ABSTRACT
Breast surgery under epidural procedure is a secure, accepted alternative to general anesthesia and can increase the outcome postoperatively with less cost. Postoperative analgesia provides a better outcome, early regain of activity with the least side effects, and early discharge. This study compares the analgesic efficacy of thoracic epidural ketamine versus thoracic epidural tramadol when added to bupivacaine at 0.5% for patients who underwent mastectomy under epidural anesthesia.
Methods
Our study included 50 female participants belonging to the 18–60-year-old age group who were epidurally anesthetized for mastectomy operation. Participants were randomly assigned to the ketamine group (epidural bupivacaine and ketamine 50 mg) (KG) and the tramadol group (epidural bupivacaine and tramadol 1 mg/kg) (TG). Pain was measured using the visual analog scale (VAS), and the consumption of rescue analgesia was recorded as well. Nausea and vomiting, sedatives, and vasopressors were recorded and compared between both groups.
Results
KG showed a decrease in VAS scores, less consumption of rescue analgesia, lesser need for antiemetic, and has demanded for more sedation in comparison with TG when observed in the post-anesthesia care unit (PACU) for 24 h postoperatively. Our study could not detect significant differences between groups among recorded demographic data, hemodynamic parameters, or the need for vasopressors.
Conclusion
Epidural ketamine provided a better analgesia and less need to rescue analgesia, less incidence of nausea and vomiting, but more sedation when compared with epidural tramadol at a dose of 1 mg/kg
1. Introduction
The classic procedure for breast surgery is general anesthesia, which has many drawbacks, including incomplete pain control, more nausea and vomiting, late hospital discharge, and depression of the immune system [Citation1]. Regional anesthesia has the advantage of prolonged postoperative analgesia, less incidence of thromboembolism, cost-effectiveness, in addition to hemodynamic stability [Citation2]. Many regional techniques are used for breast surgery, including thoracic epidural block (TEA), paravertebral block, pectoral nerve block, and erector spine block. Breast surgery under TEA is a secure, accepted alternative method to general anesthesia and can improve the outcome postoperatively with less cost [Citation1,Citation2]. TEA also blunts the stress response, avoids airway handling, provides better analgesia, and resumes feeding and home return earlier, so it has been used by several centers as an effective method of anesthesia for mastectomy [Citation1,Citation2]. Postoperative analgesia provides a better outcome, early regain of activity and function with least side effects and early hospital discharge. Several drugs are used to provide analgesia for acute postoperative pain, such as paracetamol, non-steroidal anti-inflammatory drugs, and narcotic analgesics [Citation3–5]. Perfect analgesic is one with rapid onset, prolonged effect, and without adverse effects [Citation6]. Unfortunately, there is no analgesic that can provide all these advantages. Opioids are the drugs of choice for severe pain control despite their potential side effects, including depression of respiration, addiction, and risk of overdose, which might lead to death [Citation7–9]. Accordingly, physicians are searching for safer drugs and procedures for the treatment of severe postoperative pain. Regimens of analgesia for pain relief after mastectomy differ significantly; however, many novel analgesic protocols, especially regional blocks procedures (e.g., pectoral nerves and erector spinae plane blocks), have been created during the last years in addition to epidural and paravertebral blocks. A systematic review on analgesic methods concentrating on breast operations was essentially needed. The methodology considers clinical experience, efficacy, and complications of analgesic procedures [Citation2]. Administration of analgesics through an epidural route is beneficial for postoperative pain relief, as they decrease perioperative morbidity and mortality by attenuating autonomic and neuroendocrine response to surgical trauma [Citation10]. Many epidural supplements augment and prolong postoperative analgesia beyond the local anesthetic effect, including opioids, ketamine, steroids, midazolam, clonidine, dexmedetomidine, neostigmine or epinephrine. Owing to its antagonistic activity on N-methyl-d-aspartate (NMDA) receptors, sodium channel blocking effect, monoaminergic descending inhibitory system activation, in addition to its opioid and cholinergic receptors activating effect, ketamine has been used with local anesthetic successfully to augment epidural anesthesia and analgesia [Citation11–15]. Epidural morphine offered effective postoperative pain relief; however, its use may be associated with emesis, itching, depression of respiration and urinary retention as well [Citation16–18]. Tramadol is a synthetic opioid which has a low (weak) affinity to μ opioid receptors and its mechanism of action is described as multimodal as it inhibits serotonin and epinephrine reuptake and stimulates the release of serotonin presynaptically, leading to increase in the spinal descending inhibitory system; also 5-hydroxytryptamine3 (5-HT3) receptors are present on the dorsal horn superficial lamina. This explains its analgesic potency, which is considered as effective as pethidine based on several studies [Citation19].
The aim of our study was to compare the perioperative analgesic efficacy and safety of ketamine versus tramadol when added to epidural bupivacaine 0.5% for patients who underwent mastectomy under thoracic epidural anesthesia.
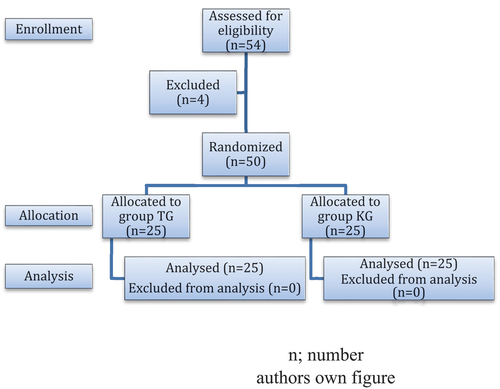
Patients and methods: After approval of medical ethics of Sohag Faculty of Medicine under IRB Registration number: Soh-Med-22-01-22 and obtaining written informed consent, 50 participants were included in this prospective, randomized and double-blind study, aged from 18 to 60 years, classified according to American Society of Anesthesiology as ASA I and II. All participants were scheduled for mastectomy under thoracic epidural anesthesia as a sole anesthetic.
Technique: Patients were randomly divided into two groups using sealed envelopes technique; ketamine group (KG) (n = 25) received 10 mL of 0.5% bupivacaine with 50 mg ketamine and tramadol group (TG) (n = 25) received 10 mL of 0.5% bupivacaine with 1 mg/kg tramadol (). Exclusion criteria include the refusal of participation, puncture site infection, coagulopathy, anatomical anomalies, technical difficulties, and uncorrected hypovolemia.
Participants did not receive any premedication. Upon arrival to the operation room and after insertion of the venous catheter, patients were infused with a crystalloid solution, and basic monitoring was applied (SpO2%, noninvasive blood pressure, electrocardiogram, and temperature). Under aseptic technique and while the patient was in a sitting position, a thoracic epidural catheter was inserted by an experienced anesthetist, who was blinded to the additive mixed with bupivacaine at T5–T6 or T6–T7 using 18-G Tuohy needle with loss of resistance to saline technique followed by fixation. Patients returned to the supine position after a negative test dose (3 ml of lidocaine 1% with epinephrine 1:200,000); all patients received sedation with intravenous midazolam 3 mg and oxygen supply with the nasal cannula at a rate of 2–3 L/min. After confirming a successful block using the pinprick method, surgery was started. Top-up doses of bupivacaine 0.5% 1–2 ml/h were given during surgery or if there were signs of insufficient anesthesia (patient pain complaint, tachycardia, hypertension). Also, insufficient sedation was treated with intermittent doses of midazolam. For sedation assessment, we used the Filos et al. [Citation20] numeric scale, which consists of 4 degrees of consciousness shown in , and our target was level 2 or 3.
Table 1. Filos et al. [Citation20] sedation scoring.
Ephedrine bolus 5–10 mg was given intravenously to correct hypotension if systolic blood pressure decreased below 90 mmHg or 20% less than the baseline measurement. Heart rate decrease below 50 beats/minute will be treated with atropine 0.6 mg boluses intravenously. The severity of postoperative pain was assessed immediately on admission to the PACU, then 1, 6, 12, 18, and 24 h postoperatively using the visual analog scale (VAS) varying from 0, which means no pain, to 10, analogous to worst imaginable pain. Paracetamol 1 g intravenous infusion every 6 h was administered to all participants. Participants were allowed to receive intravenous nalbuphine 2 mg if VAS was >3. Metoclopramide 10 mg intravenous was given to those complaining of nausea and vomiting. Both participants and investigators who assessed the outcome measurements were blinded to the type of additive.
2. Data collection
Primary outcome: Comparison of postoperative visual analog scale (VAS), between KG and TG.
Secondary outcome: Comparison of total consumption of nalbuphine and metoclopramide, intraoperative consumption of ephedrine, atropine, midazolam and bupivacaine and postoperative vomiting, systolic and diastolic blood pressure and heart rate, between KG and TG.
Data were analyzed using STATA version 14.2 (Stata Statistical Software: Release 14.2 College Station, TX: StataCorp LP.).
Sample size was calculated based on a previous similar study, but they used morphine instead of tramadol. Mean VAS after 24 h postoperativly was 2.25 ± 1.6 for the subgroup of morphine [Citation21]. Twenty-two participants from every group were enough to detect the decrease in VAS with type I error of 0.05 and power of 80%. The number was elevated to 25 patients.
Quantitative data were expressed as median, mean, standard deviation and range. Data were analyzed by a t-test of normally distributed data. When the values were not spread normally, the Mann–Whitney test was used in comparison between two different groups. Qualitative data were expressed as numbers and percentages and compared using the Chi-square test. Graphs were produced by using Excel or STATA program. pValue was considered significant if it was less than 0.05.
3. Results
Our results showed that KG had lower statistically significant pain scores (VAS) than the TG at PACU admission (p-value <0.05) at 1, 6, 12, 18, and 24 h (p-value <0.05) after surgery completion (). Also, the KG had lower consumption of nalbuphine doses than TG (2.96 ± 5.07 in KG and 9.44 ± 6.15 mg in TG with p-value <0.05) for rescue analgesia (), and a lower incidence of postoperative vomiting (only 1 patient in KG and 7 patients in TG with p-value <0.05 () with lower metoclopramide (p < 0.05) consumption as compared to TG (). Sedation score was lower in KG than TG (1 ± 0), (1.44 ± 0.71), respectively (p-value = 0.004) (), so consumption of midazolam was higher in the KG than in the TG, with a statistically significant difference () (p-value <0.05). For intraoperative medications (bupivacaine, atropine, and ephedrine), there was no statistically significant difference between both groups () (p-value >0.05).
Table 2. VAS of studied population.
Table 3. Intraoperative medications and postoperative medications.
Table 4. Intraoperative side effects.
Table 5. Intraoperative sedation score.
There was no statistically significant difference between both groups regarding demographic data, surgery duration, and ASA classification (p-value >0.05) ().
Table 6. Demographic data of the studied population.
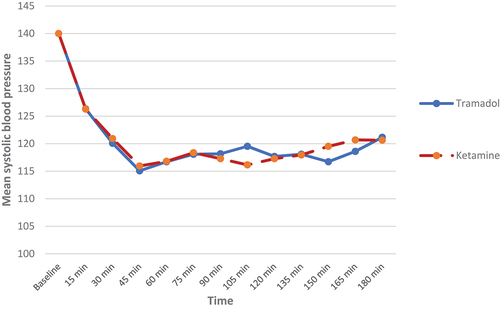
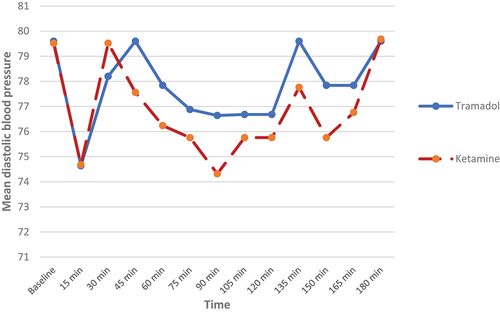
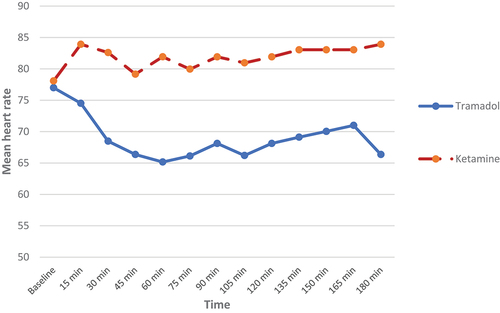
Also, there was no statistically significant difference between both groups regarding the hemodynamic parameters (systolic and diastolic blood pressures) (p > 0.05) ). As regards heart rate (HR) changes, our results showed that patients in the KG had statistically significantly higher heart rate as compared to the TG until 180 min after surgery, but without tachycardia (p-value <0.05) ().
4. Discussion
The main outcome of our study was the superiority of a single dose of 50 mg ketamine as a postoperative analgesic when compared with a single dose of 1 mg/kg tramadol as an additive to bupivacaine 0.5% in TEA for females who underwent mastectomy under TEA. This was noticed as low VAS; at admission to PACU, after 1, 6,12, 18, and 24 h (p-value ˂ 0.0001) after admission to PACU with less analgesic consumption as noticed by low nalbuphine (2.96 ± 5.07 mg for KG and 9.44 ± 6.15 mg for TG) used 24 h postoperatively (p-value ˂ 0.0001). Also, our results showed that epidural ketamine was associated with less incidence of nausea and vomiting (only one patient in KG compared with seven patients in TG). Therefore, less antiemetic drug (Metoclopramide) was used. As regards intraoperative sedation score; it was lower in KG than TG (1 ± 0), (1.44 ± 0.71) respectively (p-value = 0.004), so more sedative (midazolam 4.2 ± 1.12 mg in KG compared with 2.52 ± 2.06 mg in TG) was needed. Epidural ketamine (EK) and epidural tramadol (ET) were used for postoperative analgesia in various types of surgeries. Archana et al. [Citation22] studied the use of ketamine (30 mg) versus tramadol epidurally (100 mg) for 40 patients with lower abdominal and lower limbs surgeries divided into two equal groups and also compared the side effects of both drugs, and they concluded that analgesic time with tramadol was longer than that with ketamine, but nausea and vomiting were significant with tramadol and sedation was significant with ketamine. The findings of Archana et al., unlike our results, showed that the duration of analgesia was longer in the tramadol group, which might be explained by using a higher fixed dose of tramadol (100 mg) (1 mg/kg in our study) and a lesser dose of ketamine (30 mg) (50 mg in our study), which matched with the results of Reddy et al. [Citation23], who studied the analgesic properties of one dose of ketamine (30 mg) versus one dose of tramadol epidurally (100 mg) for patients underwent lower abdominal surgeries and they concluded that analgesic time with EK was shorter than that with ET. Though, they recorded that tramadol is superior to ketamine when used epidurally for lower abdominal surgeries as a postoperative analgesic. Salwa et al. [Citation19] concluded that adding tramadol to levobupivacaine after beginning of general anesthesia for block of pectoral nerve before surgery and in females underwent modified radical mastectomy causing bitter analgesia with significantly lower pain scores postoperatively, longer time to give first analgesic and more reduction in total analgesic dose than using levobupivacaine alone with no side effects. Mohamed et al. [Citation24] used ET in a dose of 75 mg, and their results were comparable to ours in lowering the VAS score in the first 24 h postoperatively. As regards Ek, many investigators proved its analgesic effect as Ozyalcinetal [Citation16], who evaluated the benefits of intramuscular ketamine and epidural ketamine for analgesic control on 60 patients submitted for thoracotomy under general anesthesia, concluded that pre-emptive epidural ketamine is beneficial in decreasing total analgesic needs. Patrical et al. [Citation25], who studied epidural ketamine versus intravenous opioids on 85 patients submitted for digestive surgery (operable colonic cancer), concluded that epidural ketamine, provided significant pain control after major digestive surgery. Another study evaluated the EK by Fabrício et al. [Citation21]. They compared 50 mg (s)ketamine versus 2 mg morphine when added to ropivacaine in two groups of patients submitted to mastectomy. They found that the EK had less VAS scores up to 24 h postoperative (p-value = 0.0018) and less use of total analgesic dose than the morphine group and more midazolam dose in Ketamine group (8.77 ± 3.46 mg). Their findings are in agreement with ours as they used the same dose of ketamine in our study. In a study by Sethi et al. [Citation26], they assessed the analgesic effect when ketamine was added to bupivacaine and epinephrine by patient-controlled epidural analgesia (PCEA) in participants who underwent upper abdominal and thoracic surgeries. They concluded that adding ketamine to PCEA provided a better analgesic effect and reduced rescue analgesia consumption. The local anesthetic-like action of ketamine might explain the mechanism by which it exerts its effect when added to local anesthetics in neuraxial or peripheral nerve blocks which was confirmed and explained by Wagner et al. [Citation27], who found that ketamine has sodium channel blocking action on the myocytes of the experimental rats which is similar to local anesthetic effect. Coggeshall et al. [Citation28] concluded that ketamine has been found to antagonize N-methyl-d-aspartate receptors which is thought to have an important role in pain sensation. Weber et al. [Citation29], in their study on the toad, reported that painful and thermal sensations were lost after subcutaneous infiltration of sciatic nerve with ketamine 0.5%. Mitra et al. [Citation30] concluded that after tissue trauma, opioids stimulate NMDA receptors producing central sensitization and pain sensation. Weinbroum et al. [Citation31] reported that single small dose of postoperative ketamine potentiates the analgesia produced by epidural morphine through its antagonizing effect on NMDA receptors in the posterior horn of the spinal cord, hence, attenuating central sensitization and hyperalgesia.
Baraka et al. [Citation3] found that administration of 100 mg of ET resulted in a more decrease in VAS score in all times of scoring than our results. The difference can be due to the use of a larger dose of tramadol in their study compared with our study (1 mg/kg) and due to the different times of injection, as they gave ET at skin closure. In contrast, we gave epidural tramadol before the start of surgery. In other studies, Siddik et al. [Citation32] compared two doses of tramadol dose (100 and 200 mg) with the control group who received no tramadol. They found that prolonged duration of analgesia was dose-dependent with tramadol but with a higher incidence of side effects especially vomiting. Also, a study done by Singh et al. [Citation33], who compared two doses of thoracic ET, 1 and 2 mg/kg, added to ropivacaine 0.2% for providing analgesia after upper abdominal surgeries under general anesthesia found that lowering the VAS score and prolonged analgesia duration with the dose 2 mg/kg but associated with more side effects (nausea and vomiting).
In our study, vomiting was significantly higher in the tramadol group reached 28% compared with 4% in ketamine group (p-value <0.05). Baraka et al. [Citation3] reported that nausea and vomiting incidence was 20% (2 of 10 patients) among participants who received 100 mg tramadol epidurally. Surprisingly, nausea and vomiting incidence was much higher with epidural tramadol at 50%, as reported by Coluzzi et al. [Citation34] study; however, with the use of smaller doses, the incidence was less. Postoperative nausea and vomiting with ET could be explained by stimulation of the chemoreceptor trigger zone in the brain stem and vestibular apparatus as well. Furthermore, slowing of intestinal peristalsis [Citation35–37].
Our study revealed that both EK and ET when used with bupivacaine are safe as regards hemodynamics (HR and BP) when administered with bupivacaine for TEA as hemodynamic stability was maintained, although higher HR values were found in GK but without tachycardia.
In agreement with our results, Fabricio et al. [Citation21] reported that when ketamine added to bupivacaine during thoracic epidural anesthesia for mastectomy; it provided hemodynamic stability with significant increase of HR when compared with morphine.
It is known that ketamine leads to elevation in HR and BP due to sympathetic stimulation and inhibition of reuptake of catecholamines, by central and peripheral mechanisms. The mechanism through which ketamine acts on the vascular system is complex. This drug also promotes adrenergic bundles norepinephrine release, increasing its venous blood concentration. Block epidurally and benzodiazepines may abolish these results [Citation26].
In a study done by Wagner et al [Citation27], they concluded that ketamine is a sympathetic stimulant and has catecholamine reuptake inhibitory effect, which is why it was expected to increase BP and HR. The complex mechanism of ketamine’s effect on the cardiovascular system is explained by the attenuation of baroreceptors’ function through its effect on NMDA receptors in solitary tract nuclei. Furthermore, it increases catecholamine levels by promoting their release.
In contrast to our results, Mohamed et al. [Citation24] reported significant reduction in BP and HR in TG and this can be explained as they used a fixed dose of tramadol 75 mg in all patients and 15 ml of bupivacaine 0.5% (10 ml in our study). Also, a study done by Paranjpe et al. [Citation38] studied the addition of 50 mg tramadol to a mixture of lidocaine and bupivacaine in epidural anesthesia in lower limb surgeries, and found that there was significant reduction in HR and BP.
5. Conclusion
Ketamine in 50 mg single dose had a satisfactory analgesic effect when added to bupivacaine for patients who underwent mastectomy under thoracic epidural anesthesia, owing to its lower VAS scores, less consumption of rescue analgesia, and hemodynamic stability with less incidence of nausea and vomiting when compared to tramadol.
6. Limitations
We did not use a control group due to ethical factors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aarti S, Shaista J, Ankur K. Thoracic epidural anesthesia for modified radical mastectomy in a high-risk patient: a case report with literature review. Cureus. 2021;13(6). 10.7759/cureus.15822.
- Jacobs A, Lemoine A, Joshi GP, et al., on behalf of the PROSPECT Working Group collaborators. PROSPECT guideline for oncological breast surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2020; 75(5):664–673.
- Baraka A, Jabbour S, Ghabash M, et al. A comparison of epidural tramadol and epidural morphine for postoperative analgesia. Can J Anaesth. 1993;40(4):308–313.
- Helander EM, Menard BL, Harmon CM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21(1). 10.1007/s11916-017-0607-y.
- Faridaalaee G, Mohammadi N, Merghati SZ, et al. Intravenous morphine vs intravenous ketofol for treating renal colic; a randomized controlled trial. Emerg (Tehran). 2016;4(4):202–206.
- Subedi M, Bajaj S, Kumar MS, et al. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019;111:443–451.
- Todd KH. A review of current and emerging approaches to pain management in the emergency department. Pain Ther. 2017;6(2):193–202.
- Cisewski DH, Motov SM. Essential pharmacologic options for acute pain management in the emergency setting. Turk J Emerg Med. 2019;19(1):1–11.
- Gomes T, Mamdani MM, Dhalla IA, et al. The burden of premature opioid-related mortality. Addiction. 2014;109(9):1482–1488.
- Bérubé M, Moore L, Lauzier F, et al. Strategies aimed at preventing chronic opioid use in trauma and acute care surgery: a scoping review protocol. BMJ Open. 2020;10(4):e035268.
- Kehlet H. The stress response to surgery: release mechanisms and the modifying effect of pain relief. Acta Chir Scand Suppl. 1989;550:22–8.12.
- White PF, Schuttler J, Shafer A. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth. 1985;57(2):197–203.
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new trick. Anesth Analg. 1998;87(5):1186–1193.
- Pfenninger EG, Durieux ME, Himmelseher S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology. 2002;96(2):357–366.
- Iida H, Dohi S, Tanahashi T, et al. Spinal conduction block by intrathecal ketamine in dogs. AnesthAnalg. 1997;85(1):106–110.
- Ozyalcin NS, Yucel A, Camlica H, et al. Effect of pre-emptive ketamine on sensory changes on postoperative pain after thoracotomy: comparison of epidural and intramuscular routes. Br J Anaesth. 2004;93(3):356–361.
- Krane EJ, Dalens BJ, Murat I, et al. The safety of epidurals during general anesthesia. RegAnesth Pain Med. 1998;23:433–438.
- Senel AC, Akyol A, Dohman D, et al. Caudal bupivacaine-tramadol combination for postoperative analgesia in pediatric herniorrhaphy. Acta Anaesthesiol Scand. 2001;45:786–789.
- Salwa MSH, El Raouf R, Emad El DeenHamed. Efficacy of adding tramadol as an adjunctive analgesic with levobupivacaine in modified pectoral nerve block for modified radical mastectomy surgery. Egyptian J Anesthesia. 17 May 2019;33, 339–343. Published online.
- Filos KS, Goudas LC, Patroni O. Intrathecal clonidine as a sole analgesic for pain relief after cesarean section. Anesthesiology. 1992;77(2):267–274.
- M FT, Manuela FC, Cristina CR. Comparative study between epidural ketamine and morphine in patients submitted to mastectomy. Rev Dor. São Paulo. 2013 Jul-Sep;14(3):169–173.
- Dhamija A, Garg R, Maratha V. Epidural ketamine & epidural tramadol for post-operative analgesia. Int J Med Res Prof. 2019 May;5(3):11–16.
- Reddy BS, Reddy GP. Efficacy of ketamine Hcl and tramadol Hcl by epidural route for lower abdominal surgeries. Int J Adv Med. 2019 Aug;6(4):1188–1192.
- Mohamed AA, Mohamad HH, Mostafa AA, et al. Epidural dexmedetomidine, tramadol, or neostigmine for postoperative pain after major breast surgeries. Ain Shams J Anesthesiol. July-September 2015;8(3):370–376.
- PatricalL MD, Hilde W, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology. 2005 October;103(4): 813–820.
- Sethi M, Sethi N, Jain P, et al. Role of epidural ketamine for postoperative analgesia after upper abdominal surgery. Indian J Anaesth. 2011;55(2):141–145.
- Wagner LE, Gingrich KJ, Kulli JC, et al. Ketamine blockade of voltage-gated sodium channels, evidence for a shared receptor site with local anesthetics. Anesthesiology. 2001;95(6):1406–1413.
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Rev. 1997;24(1):28–66.
- Weber WV, Jawalekar KS, Jawalekar SR. The effect of ketamine on the nerve conduction in isolated sciatic nerves of the toad. Neurosci Lett. 1975;1(2):115–120.
- Mitra S. Opioid-induced hyperalgesia: pathophysiology and clinical implications. J Opioid Manag. 2008;4(3):123–130.
- Weinbroum AA. A small dose of postoperative ketamine provides rapid and sustained improvement in morphine analgesia in the presence of morphine-resistant pain. AnesthAnalg. 2003;96:789–795.
- Siddik-Sayyid S, Aouad-Maroun M, Sleiman D, et al. Epidural tramadol for postoperative pain after Cesarean section. Can J Anaesth. 1999;46(8):731–735.
- Singh AP, Singh D, Singh Y, et al. Postoperative analgesic efficacy of epidural tramadol as adjutant to ropivacaine in adult upper abdominal surgeries. Anesth Essays Res. 2015 Sep-Dec;9(3):369–373. PMID: 26712976; PMCID: PMC4683493.
- Coluzzi F, Rocco A, Mandatori I. Non-Analgesic Effects Of Opioids: opioid-induced nausea and vomiting: mechanisms and strategies for their limitation. Curr Pharm Des. 2012;18(37):1–10.
- Delilkan AE, Vijayan R. Epidural tramadol for postoperative pain relief. Anesthesia. 1993;48(4):328–331.
- Rathie P, Verma RS, Jatav TS, et al. Postoperative pain relief by epidural tramadol. Indian J Anaesth. 1998;42:26–31.
- Laffita ZJ, Echazábal MJ, Mora GS. Tramadol versus morphine for postoperative peridural analgesia in patients with abdominal hysterectomy. Rev Cub Med Mil. 2012;41(2 175–182).
- Paranjpe JS, Gaikwad SA, Patil MS, et al. Epidural tramadol with low dose local anesthetics for anesthesia and postoperative analgesia in NYHA class III orthopaedic patients - a prospective randomized controlled study. J Pharm Biol Sci. 2013;5:18–22.