Abstract
This study updates the distribution and trends of Gram-positive organisms causing bloodstream infections (BSIs) in the United States (US) and Europe during 2010–2016. In vitro activities of oritavancin and comparators were also evaluated. Staphylococcus aureus, Enterococcus spp., and coagulase-negative staphylococci (CoNS) were the most common organisms in both regions. The proportion of methicillin-resistant S. aureus (MRSA) among all isolates declined (from 11.5% to 9.9%) in the US, a trend also noted for methicillin resistance rates within S. aureus (from 45.7% to 41.9%). MRSA rates (4.1% to 4.2%) in Europe remained stable during 2013–2016. Enterococcus faecalis (7.0–5.2%) and E. faecium (5.1–3.0%) rates declined in the US while remaining stable in Europe (4.5–5.7% and 3.3–4.7%, respectively). Rates for CoNS increased in the US; no temporal trends were noted in Europe. Oritavancin (MIC50/90, 0.03/0.06 µg/mL) inhibited 99.7–99.8% of S. aureus from both regions at ≤0.12 µg/mL and inhibited 96.8–97.9% of E. faecalis and 99.1–99.9% of E. faecium at this concentration. Oritavancin inhibited 99.7% of streptococci at the susceptible breakpoint. This study updates the distribution of pathogens causing BSI in the US and Europe. The in vitro activity for oritavancin against BSI pathogens supports its further development for bacteraemia.
Introduction
Bloodstream infections (BSIs) and the healthcare-associated subset of BSIs are associated with morbidity, mortality, and increased healthcare costs in hospitalized patients.Citation1,Citation2 A recent review estimated that 575,000–677,000 episodes of BSI occur annually in North America (536,000–628,000 in the US and 40,000–49,000 in Canada) and over 1.2 million episodes occur each year in Europe.Citation3 Approximately two-thirds of hospitalized patients with BSIs had hospital- or healthcare-associated BSI, which had higher associated morbidity and mortality than community-associated BSI.Citation4,Citation5 Although community-associated BSIs are less prevalent in hospitalized patients, recent studies indicate that the epidemiology of BSIs within US community hospitals is changing, with increasing rates of community-onset, healthcare-associated BSIs.Citation6,Citation7
A previous study of nosocomial BSIs in 49 US hospitals over a 7-year period (March 1995–September 2002) reported that Gram-positive organisms were responsible for 65% of all BSI cases.Citation8 Slightly higher rates (72%) were recently reported in the paediatric population. Coagulase-negative staphylococci (CoNS) and Staphylococcus aureus remain the most common pathogens responsible for community-onset, healthcare-associated, and hospital-associated BSIs.Citation5,Citation6,Citation8 However, Escherichia coli and S. aureus ranked as first and second in prevalence, respectively, among isolates causing community-associated BSIs.Citation5,Citation6 The high prevalence of S. aureus causing BSIs is significant since methicillin-resistant S. aureus (MRSA) isolates were associated with the highest crude mortality rate (22.5%), highest costs, and longest hospital stay.Citation4
Regardless of pathogen or origin of pathogen causing BSIs, reports indicate that approximately one-third (range of 21–71%) of BSI episodes in the US received inappropriate empiric therapy within 24 h after infection onset.Citation6 This finding may have an important impact in patient care because inappropriate therapy leads to a 60% increase in mortality.Citation9 As results from rapid diagnostic testing are usually not immediately available for patient care, additional antimicrobial options may be important for empiric therapy strategies. Oritavancin was approved by the Food and Drug Administration (FDA; 2014) and by the European Medicines Agency (2015) to treat adults with acute bacterial skin and skin structure infections. This agent has demonstrated potent in vitro inhibitory activity against Gram-positive organisms that pose treatment challenges in BSIs, including MRSA and vancomycin-resistant enterococci (VRE).Citation10 This study updates the distribution and trends over time of organisms causing BSI in US and European hospitals. In addition, in vitro activities of oritavancin and comparator agents are evaluated against these pathogens.
Methods
Bacterial isolate collection
The organisms in this study were collected as part of the SENTRY Antimicrobial Surveillance Program, which monitors antimicrobial resistance and distribution of pathogens causing BSIs, community-acquired pneumonia, pneumonia in hospitalized patients, skin and skin structure infections, urinary tract infections, and intra-abdominal infections (six main study protocols). Participating sites followed specific instructions for each of these protocols to select and include consecutive and unique (one per patient) isolates deemed clinically relevant based on local criteria until they reached a target number of pathogens per site (250–500 isolates per year from numerous infection types depending on hospital size). Most participating sites contributed isolates continuously during this study; however, both regions had sites discontinued and new sites included during the period.
Only isolates causing BSI were included in this study. Participating sites were instructed to collect 60–240 (depending on hospital size) consecutive and unique BSI isolates until reaching a target number per year during 2010–2016. The analysis of the distribution of isolates causing BSI included 24,319 US isolates and 28,046 organisms from Europe, which numbers were utilized as denominators. European isolates were collected from 33 sites in 21 European countries and regions, namely, Belarus (1 site), Belgium (1), Czech Republic (1), France (7), Germany (9), Greece (1), Hungary (1), Ireland (2), Israel (1), Italy (5), Poland (1), Portugal (1), Romania (3), Russia (3), Slovakia (1), Slovenia (1), Spain (3), Sweden (2), Turkey (3), United Kingdom (4), and Ukraine (1). Isolates were recovered from the blood of hospitalized patients and initially identified by the participating laboratory using local practices/devices before submitting the isolates to the coordinating monitoring laboratory (JMI Laboratories, North Liberty, Iowa, USA). The monitoring laboratory confirmed bacterial identifications by biochemical methods/standard algorithms as per the work by Murray et al.Citation11 and supported by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (Bruker Daltonics, Bremen, Germany).
The number of occurrences of species or organism group between US and European hospitals were compared by applying the χ2 test using the Epi Info™ Version 7.1.5.2 software package (Centers for Disease Control and Prevention, Atlanta, Georgia, USA). p values <0.05 were considered significant.
Clinical isolates for in vitro antimicrobial susceptibility analysis
The antimicrobial activity analysis used a subset of 7158 isolates from US and 6531 isolates from European hospitals (2010–2015). All non-US isolates were analyzed in aggregate and labelled as European isolates.
Antimicrobial susceptibility test methods
MIC values were obtained by broth microdilution following Clinical and Laboratory Standards Institute (CLSI)Citation12 protocols. Bacterial inoculum density was monitored by colony counts to ensure an adequate number of cells for each testing event. MIC validation was performed by concurrently testing CLSI-recommended quality control (QC) reference strains (S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Streptococcus pneumoniae ATCC 49619).Citation13 All QC results were within published acceptable ranges.Citation13 MIC interpretations were based on CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint criteria, as available.Citation13,Citation14
Results
Distribution and rank order of main Gram-positive isolates
Overall, 50.1% of US isolates and 40.6% of European isolates responsible for BSI were Gram-positive organisms (). S. aureus, followed by Enterococcus spp., and CoNS prevailed among aerobic Gram-positive isolates causing BSI in hospitals in both geographic regions (). However, distribution of S. aureus and MRSA showed notable differences (i.e. p < 0.0001 for both) between the two geographies: proportions of S. aureus (23.7%) and MRSA (10.4%) in the US were higher than in Europe (16.7 and 4.2%, respectively). In contrast, rates of CoNS among organisms causing BSI were 5.6% in the US and 7.3% in Europe. Distribution of other species and species groups for the two regions are listed in .
Table 1 Distribution of organisms causing bacteraemia in US and European hospitals from 2010 to 2016
Yearly proportion of main Gram-positive isolates
The proportion of S. aureus in the US increased slightly from 2010 through 2012 and decreased in the following years (). MRSA rates appeared to have decreased between 2011 and 2014, and remained stable in the last two years of study. The proportion of MRSA isolates within S. aureus were 45.7%, 47.3%, 43.1%, 42.2%, 39.7%, 45.4%, and 41.9%, respectively, in the US from 2010 through 2016 (data not shown).
Figure 1 Yearly distribution of S. aureus or groups of staphylococci from the SENTRY Program causing BSI in US and European hospitals from 2010 to 2016.
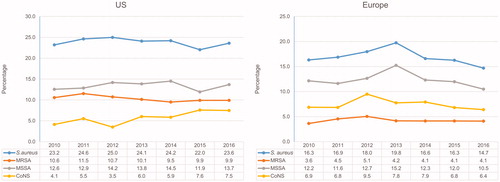
The proportion of BSI in European hospitals that was caused by S. aureus also increased consistently between 2010 and 2013 (from 16.3% to 19.8%) and declined in the following years (14.7% in 2016) (). This profile was driven primarily by the methicillin-susceptible S. aureus (MSSA) rates over the study period. The proportion of MRSA remained stable, particularly between 2013 and 2016. The methicillin resistance rates within S. aureus were 23.0% in 2010, 28.1% in 2011, 28.6% in 2012, 21.5% in 2013, 25.1% in 2014, 25.7% in 2015, and 28.0% in 2016 in European hospitals (data not shown). CoNS distribution increased from 4.1% to 7.5% in the US during 2010 through 2016. In Europe, CoNS rates peaked in 2012 (from 6.9% to 9.5%) and lower rates were noted afterward (6.4% in 2016) ().
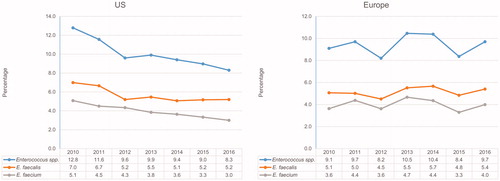
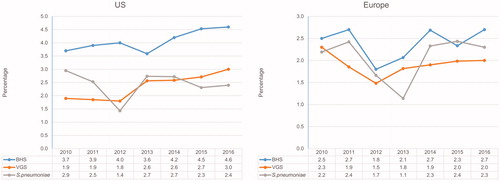
Rates of E. faecalis (from 7.0% to 5.2%), E. faecium (from 5.1% to 3.0%), and all enterococci in aggregate (from 12.8% to 8.3%) as causative organisms of BSI decreased annually in the US during the study interval (). These same organisms had more stable rates over the same time period among European sites. The proportion of vancomycin resistance (MIC, >4 µg/mL) among E. faecalis remained low in both the US (1.9–5.3%) and European regions (0.0–2.1%; data not shown). Vancomycin resistance rates among E. faecium from the US were 80.7%, 75.1%, 78.2%, 71.6%, 68.4%, 68.0%, and 66.0% for 2010, 2011, 2012, 2013, 2014, 2015, and 2016, respectively, whereas rates of 23.2%, 17.6%, 27.3%, 20.8%, 20.3%, 14.7%, and 19.4% were noted in Europe (data not shown). Streptococcal rates fluctuated over time, mainly among European hospitals (). On the other hand, the proportions of beta-haemolytic streptococci (BHS) (from 3.7% to 4.6%) and viridans group streptococci (VGS) (from 1.9% to 3.0%) increased consistently over time in the US ().
In vitro activity of oritavancin and comparator agents
Oritavancin (MIC50/90, 0.03/0.06 µg/mL) inhibited 99.7–99.8% of S. aureus from both regions at the breakpoint for susceptibility (≤0.12 µg/mL) () and had an MIC90 value 8- to 16-fold lower than daptomycin (MIC50/90, 0.25/0.5 µg/mL), ceftaroline (MIC50/90, 0.25/1 µg/mL), and vancomycin (MIC50/90, 1/1 µg/mL; ). CoNS isolates (76.5% oxacillin-resistant) demonstrated a multidrug-resistant phenotype with susceptibility rates for most agents between 23.5% and 83.8%, except for daptomycin (100.0% susceptible), linezolid (98.7–99.3% susceptible), and vancomycin (100.0% susceptible; ). Teicoplanin had acceptable (>90%) susceptibility rates when applying the CLSI breakpoint (98.2–98.9%), but lower rates when EUCAST criteria were used (87.0–88.9%). The oritavancin MIC90 value of 0.06 µg/mL against CoNS in both geographies was at least 8-fold lower than that of comparators.
Table 2 Antimicrobial activity of oritavancin against the main bloodstream infection organisms and organism groups isolated from US and European hospitals
Oritavancin inhibited 97.4–99.4% of all US and European enterococcal isolates at the susceptible breakpoint for E. faecalis (≤0.12 µg/mL for vancomycin-susceptible isolates only) (). E. faecium from Europe (MIC50/90, ≤0.008/0.015 µg/mL) showed oritavancin MIC results lower than those from the US (MIC50/90, 0.03/0.12 µg/mL). Oritavancin MIC values obtained against E. faecalis were similar in both regions (MIC50/90, 0.015/0.06 µg/mL; ). Only oritavancin, daptomycin (MIC50/90, 2/2 µg/mL), and linezolid (MIC50/90, 1/1–2 µg/mL) showed in vitro activity against E. faecium, which had vancomycin resistance rates of 23.6% and 79.0% in Europe and the US, respectively. Oritavancin showed MIC90 values that were at least 16- and 64-fold lower than comparator agents against E. faecium from the US and Europe, respectively. Oritavancin, daptomycin (MIC50/90, 1/1 µg/mL), linezolid (MIC50/90, 1/1 µg/mL), and ampicillin (MIC50/90, ≤1/2 µg/mL) were active against E. faecalis. Oritavancin MIC90 values against E. faecalis were at least 16-fold lower than those of comparators.
Table 3 Antimicrobial activity of oritavancin and comparator agents tested against main bloodstream infection organisms and organism groups from US and European hospitals
VGS and BHS were susceptible to oritavancin (MIC, ≤0.25 µg/mL) except for 1 BHS (0.3%) isolate displaying an MIC value of 0.5 µg/mL (). Most drugs tested against VGS and BHS showed high susceptibility rates (91.0–100.0%); however, clindamycin, erythromycin, and tetracycline had rates of 41.4–88.9% (). The exception was penicillin, which was fully active against BHS, but showed lower susceptibility against VGS (71.7–80.5%).
Conclusions
Overall, the US rate of S. aureus causing BSI (23.7%) was similar to rates reported in previous studies (20.2–28.7%).Citation8,Citation15 This study showed a slow but steady temporal decline in the distribution of MRSA causing BSI (11.5% to 9.9%), a trend that was also noted for methicillin-resistance rates within the S. aureus population (45.7–41.9%). A previous report from the SENTRY Antimicrobial Surveillance Program documented an escalating proportion of MRSA within S. aureus in the US, from 22.4% in 1997 to 39.1% in 2002.Citation15 Increasing proportions of methicillin resistance among S. aureus isolates causing BSI were also reported by Wisplinghoff et al.,Citation8,Citation15 from 22% in 1995 to 57% in 2001. A 2000–2004 US study reported MRSA rates among S. aureus causing BSI of 54.1%, an increase from 27.0% in 1990 through 1994.Citation16 These rates seemed to have increased during the 1990s and early 2000s. The current data presented here show MRSA numbers declining; this trend corroborates several recent population-based studies that have documented a decrease in the overall rates of MRSA causing invasive or BSI after 2005.Citation17,Citation18 However, it has been documented that the rates of community-associated invasive MRSA infections in the US remained stable from 2005 through 2011, and the overall MRSA decline results from decreasing community-onset healthcare-associated and hospital-onset healthcare-associated infections,Citation18 indicating the origin of the pathogen causing BSI can influence MRSA rates and trends.
The rate of S. aureus causing BSI (23.7%) in US hospitals in this study was significantly higher than the European rate (16.7%). Several recent studies documented that S. aureus rates causing BSI have decreased drastically in England and other European countries,Citation19–21 while other studies reported increasing trends for S. aureus BSI in Europe.Citation22–24 The European Antimicrobial Resistance Surveillance System (EARS-net) reported a slightly increased trend in S. aureus causing invasive infections in European countries from 2002 through 2008.Citation25 This latter time frame does not overlap those herein, but our data show a continuous increased trend for S. aureus causing BSIs in Europe from 2010 to 2013, which decreased consistently thereafter. A more recent report from EARS-net described a decrease in the methicillin resistance rates within S. aureus from 18.8% in 2012 to 16.8% in 2015.Citation26 These results disagree with those described here, which did not show any particular trend and varied from 21.5 to 28.0%. It is important to mention that the countries and hospitals within countries included in both EARS-net and SENTRY surveillance systems may differ, which can cause the S. aureus, especially MRSA, rates for Europe analyzed in aggregate to vary considerably. When analyzing in aggregate, any European surveillance study can be subjected to bias as a consequence of varying participating countries, populations, differences in healthcare systems, infection control measures, and quality of surveillance data.Citation22 While a more detailed analysis by country would be beneficial, the small number of hospitals representing some countries/regions herein and the total number of S. aureus submitted annually by each site would not allow for robust statistical analysis.
The data presented here confirm E. faecalis and E. faecium as important Gram-positive pathogens causing BSI in the US and Europe. In addition, the rate of E. faecium closely follows the rate of E. faecalis in both regions, which is a concern since E. faecium isolates often express multidrug resistance phenotypes.Citation27 Previous data from the SENTRY Antimicrobial Surveillance Program documented that the rate of E. faecalis and E. faecium in the US showed signs of consistent decline after 2010.Citation10 It is interesting to note that the rates presented here confirm the trend for E. faecium causing BSI, while they show that E. faecalis seems to have plateaued in the latest years. Similarly, we previously documented increasing rates of E. faecium causing BSI in Europe, while E. faecalis remained stable over the study period.Citation10 In this present study, rates for both E. faecalis and E. faecium in Europe did not demonstrate any trends, suggesting that rates for both species have become more stable in the last years. Moreover, we reported previously that the VRE rates among E. faecium in the US increased from 60.0% in 2001 to 80.7% in 2010, subsequently decreasing to 68.4% in 2014.Citation10 This trend seems to have continued herein; decreasing rates were noted in 2015 (68.0%) and 2016 (66.0%). In Europe, vancomycin resistance rates in E. faecium were previously reported to have increased from 4.7% in 2001 to 27.3% in 2012, followed by similar occurrences (20.3–20.8%) in 2013 – 2014.Citation10 The data shown here confirms more stable VRE rates for E. faecium in Europe. EARS-net reported a significant increase in the percentage of E. faecium invasive isolates that were VRE in 12 out of the 26 countries between 2012 and 2015.Citation26 However, a similar increasing trend was not observed in aggregate, which corroborates the data presented here.
CoNS isolates remained the third most common Gram-positive group of organisms after S. aureus and enterococci. These species have been recognized as important clinical pathogens, especially among patients with central venous catheters and immunocompromised patients when the integrity of the skin barrier is disturbed.Citation28 The previous US rates documented by Wisplinghoff et al.Citation8 and Karlowsky et al.Citation29 for CoNS causing BSI in the US were 31.3% and 42.0%, respectively. However, these study designs might have led to overestimating BSIs due to CoNS. An earlier report by the SENTRY Program documented an overall rate of 11.5% for BSIs caused by CoNS in the US during 1997–2002.Citation15 Interestingly, the rates of CoNS causing BSI in the US in this previous SENTRY study declined from 12.9% in 1997 to 9.3% in 2002. It is tempting to speculate that these rates could have dropped during the early 2000s and began to increase again from 2011 through 2016.
The CoNS rate of causing BSI in Europe (7.3%) was higher than that for the US (5.6%). Previous local studies in Europe reported rates of 8.2–12.6%,Citation30 and these rates tended to be higher in haematologic patients.Citation31 The CoNS rate observed here for Europe (7.3%) was much lower than the rate (19.4%) the SENTRY Program reported during 1997–1998.Citation32 Reasons for these variations in the CoNS distribution may be multifactorial, including the staff assessing the clinical significance of CoNS blood cultures; blood culture practices and techniques; and the complexity of hospitals participating in the study, respective services, and patient populations.Citation33 Thus, these results need careful evaluation because they could be associated with several confounding factors.Citation34 Nevertheless, it is important to emphasize that the CoNS rates in US hospitals seem to be increasing and the consistency observed favours a valid trend.
The distribution of streptococcal isolates causing BSI was consistent in Europe from 2010 to 2016, but consistent increases were observed in the US for VGS and BHS. VGS gained considerable attention as a cause of BSIs during neutropenia in adults and children.Citation31,Citation35,Citation36 Several studies have associated febrile neutropenic bacteraemia caused by VGS with high-dose chemotherapy, quinolone/trimethoprim prophylaxis, and chemotherapy-induced mucositis.Citation31,Citation35,Citation37 The VGS trend in the US parallels the CoNS trend; both are important groups of organisms causing BSI in neutropenic patients with cancer and the VGS and CoNS trends may be a consequence of increasing neutropenic patient populations.Citation38 The BHS group showed a positive temporal trend in the US. While rates for Streptococcus pyogenes and Streptococcus agalactiae remained constant over time, rates for Streptococcus dysgalactiae increased from 0.6% to 1.1% (data not shown). This S. dysgalactiae trend resembles the data reported by Sylvetsky et al.Citation39 The trend may result from an increase in clinical recognition of this organism as a human pathogen.
Overall, this study provides an analysis of Gram-positive isolates causing BSI in the US and Europe and in vitro activity analysis for oritavancin and other clinically important agents. The study extends important trends described in previous studies, including the parallel decline of MRSA and enterococcal rates in the US and temporal increases for CoNS and VGS in the US. While the former may be associated with broader screening practices and contact precaution measures implemented within the healthcare system,Citation40 the latter may be due to the increased number of centres attending to neutropenic patients. Reasons for increased S. dysgalactiae rates in US hospitals are unclear, thus requiring continued monitoring to identify trends over time and more studies to better understand the causes associated with BSI due to S. dysgalactiae.
This study has several limitations, including the lack of hospital admissions as denominators for prevalence calculations, lack of data associated with the hospital, hospital size, and complexity or origin of isolates. In addition, the small number of sites per European country complicates a more granular analysis. Moreover, most sites contributed isolates continuously during this study; however, sites from both regions were discontinued and new sites were included during the period, and variation in participating sites can also impact the prevalence analysis. However, a robust analysis is provided for the US and the combined European data that offers an update on isolates causing BSI and their distribution. Oritavancin demonstrated potent in vitro activity against the Gram-positive isolates from US and European regions. In addition, oritavancin in vitro activity was generally equivalent between regions. Moreover, oritavancin in vitro activity was usually greater than comparator agents commonly used for treating patients with invasive Gram-positive infections.
Notes on contributors
All authors were responsible for the study design, data collection and validation of results/database. REM was responsible for data analysis and drafted all Figures, Tables and manuscript text. All co-authors reviewed the manuscript and provided input. REM was also responsible for collating all comments/edits received from co-authors.
Acknowledgements
The authors wish to thank the following staff members at JMI Laboratories (North Liberty, Iowa, USA): L.M. Deshpande, L.R. Duncan, L. Flanigan, M.D. Huband, M. Janechek, J. Oberholser, T. Reynolds, P.R. Rhomberg, J. Schuchert, J.M. Streit, and L.N. Woosley for technical support and/or assistance with manuscript preparation. The authors also wish to thank F.F. Arhin and G. Moeck, former employees of The Medicines Company (Ville Saint Laurent, Quebec, Canada), for editorial support.
Disclosure statement
JMI Laboratories contracted to perform services in 2017 for Achaogen, Allecra Therapeutics, Allergan, Amplyx Pharmaceuticals, Antabio, API, Astellas Pharma, AstraZeneca, Athelas, Basilea Pharmaceutica, Bayer AG, BD, Becton, Dickinson and Co., Boston, CEM-102 Pharma, Cempra, Cidara Therapeutics, Inc., CorMedix, CSA Biotech, Cutanea Life Sciences, Inc., Entasis Therapeutics, Inc., Geom Therapeutics, Inc., GSK, Iterum Pharma, Medpace, Melinta Therapeutics, Inc., Merck & Co., Inc., MicuRx Pharmaceuticals, Inc., N8 Medical, Inc., Nabriva Therapeutics, Inc., NAEJA-RGM, Novartis, Paratek Pharmaceuticals, Inc., Pfizer, Polyphor, Ra Pharma, Rempex, Riptide Bioscience Inc., Roche, Scynexis, Shionogi, Sinsa Labs Inc., Skyline Antiinfectives, Sonoran Biosciences, Spero Therapeutics, Symbiotica, Synlogic, Synthes Biomaterials, TenNor Therapeutics, Tetraphase, The Medicines Company, Theravance Biopharma, VenatoRx Pharmaceuticals, Inc., Wockhardt, Yukon Pharma, Zai Laboratory, Zavante Therapeutics, Inc. There are no speakers’ bureaus or stock options to declare.
Additional information
Funding
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10.
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19:501–9.
- Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34:2588–95.
- Kollef MH, Zilberberg MD, Shorr AF, Vo L, Schein J, Micek ST, et al. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect. 2011;62:130–5.
- Anderson DJ, Moehring RW, Sloane R, Schmader KE, Weber DJ, Fowler VG Jr, et al. Bloodstream infections in community hospitals in the 21st century: a multicenter cohort study. PLoS One 2014;9:e91713.
- Wang SH, Hines L, van Balen J, Mediavilla JR, Pan X, Hoet AE, et al. Molecular and clinical characteristics of hospital and community onset methicillin-resistant Staphylococcus aureus strains associated with bloodstream infections. J Clin Microbiol. 2015;53:1599–608.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17.
- Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54:4851–63.
- Mendes RE, Castanheira M, Farrell DJ, Flamm RK, Sader HS, Jones RN. Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J Antimicrob Chemother. 2016;71:3453–8.
- Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of clinical microbiology. 9th ed. Washington D.C.: ASM Press; 2007.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 25th informational supplement. M100-S25. Wayne, PA, USA: CLSI; 2015.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 27th informational supplement. M100-S27. Wayne, PA, USA: CLSI; 2017.
- EUCAST. Breakpoint tables for interpretation of MIC's and zone diameters. Version 7.1, March 2017, 2017. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf. Accessed March 2017.
- Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002). Diagn Microbiol Infect Dis. 2004;50:59–69.
- Klevens RM, Edwards JR, Gaynes RP; National Nosocomial Infections Surveillance System. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–30.
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010;304:641–8.
- Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970–8.
- Liebowitz LD. MRSA burden and interventions. Int J Antimicrob Agents 2009;34:S11–S13.
- Johnson AP, Davies J, Guy R, Abernethy J, Sheridan E, Pearson A, et al. Mandatory surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in England: the first 10 years. J Antimicrob Chemother. 2012;67:802–9.
- Nielsen SL, Pedersen C, Jensen TG, Gradel KO, Kolmos HJ, Lassen AT. Decreasing incidence rates of bacteremia: a 9-year population-based study. J Infect. 2014;69:51–9.
- Blomfeldt A, Eskesen AN, Aamot HV, Leegaard TM, Bjornholt JV. Population-based epidemiology of Staphylococcus aureus bloodstream infection: clonal complex 30 genotype is associated with mortality. Eur J Clin Microbiol Infect Dis. 2016;35:803–13.
- Asgeirsson H, Gudlaugsson O, Kristinsson KG, Heiddal S, Kristjansson M. Staphylococcus aureus bacteraemia in Iceland, 1995-2008: changing incidence and mortality. Clin Microbiol Infect. 2011;17:513–l8.
- Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–63.
- de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect. 2013;19:860–8.
- ECDC. Summary of the latest data on antibiotic resistance in the European Union, EARS-NET surveillance data, 2016. Available from: http://ecdc.europa.eu/en/eaad/Documents/antibiotics-EARS-Net-summary-2016.pdf.
- Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray BE. Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. Clin Infect Dis. 2012;54:S233–S238.
- Garcia-Vazquez E, Fernandez-Rufete A, Hernandez-Torres A, Canteras M, Ruiz J, Gomez J. When is coagulase-negative Staphylococcus bacteraemia clinically significant? Scand J Infect Dis. 2013;45:664–71.
- Karlowsky JA, Jones ME, Draghi DC, Thornsberry C, Sahm DF, Volturo GA. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann Clin Microbiol Antimicrob. 2004;3:7.
- Elouennass M, Sahnoun I, Zrara A, Bajjou T, Elhamzaoui S. Epidemiology and susceptibility profile of blood culture isolates in an intensive care unit (2002–2005). Med Mal Infect. 2008;38:18–24.
- Syrjala H, Ohtonen P, Kinnunen U, Raty R, Elonen E, Nousiainen T, et al. Blood stream infections during chemotherapy-induced neutropenia in adult patients with acute myeloid leukemia: treatment cycle matters. Eur J Clin Microbiol Infect Dis. 2010;29:1211–18.
- Fluit AC, Verhoef J, Schmitz FJ; European SP. Frequency of isolation and antimicrobial resistance of gram-negative and gram-positive bacteria from patients in intensive care units of 25 European university hospitals participating in the European arm of the SENTRY Antimicrobial Surveillance Program 1997–1998. Eur J Clin Microbiol Infect Dis. 2001;20:617–25.
- Thylefors JD, Harbarth S, Pittet D. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality? Infect Control Hosp Epidemiol. 1998;19:581–9.
- Wang IK, Chang YC, Liang CC, Chuang FR, Chang CT, Lin HH, et al. Bacteremia in hemodialysis and peritoneal dialysis patients. Intern Med. 2012;51:1015–21.
- Aust C, Tolfvenstam T, Broliden K, Ljungman P, Kalin M, Giske CG, et al. Bacteremia in Swedish hematological patients with febrile neutropenia: bacterial spectrum and antimicrobial resistance patterns. Scand J Infect Dis. 2013;45:285–91.
- Han SB, Bae EY, Lee JW, Lee DG, Chung NG, Jeong DC, et al. Clinical characteristics and antimicrobial susceptibilities of viridans Streptococcal bacteremia during febrile neutropenia in patients with hematologic malignancies: a comparison between adults and children. BMC Infect Dis. 2013;13:273.
- Felsenstein S, Orgel E, Rushing T, Fu C, Hoffman JA. Clinical and microbiologic outcomes of quinolone prophylaxis in children with acute myeloid leukemia. Pediatr Infect Dis J. 2015;34:e78–e84.
- Gudiol C, Bodro M, Simonetti A, Tubau F, Gonzalez-Barca E, Cisnal M, et al. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect. 2013;19:474–9.
- Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112:622–6.
- Weber SG, Huang SS, Oriola S, Huskins WC, Noskin GA, Harriman K, et al. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: position statement from the Joint SHEA and APIC Task Force. Am J Infect Control. 2007;35:73–85.