 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Various outcomes of mortality, medical costs, and antimicrobial usage result from antimicrobial stewardship (AS) programmes. Here, we clarified the effects of AS implementation by a well-trained pharmacist in an open intensive care unit (open ICU) through a retrospective, comparative study of 5123 open ICU patients of Tokai University Hospital. The 12 months before and after AS implementation were considered the control and study periods, respectively. After AS implementation, the number of AS cases increased significantly. The period until the implementation of therapeutic drug monitoring was significantly shortened, and antimicrobial drug usage increased significantly. The methicillin-resistant Staphylococcus aureus (MRSA) detection rate decreased significantly. Earlier and more frequent AS implementation could enhance treatment effects, possibly decreasing the MRSA incidence. Despite active AS implementation, antimicrobial drug usage did not necessarily decrease. ICU pharmacists with experience in AS should take on leadership roles and implement active AS strategies in open ICU settings.
Introduction
Over the recent years, drug-resistant bacteria incidence has increased to become a major global threat. It is estimated that the number of deaths due to drug resistance worldwide will reach approximately 10 million annually by 2050 unless counteractive measures are taken [Citation1]. Thus, it is necessary to promote the proper use of antimicrobial drugs worldwide. Antimicrobial stewardship (AS) teams (ASTs) have been organised worldwide to promote the proper use of antimicrobial drugs. AS programmes have been shown to result in many beneficial outcomes. Mortality is reduced and pressure on the healthcare systems is alleviated as medical costs fall, and lengths of hospital stays become shorter [Citation2, Citation3]. In Japan, ASTs have been launched in many medical institutions and an environment has been fostered to promote the appropriate use of antimicrobial drugs. However, it has been noted that there is a large gap between the number of pharmacists required for AS programmes and the number presently engaged. Indeed, the human resources available are insufficient, even in large-scale hospitals [Citation4]. Consequently, a new strategy is required to effectively promote the appropriate use of antimicrobial drugs with limited human resources.
Intensive care medicine is a potential target for a strategy of appropriate use of antimicrobial drugs to focus upon [Citation5]. Early intervention and mechanisms that allow feedback to be acted on are essential, as patients requiring intensive care report severe and various underlying diseases, with invasive devices often being used, and high infection incidence and case fatality rates being high [Citation6]. In addition, 51% of patients admitted to the intensive care unit (ICU) are reportedly diagnosed with infectious complications [Citation7]. In patients with severe infections, such as septic shock, frequent use of broad-spectrum antimicrobial drugs and their combined use has been reported, thereby increasing the risk of drug-resistant bacteria occurrence [Citation8]. The metabolic and excretory functions of such ICU patients change over time because of the augmented renal clearance in patients with organ damage or sepsis. Thus, fine-tuned usage and dosage adjustments of antimicrobial drugs according to patient characteristics, disease state, types of antimicrobial drugs, and renal replacement therapy are necessary [Citation9, Citation10].
It has been demonstrated that in the treatment of sepsis, early implementation of an appropriate antimicrobial drug treatment can suppress the increase in mortality [Citation11, Citation12]. The ICU includes an open ICU, which is managed by the attending physicians of each hospital department, and a closed ICU, which is constantly managed by intensive care specialists. Unlike in closed ICUs, the management system in open ICUs differs for each department. Given the need to balance the demands of surgery and outpatient care, attending physicians and doctors-in-charge can, find it difficult to rapidly assess and handle infection treatment in many cases. When mortality in open and closed ICUs is compared, it has been shown that mortality is significantly higher in open ICUs than in closed ICUs, where close communication with doctors is possible [Citation13]. To prevent delays in treatment of infection in open ICUs, active and rapid implementation of antimicrobial drug administration plans is vital. These plans should include therapeutic drug monitoring (TDM) based on pharmacokinetic–pharmacodynamic analysis by ICU pharmacists with experience in AST programmes.
In this study, we aim to clarify the effects of AS implementation by a well-trained ICU pharmacist, who is experienced in AST, without otherwise increasing the number of staff, as a human resource strategy.
Materials and methods
A retrospective and comparative study was performed.
Subjects
A total of 5123 patients were admitted to the open ICU of Tokai University Hospital between April 1, 2016, and March 31, 2018. The control period included 1559 males and 1024 females, with a median age of 68 years. The introduction period included 1485 males and 1055 females, with a median age of 68 years. There was no significant difference between the two groups in the number of patients admitted, median age, number of patients using antimicrobial drugs, total length of open ICU stays, number of surgeries, or mean length of ICU stay.
Survey period
One year before the introduction of the AS pharmacist (April 2016 to March 2017) was set as the control period and compared with the year after the introduction of the AS pharmacist (April 2017 to March 2018). In both periods, there were three ICU pharmacists (about two full-time equivalents per day) with zero to two years of experience in management of the ICU. The AS pharmacist introduction period was the period when a well-trained pharmacist, who had experience serving as a core AST member, was engaged as an ICU pharmacist. This pharmacist had experience in implementing AS for the entire hospital with doctors over the past five years or more. The pharmacist was trained by doctors through actual AS to be able to read blood-test data and imaging data, such as ultrasonography, radiography, computed tomography, and magnetic resonance imaging.
Characteristics of the ICU and duties of an ICU pharmacist
The ICU at Tokai University Hospital is a 32-bed open ICU that admits patients from infants to the elderly. The main reasons for admission were scheduled surgery, sudden deterioration of disease conditions in the general ward, and acute cardiovascular diseases in patients transported to the emergency department. There is a second emergency and critical care centre ICU (36-bedded) for severe trauma, burns, and poisoning. Stationed ICU pharmacists on Mondays to Saturdays (only the first, third, and fifth Saturdays) performed duties, such as pharmaceutical inventory control and aseptic mixture preparation of injectable drugs. In addition, TDM and non-TDM duties require the confirmation of bacterial detection and antimicrobial drug use.
AS implementation contents
In case of doubts regarding the appropriate use of antimicrobial drugs or when confirmation were deemed necessary, ICU pharmacists promptly contacted attending physicians and doctors-in-charge to propose improvement. The AS pharmacist took on a leadership role and implemented the following activities during the introduction period:
Setting ‘active AS implementation’ as a goal of ICU pharmacists and performing on-the-job training.
Utilisation of the Hospital Information System for Infection Prevention, La-vietal IS (Sysmex Corporation, Kobe, Japan). The La-vietal IS enables the visual understanding of antimicrobial drug use (type, dose, and days of administration related to antimicrobial drugs), bacterial test results (specimens and bacterial species), use of medical devices (central venous catheter, urethral catheter, and others), and infection treatment effects (body temperature, leukocyte count, and C-reactive protein) on one screen.
Creation and utilisation of a monitoring sheet by organ system.
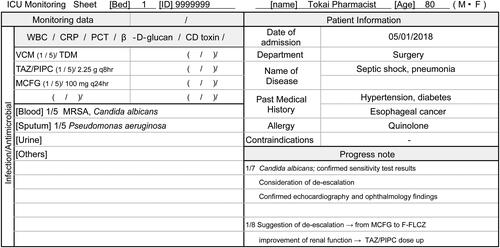
One for each patient, shared information about notes taken by ICU pharmacists concerned with renal function (including renal replacement therapy) and liver function that affect usage and dosage of antimicrobial drugs. In addition, they confirmed the side effects of the antimicrobial drugs used and examined the treatment provided for the infection through focused monitoring ().
Figure 1. Monitoring sheet by organ system in the intensive care unit, and examples of descriptions (partial excerpt). This sheet was created to enable systematic evaluation of drug therapy by organs. It allows for the checking of antimicrobial drug type and dose, bacterial detection, and inflammatory markers related to infection. By sharing monitoring points for each patient among ICU pharmacists, early AS can be implemented. ICU: Intensive care unit, WBC: White blood cell, CRP: C-reactive protein, PCT: Procalcitonin, CD: Clostridioides difficile, VCM: Vancomycin, TDM: Therapeutic Drug Monitoring, TAZ/PIPC: Tazobactam/Piperacillin, MCFG: Micafungin, MRSA: methicillin‐resistant Staphylococcus aureus, F-FLCZ: Fosfluconazole.
Consultation with the AST. Established a system that enabled prompt confirmation and consultation by phone calls to the AST for cases with diagnosis-related judgement in complicated cases.
Indicators of effective measures
Activities were evaluated by the following measures:
Antimicrobial use density (AUD)
The value specified in the Anatomical Therapeutic Chemical/defined daily dose (DDD) index 2019 (https://www.whocc.no/atc_ddd_index/) was used for the DDD.
Days of therapy (DOT)
Detection rate of drug-resistant bacteria (DRdrB) and Clostridioides difficile (DRCD) toxins per 1000 bed days.
Detections within two days after ICU admission were excluded from the aggregation of the detection rate. Pseudomonas aeruginosa strains exhibiting intermediate resistance or resistance to the minimum inhibitory concentration (MIC) of two classes of carbapenems, aminoglycosides, and quinolones were defined as dual-drug-resistant strains, and strains exhibiting intermediate resistance or resistance to the MIC of the three classes were defined as multidrug-resistant strains. To confirm the effects over time, the detection rate of CD toxins was calculated after dividing both the control and intervention periods by six months (early and late).
30-day mortality
To confirm the effects over time, 30-day mortality was calculated after dividing both the control and intervention period by six months (early and late).
Statistical analyses
The statistical software used was Microsoft Excel and IBM SPSS Statistics 26. The χ2 test was used for the comparison of categorical variables (Fisher’s exact test was used when the expected frequency was less than 5), the Mann–Whitney U test was used for the comparison of continuous variables, and P<.05 was set as the significance level.
Ethical considerations
We have disclosed informational documents on our website and provided the opportunity to refuse to participate in this study. This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board for Clinical Research of Tokai University (No. 21R262).
Results
Dynamics of patients admitted to the ICU
There was no significant difference between the control and introduction periods in terms of sex, age, number of patients using antimicrobial drugs, total length of ICU admission, number of surgeries, and mean length of ICU stay ().
Table 1. Characteristics of patients admitted to the ICU.
Number of patients undergoing as and the number of AS cases
The number of patients who underwent TDM by ICU pharmacists was 102 in the control period and 118 in the introduction period. No significant increase was observed (P=.219). The number of patients who underwent non-TDM AS was 19 in the control and 116 in the introduction period. The number of patients who underwent non-TDM AS increased significantly during the introduction period (P<.001). The total number of TDM cases was 322 and 514 in the control and introduction periods, respectively. The total number of non-TDM cases was 20 and 193 in the control and introduction periods, respectively. The number of AS cases increased significantly in both TDM and non-TDM groups (P<.001, P<.001). The mean frequency of AS implementations per patient was 3.41 times for TDM and 1.05 times for non-TDM in the control period, and 4.38 times for TDM and 1.66 times for non-TDM in the introduction period, which significantly increased for non-TDM(P=.020). The mean number of days required from ICU admission to AS implementation was 7.87 days for TDM and 15.35 days for non-TDM in the control period, and 5.54 days for TDM and 7.34 days for non-TDM in the introduction period, which was significantly shortened for TDM (P=.021).
The percentage of AS implemented by ICU pharmacists that was changed as proposed (proposal acceptance rate) was 90.4% for TDM and 75.0% for non-TDM in the control period and 95.7% for TDM and 78.8% for non-TDM in the introduction period, with no significant difference between the two periods ().
Table 2. Number of patients undergoing AS and the number of AS implementations.
Breakdown of AS
TDM
There was no significant difference (P=.573) in the number of proposals for drug change or discontinuation between the two periods, with six proposals in the control period and eight in the introduction period. The number of proposals for initial design, dose reduction, and dose increase significantly increased in the introduction period (P<.001, P<.001, and P=.005, respectively) ().
Table 3. Types of AS.
Non-TDM
In the control period, the number of proposals for dosage change (dose reduction and dose increase) was 12 (60.0%), accounting for the majority, whereas the number of other proposals was only six (30.0%) for drug change and two (10.0%) for administration methods. In contrast, in the introduction period, while the number of proposals for dosage change was the highest at 88 (45.6%), there were 105 (54.4%) non-dosage proposals, covering a wide variety of content, including drug change, discontinuation, administration method, drug addiction and test addition ().
Antimicrobial drug use (AUD and DOT)
The AUD and DOT of all antimicrobial drugs, including antifungal drugs, were 61.71 ± 9.63 and 98.68 ± 11.09 in the control period and 74.06 ± 9.82 and 109.80 ± 11.09 in the introduction period, respectively. Both the AUD and DOT increased significantly during the introduction period (P=.017 and P=.014, respectively). By class, the AUD of anti-P. aeruginosa penicillin, monobactam, metronidazole and daptomycin, and antifungal drugs increased significantly, while the DOT of monobactam, glycopeptides, and antifungal drugs increased significantly. By contrast, no class had significantly decreased antimicrobial drug use during the introduction period (). There was no significant difference in the AUD of broad-spectrum antimicrobial drugs (the total of anti-P. aeruginosa penicillin, fourth-generation cephems, carbapenems, and quinolones) between the two periods (24.53 ± 5.71 in the control period and 24.88 ± 5.61 in the introduction period [P=.887]). There was no significant difference in DOT, with the value being 37.70 ± 7.21 in the control period and 38.01 ± 6.66 in the introduction period (P=.887). However, the proportion of broad-spectrum antimicrobial drugs decreased by 5.2% in AUD and 3.0% in DOT ().
Table 4. AUD and DOT of antimicrobial drugs by class.
Table 5. AUD and DOT of broad-spectrum antimicrobial drugs.
Detection rate of resistant bacteria and detection rate of CD toxins
The detection rate of methicillin-resistant Staphylococcus aureus (MRSA) (‰) decreased significantly to 2.61 and 1.30 in the control and introduction periods, respectively (P=.042). No significant changes were observed in extended-spectrum β-lactamase- (ESBL-) producing bacteria, dual-drug-resistant P. aeruginosa, and multidrug-resistant P. aeruginosa (). The detection rate of CD toxins was 1.64‰ in the early control period and 0.39‰ in the late introduction period (P=.060) ().
Table 6. Bacterial test implementation and resistant bacteria detection.
Table 7. Detection of CD toxins.
Thirty-day mortality
The 30-day mortality was 3.93% in the late introduction period, compared with 5.41% in the late control period (P=.074) ().
Table 8. Thirty-day mortality.
Discussion
In this study, it was shown that the introduction of a pharmacist with experience in AS to an open ICU might have decreased the incidence of drug-resistant bacteria. This could be explained by the fact that the experience of the AST brought about the following five advantages. The first was the ability to learn the highlights for the diagnosis and treatment of infection by doctors. The second was the ability of clinical laboratory technicians to interpret microbial and blood biochemical tests. The third was the ability to cultivate insights based on AST consultations and learning skills to better communicate with attending physicians. The fourth was the ability to learn effective methods in utilising the Hospital Information System for Infection Prevention. Finally, the fifth was the ability to build a human network with AST members. Based on the aforementioned factors, it is desirable to introduce pharmacists with experience in AS to open ICUs, where there are many patients with severe infections.
The acceptance rate of AS proposals by ICU pharmacists was high during both the control and introduction periods. This could be because stationed pharmacists in the open ICU acquired information about infection treatment, such as vital signs, blood tests, and bacterial tests, earlier than doctors and could suggest the appropriate use of antimicrobial drugs. Despite the significant increase in the number of AS cases in the introduction period, the proposal acceptance rate was maintained. Thus, it was inferred that active AS by pharmacists in the open ICU was widely accepted by doctors.
In the introduction period, the total number of TDM implementations increased significantly, whereas the period required until TDM implementation was significantly shortened. This could be due to “improvement of treatment effects”, whereby active recommendation of initial high dosage to increase blood concentration from early treatment was implemented, which was then reduced according to subsequent situations. Second, ICU pharmacists accurately adjusted the dosage according to renal function that changed from time to time, were constantly aware of “reducing the occurrence risk of adverse effects”, and practiced active AS.
In addition, the number of non-TDM AS implementations increased significantly during the introduction period for three reasons. First, changes and signs of infection were promptly noticed with active utilisation of the Hospital Information System for Infection Prevention. It has been reported that the Hospital Information System for Infection Prevention is effective for AST programmes [Citation14], and it was an extremely useful tool in cases where ICU pharmacists practiced AS within a limited time in this study. Second, all ICU pharmacists shared and monitored patients for points to note using the monitoring sheet by organ system; thus, AS could be implemented at appropriate times (). Third, the AST could be consulted in complicated cases and AS could be practiced.
With respect to resistant bacteria, there were many brought-in cases of MRSA in the introduction period, but the incidence of MRSA, excluding carried-in cases, significantly decreased. A meta-analysis based on 19 papers in 2017 reported that AS significantly reduced the infection and carrier rates of MRSA [Citation15]. In the introduction period of this study, there were many proposals for antimicrobial drug addition, initial TDM designs, and proposals for dose reduction. In addition, in the introduction period, since AS had been implemented early after ICU admission, treatment effects could be enhanced, and had turned MRSA negative early, possibly resulting in a decrease in MRSA incidence. Although the cause-and-effect relationship is unclear owing to the effects of horizontal transmission prevention via hand hygiene, it is likely that active AS by ICU pharmacists reduced MRSA incidence.
The reduction of antimicrobial drug use, which contributes to the risk of CD infection, has been recommended as one of the goals of AS [Citation16]. The clinical practice guidelines of many countries have also strongly recommended AS to reduce the incidence of CD infection [Citation17, Citation18]. A meta-analysis of 11 papers indicated that AS significantly reduced the incidence of CD infection [Citation15]. It has been clarified that antimicrobial drug use within the past 90 days is a risk factor for the occurrence of CD infection [Citation19]. Considering the possibility of delayed AS effect manifestation, we performed the analysis after dividing both the control and introduction periods into two stages. There was no significant difference between the late introduction and early control periods (P=.060). The probable reason behind this is that the occurrence of CD was relatively suppressed, even during the control period, and no clear intervention effect was observed. As the detection rate of CD toxins was low in the late introduction period, most strongly influenced by AS, it was suggested that AS by ICU pharmacists might have contributed to the decreasing tendency in the detection rate of CD toxins.
Taggart et al. reported that AS significantly reduced the use of all antimicrobial drugs in ICU [Citation20]. In this study, although the number of patients using antimicrobial drugs did not change in the control and introduction periods, the AUD and DOT of all antimicrobial drugs increased during the introduction period, where the number of AS implementations was significantly higher. To date, there have been no paradoxical reports associating the significant increase in AS with an increase in antimicrobial drug usage. One of the reasons why antimicrobial drug use increased in the introduction period of this study is that there tended to be more admissions of cases where broad-spectrum antimicrobial drugs had to be used, such as for ESBL-producing bacteria, dual-drug-resistant P. aeruginosa, and multidrug-resistant P. aeruginosa. When using antimicrobial drugs use as a measured outcome, it is important to determine whether the drug-resistant bacteria have been detected.
Despite the lack of a significant change, the proportion of broad-spectrum antimicrobial drug usage (AUD and DOT) tended to decrease in the introduction period. The AUD and DOT of penicillin, first-generation cephems, and second-generation cephems, which are narrow-spectrum antimicrobial drugs, showed a tendency to increase. Moreover, the AUD and DOT of carbapenems tended to decrease, while the AUD and DOT of anti-P. aeruginosa penicillin increased. It is believed that these beneficial effects were because ICU pharmacists proposed test requests and de-escalation which could promote early transition to targeted treatment.
The AUD and DOT are effective measures of AS. Even if active AS has been promoted, antimicrobial drug usage does not necessarily decrease, especially in the ICU. This is because it may be affected by changes in the number of patients requiring high-dose and long-term administration of antimicrobial drugs, percentage of patients with decreased renal function, and detection of resistant bacteria, including the carried-in infection. Therefore, AUD and DOT alone should not be used as indicators of AS effectiveness. When evaluating indicators of effectiveness, it is important to consider not only the quantity, but also the quality and background.
There was no significant difference in mortality 30 days after admission to the ICU (p=.074). During the introduction period, ICU pharmacists actively implemented AS early in many patients. Active AS during the intervention period significantly reduced MRSA incidence. However, the effects of death from causes other than infectious diseases cannot be ruled out, and we speculate that the difference may not have been significant.
Based on the results of this study, the introduction of a well-trained pharmacist with experience in AS to the open ICU might have decreased the incidence of resistant bacteria, including MRSA, the detection rate of CD toxins, and 30-day mortality. Nevertheless, this study had some limitations. This was a single-centre retrospective observational study. Multi-centre verification is necessary in the future. In addition, patients were not stratified by specific types of infection to accurately compare the effectiveness of the programme. The Acute Physiology and Chronic Health Evaluation II score was not used for evaluation. Moreover, the necessity of long-term administration of antimicrobial drugs was not evaluated based on each disease. The detection rate of resistant bacteria could also be affected by hand hygiene and the appropriate use of personal protective equipment. Therefore, detection cannot be concluded to depend only on the appropriate use of antimicrobial drugs. Finally, the number of patients might have been insufficient to reach statistical significance for some outcomes (for example CD toxin detection and mortality). In the future, it will be necessary to consider studies that have taken these caveats into account.
Based on our present results, we reported a paradoxical phenomenon, where a significant increase in antimicrobial drug usage was noted, despite the active implementation of AS by a well-trained pharmacist in the open ICU. Antimicrobial drug use alone should not be used as an indicator of the effectiveness of AS in open ICUs. Conversely, in addition to conventional indices such as AUD and DOT, as well as 30-day mortality, it is imperative to perform comprehensive analyses that incorporate microbiological test data, such as resistant bacteria carried-in and the occurrence of CD infection. Thus, as a strategy to promote the appropriate use of antimicrobial drugs in the open ICU, ICU pharmacists with experience in AST programmes should take on leadership roles and implement active AS strategies.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [S.A], upon reasonable request.
References
- Antimicrobial resistance: tackling a crisis for the health and wealth of nations. The Review on antimicrobial Resistance Chaired by Jim O’ Neill; 2014. https://amr-review.org/Publications.html. Accessed Mar 23, 2020.
- Nathwani D, Varghese D, Stephens J, et al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8:35.
- Gentry CA, Greenfield RA, Slater LN, et al. Outcomes of an antimicrobial control program in a teaching hospital. Am J Health Syst Pharm. 2000;57(3):268–274.
- Maeda M, Muraki Y, Kosaka T, et al. Essential human resources for antimicrobial stewardship teams in Japan: estimates from a nationwide survey conducted by the Japanese society of chemotherapy. J Infect Chemother. 2019;25(9):653–656.
- Japan Chemotherapy Society. Guidance for implementing an antimicrobial stewardship program in Japan. Journal of the Japanese Society of Chemotherapy. 2017;65(5):650–687. Available from http://www.chemotherapy.or.jp/guideline/kobiseibutuyaku_guidance.pdf
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329.
- Luyt CE, Bréchot N, Trouillet JL, et al. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18(5):480.
- Hobbs AL, Shea KM, Roberts KM, et al. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy. 2015;35(11):1063–1075.
- Li L, Li X, Xia Y, et al. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol. 2020;11:786.
- Li Z, Cheng B, Zhang K, et al. Pharmacist-driven antimicrobial stewardship in intensive care units in East China: a multicenter prospective cohort study. Am J Infect Control. 2017;45(9):983–989.
- Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244.
- Yang Q, Du JL, Shao F. Mortality rate and other clinical features observed in open vs closed format intensive care units: a systematic review and Meta-analysis. Medicine (Baltimore). 2019;98(27):e16261.
- Ohashi K, Matsuoka T, Shinoda Y, et al. Effectiveness of a computer-facilitated, pharmacist-driven antimicrobial stewardship program for infection management. Yakugaku Zasshi J Pharm Soc Jpn. 2017;137(5):643–650.
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and clostridium difficile infection: a systematic review and Meta-analysis. Lancet Infect Dis. 2017;17(9):990–1001.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48.
- Tschudin-Sutter S, Kuijper EJ, Durovic A, Committee, et al. Guidance document for prevention of clostridium difficile infection in acute healthcare settings. Clin Microbiol Infect. 2018;24(10):1051–1054.
- Zilberberg MD, Shorr AF, Wang L, et al. Development and validation of a risk score for clostridium difficile infection in medicare beneficiaries: a population-based cohort study. J Am Geriatr Soc. 2016;64(8):1690–1695.
- Taggart LR, Leung E, Muller MP, et al. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480.
