4. Fetal side effects.
4. Discussion
In the current study, reduction of blood pressure at 90 minutes to the desired end point was achieved in 96% and 87% of the patients by nitroglycerin and labetalol infusion respectively. Nitroglycerin showed faster control and lower incidence of persistent hypertension. However, it caused more tachycardia, headache, and flushes than labetalol which caused more bradycardia. Both drugs showed comparable fetal tolerability.
Acute severe pre-eclampsia requires urgent antihypertensive therapy to reduce the incidence of adverse outcome [Citation8]. Intravenous hydralazine and labetalol as well as oral nifedipine have been considered first line therapy for the management of acute severe hypertension in PE [Citation4]. Cochrane database review reported lack of evidence regarding choice of antihypertensive [Citation9]. Later systematic review has declared all three drugs equally effective in controlling blood pressure in hypertensive emergencies in pregnant women [Citation2].
Although antihypertensive therapy may diminish the maternal complications, it may also impair utero placental circulation. As rapid or excessive falls in maternal blood pressure may decrease placental perfusion and lead to placental abruption, CS, and low Apgar scores [Citation10]. Consequently, identifying optimal antihypertensive therapy is warranted to ameliorate these risks.
Labetalol is a non-selective beta blocker and post synaptic alpha-1 blocking agent. The hypotensive effect of labetalol begins within 2–5 min after its intravenous administration, reaching a peak at 5–15 min after administration and lasting for about 2–4 hours [Citation11]. Intravenous labetalol may be considered as first line drug treatment in severe PE. Labetalol preserves uteroplacental flow unchanged. Parental labetalol may cause neonatal bradycardia even though little placental transfer occurs that is mainly attributable to its negligible lipid solubility [Citation12]. Labetalol should be avoided in women with asthma, heart disease or congestive heart failure [Citation13]. Thus, labetalol would not be suitable as an antihypertensive in considerable part of the population. In this study, a slower rate of control of blood pressure was achieved by labetalol than that with nitroglycerin. This is consistent with several studies in which labetalol has slower achievement of goal BP with more use of rescue antihypertensive agents [Citation14,Citation15].
Endothelial dysfunction and disruption of nitric oxide bioavailability are major contributors to the manifestations of PE [Citation3]. Therapeutic options that focus on reversing the underlying endothelial dysfunction would potentially be translated into new treatment modalities for this condition. There is currently very limited evidence for the role of nitric oxide donors as antihypertensive therapy for acute severe PE. The most established nitric oxide donors that have been studied in the context of pre-eclampsia is organic nitrates in particular nitroglycerin [Citation5,Citation6,Citation16–18].
Nitroglycerin is a nitric oxide donor that produces a marked vasodilator effect on veins and a modest vasodilator effect on arteries [Citation19]. It has the advantage of targeting the underlying pathophysiological changes of PE and reverse the endothelial dysfunction. The use of the intravenous nitroglycerin results in an immediate peak effect, a duration of 3–5 minutes, with a half-life of 1–4 minutes. Owing to its rapid onset and brief half-life, nitroglycerin antihypertensive effect is predictable and can be titrated easily by adjusting the dose according to the patient response to avoid excessive reduction in blood pressure [Citation5]. Uncontrolled reduction of maternal blood pressure can cause reduction in utero placental circulation and fetal distress.
Nitroglycerin has been previously studied in several obstetric complications, including premature labor [Citation20–22], prevention of pre-eclampsia [Citation23], intrauterine growth restriction [Citation24].
The development of tolerance with nitroglycerin can be a restriction against its use for long-term control of blood pressure in women with pre-eclampsia. However, in the case of acute severe elevation of the blood pressure before delivery transient administration for a limited time would allow its use without tolerance [Citation25].
In one study, intravenous nitroglycerin infusion was effective in lowering maternal blood pressure and in blunting the hemodynamic responses to endotracheal intubation in women with severe PE [Citation26].
In one more study, continuous infusion of intravenous nitroglycerine reduced blood pressure faster, to a greater extent, and more precisely with less variability than sublingual nifedipine administered to women with severe pre-eclampsia. Heart rate also increased in the nifedipine-treated group; it was almost two-fold that in the nitroglycerin-treated group even though both are vasodilatory therapy. There were no significant changes in fetal heart rate in response to vasodilator therapy. The frequency of perinatal fetal–maternal adverse effects was similar in both groups [Citation5]. These findings are consistent with the present study.
In another study, although both hydralazine and nitroglycerin infusions were effective in acute control of hypertension in patients with severe PE before delivery, the therapeutic goal was reached faster with nitroglycerin and with greater safety profile than with hydralazine [Citation6].
The European society of cardiology recommended the use of nitroglycerin infusion in patients with PE complicated with pulmonary edema [Citation27].
As women with pre-eclampsia are usually intravascularly volume depleted, theoretically it may be prudent to choose an antihypertensive that is a rapid acting venodilator like nitroglycerin [Citation28]. However, hypotensive attacks were comparable between the nitroglycerin group and the labetalol group in the current study. Likewise, in a previous study both nitroglycerin and hydralazine groups were comparable regarding the incidence of hypotension throughout the acute controlling of hypertension in patients with severe PE before delivery [Citation6].
Maternal headache, flushes, and tachycardia were more frequent in nitroglycerin group which were attributed to its potent vasodilator effect and its unique mechanism of action which was consistent with other studies [Citation17,Citation18], while maternal bradycardia was more frequent in labetalol group due to its alpha and beta blocking effect which was reported by other researchers [Citation29]. Fetal side effects including fetal distress, Apgar scores and time to delivery were comparable between the two groups. These results are consistent with other study [Citation6]. Future research should further explore the effect of nitroglycerine on fetal liver and methemoglobinemia.
In conclusion, labetalol is an effective and safe antihypertensive drug in severe pre-eclampsia with minimal side effects. However, nitroglycerin showed faster control, limited adverse effects, and lower incidence of failure presented by persistent hypertension. Therefore, it can be considered an important alternative to labetalol in acute severe pre-eclampsia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Duley L, Meher S, Jones L, Cochrane Pregnancy and Childbirth Group. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2013;9(73).
- Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(9):1906–1916.
- Johal T, Lees CC, Everett TR, et al. The nitric oxide pathway and possible therapeutic options in pre-eclampsia. Br J Clin Pharmacol. 2014;78(2):244–257.
- American College of Obstetricians and Gynecologists. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. ACOG Prac Bull. 2019;767.
- Manzur-Verástegui S, Mandeville PB, Gordillo-Moscoso A, et al. Efficacy of nitroglycerine infusion versus sublingual nifedipine in severe pre-eclampsia: a randomized, triple-blind, controlled trial. Clin Exp Pharmacol Physiol. 2008 May;35(5–6):580–585.
- Ali RM, Salah D, Mansour DY. The effect of nitroglycerin infusion versus hydralazine infusion as antihypertensive therapy in acute management of patients with severe pre-eclampsia. Ain-Shams J Anaesthesiol. 2015;8:499–504.
- American College of Obstetricians and Gynecologists (ACOG). Practice Bulletin No. 222: gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135:e237.
- Odigboegwu O, Pan L, Chatterjee P. Use of antihypertensive drugs during preeclampsia. Front Cardiovasc Med. 2018;5:50.
- Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2006;3.
- Magee LA, Cham C, Waterman EJ, et al. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:955.
- Varon J, Marik PE. Clinical review: the management of hypertensive crises. Crit Care. 2003;7(5):374–384.
- Vigil-De Gracia P, Ruiz E, López JC, et al. Management of severe hypertension in the postpartum period with intravenous hydralazine or labetalol: a randomized clinical trial. Hypertens Pregnancy. 2007;26(2):163–171.
- Khan A, Hafeez S, Nasrullah FD. Comparison of hydralazine and labetalol to lower severe hypertension in pregnancy. Pak J Med Sci. 2017 Mar-Apr;33(2):466–470.
- Liu-DeRyke X, Janisse J, Coplin WM, et al. A comparison of nicardipine and labetalol for acute hypertension management following stroke. Neurocrit Care. 2008;9:167–176.
- Liu-DeRyke X, Levy PD, Parker D Jr, et al. A prospective evaluation of labetalol versus nicardipine for blood pressure management in patients with acute stroke. Neurocrit Care. 2013;19:41–47.
- Grunewald C, Kublickas M, Carlström K, et al. Effects of nitroglycerin on the uterine and umbilical circulation in severe preeclampsia. Obstet Gynecol. 1995;86:600–604.
- Cetin A, Yurtcu N, Guvenal T, et al. The effect of glyceryl trinitrate on hypertension in women with severe preeclampsia, HELLP syndrome, and eclampsia. Hypertens Pregnancy. 2004;23:37–46.
- Nevo O, Thaler I, Shik V, et al. The effect of isosorbide dinitrate, a donor of nitric oxide, on maternal cerebral blood flow in gestational hypertension and preeclampsia. Am J Obstet Gynecol. 2003 May;188(5):1360–1365.
- López-Rivera F, Cintrón Martínez HR, Castillo Latorre C, et al. Treatment of hypertensive cardiogenic edema with intravenous high-dose nitroglycerin in a patient presenting with signs of respiratory failure: a case report and review of the literature. Am J Case Rep. 2019;20:83–90. Published 2019 Jan 21.
- Nassar AH, Usta IM. Randomized, double-blind, placebo-controlled trial of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2007;197:325–326.
- Smith GN, Guo Y, Wen SW, et al.; Canadian Preterm Labor Nitroglycerin Trial Group. Secondary analysis of the use of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2010;203:565.e1– 6.
- Shah K, Gupta B, Sharma R. “Dermal nitroglycerin patch” in Treatment of Preterm Labour. J Biosci Med. 2015;3:82–90.
- Trapani A Jr, Gonçalves LF, Pires MM. Transdermal nitroglycerin in patients with severe pre-eclampsia with placental insufficiency: effect on uterine, umbilical, and fetal middle cerebral artery resistance indices. Ultrasound Obstet Gynecol. 2011;38:389–394.
- Oyelese KO, Black RS, Lees CC, et al. A novel approach to the management of pregnancies complicated by uteroplacental insufficiency and previous stillbirth. Aust N Z J Obstet Gynaecol. 1998;38:391–395.
- Hashimoto S, Kobayashi A. Clinical pharmacokinetics and pharmacodynamics of glyceryl trinitrate and its metabolites. Clin Pharmacokinet. 2003;42:205–221.
- Longmire S, Leduc L, Jones MM, et al. The hemodynamic effects of intubation during nitroglycerine infusion in severe pre-eclampsia. Am J Obstet Gynecol. 2010;154:551–556.
- Williams B, Mancia G, Spiering W, et al. 2018 practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(12):2284–2309.
- Magee LA, Abalos E, von Dadelszen P, et al.; and for the CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol. 2011;72:394–401.
- Delgado De Pasquale S, Velarde R, Reyes O, et al. Hydralazine vs Labetalol for treatment of severe hypertensive disorders of pregnancy. A randomized, controlled trial. Preg Hyper. 2013;4:19.