Abstract
An accurate survey of the earthworm fauna of Gomera produced 27 species representing five families. Five species are new records for Gomera, and another (Murchieona minuscula) is new for the Canary Islands. The chorological data, obtained from 62 sampling localities, reveal the affinity of each species for the different vegetation zones. The group of taxa Amynthas, Metaphire californica and Pithemera bicincta is widespread throughout the banana groves in the arid basal belt, whereas lumbricid species like Dendrobaena cognettii, Lumbricus rubellus, and Octolasion lacteum are common in the laurel forest of the subhumid montane zone. The origin of the Gomera fauna is clearly linked to human action, and less so to possible remote natural means. The faunistic affinity with other islands of Macharonesia, with the Maghreb and the southwestern biogeographic zones of the Mediterranean is discussed.
Keywords:
Introduction
Gomera is a volcanic island in the Canary archipelago situated in the Atlantic Ocean approximately 300 km from the African continent, influenced by subtropical high pressures (Figure ). Its forests are well conserved on the central plateau containing the Garajonay National Park, with its lush evergreen laurel forest, while the deep valleys have been considerably altered by man. The geographical position of the island within Macharonesia and the proximity of the Maghreb make a biogeographical study of the Gomera lumbricofauna particularly interesting. In two previous studies devoted to this specific topic, the records of species were insufficient. May (Citation1912) mentioned six lumbricid species, while Bacallado and Talavera (Citation1980) in a work on the Garajonay National Park increased the list to 11 species (10 lumbricids and one acanthodrilid). Later, Talavera (Citation1990) recorded five megascolecid species, common in banana groves, but less so in nearby areas rich in herbaceous plants.
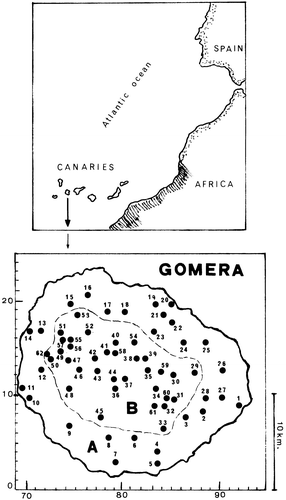
Figure 1. Position of the Canary Islands and map of Gomera showing the collecting sites.A, basal belt. B, montane zone.
The new collecting campaigns were carried out by myself in a laurel forest, banana groves, a pine forest, Myrica–Erica and Euphorbia communities, with the aim of increasing the number of sampling localities, verifying past records and evaluating diversity. The present paper includes a new checklist of species, together with the most common biotopes they inhabit (located in basal and montane zones), and observations on the origin, means of dispersion, and faunistic relationships with other biogeographic areas.
Materials and methods
The worms were collected mainly by manual extraction using a geologist's hammer. Such a procedure, the most suitable in volcanic soils, assures the inclusion of different microhabitats at each location. The list of localities comprises those prospected personally, those of the bibliography confirmed by my sampling and those that contribute non‐repeated information on diversity.
Sixty‐two sampling localities were selected from the most representative vegetation zones and registered on a 1:5000 scale Universal Transverse Mercator (UTM) map with 1×1 km grid (Figure ). The collecting sites with their corresponding geographical coordinates are grouped into the following two zones: a basal belt, up to 600 m a.s.l., occupied by Euphorbia communities and banana groves; a central montane zone, with lush laurel forest between 600 and 1100 m; Myrica–Erica communities up to 1400 m and a few small pine plantations (for details see the Appendix).
Results
Twenty‐seven species were identified from the most representative Gomera biotopes (Table ), the laurel forest of the Garajonay National Park contributing the largest number of Palearctic elements. Although it shows no endemics, the Park constitutes the best refuge for the native lumbricids. Of these, one is reported for the first time in the Canaries, Murchieona minuscula (Rosa, 1905) and another five are new records for Gomera: Dendrobaena hortensis (Michaelsen, 1890), Dendrobaena cognettii (Michaelsen, 1903), Amynthas corticis (Kinberg, 1867), Microscolex phosphoreus (Dugès, 1837), and Pontodrilus litoralis (Grube, 1855).
Table I. Checklist and distribution of Gomera earthworms: BG = banana groves; EC = Euphorbia communities; LF = laurel forest; ME = Myrica–Erica; PF = pine forest.
M. minuscula, originating from a nearby Mediterranean area, was only found in Barranco de Juel at 400 m a.s.l., near surviving relicts of mixed laurel forest and Myrica–Erica communities. However, D. cognettii, Lumbricus rubellus (Hoffmeister, 1843) and Octolasion lacteum (Örley, 1881) are generally common in the laurel forest, where they find their ideal habitat in soils with sufficient moisture and forest litter. El Cedro seems to be the focal point from which L. rubellus has spread throughout the Garajonay National Park, moving about over short distances during rainy periods and by passive transport in the humus layers washed away by surface water and streams. Allolobophora caliginosa (Savigny, 1826), Allolobophora chlorotica (Savigny, 1826) and Eisenia andrei Bouché, 1972 also seem to prefer the laurel forest and one of their most active dispersal nuclei is considered to be forest clearings transformed into cultivated plots. Another three species, Allolobophora trapezoides (Dùges, 1828), Dendrodrilus rubidus (Savigny, 1826), and Eisenia eiseni (Levinsen, 1884), less selective in habitat, can be found in both deforested and wooded zones between 300 and 1400 m (rarely lower), and in anthropized areas. The two last species are common in the pine forest, and E. eiseni deserves mention apart since it also dwells under the leaf litter and decaying logs of the laurel forest.
The six megascolecids mainly inhabit the banana groves from 5 to 300 m in the arid and semiarid basal belt. Amynthas morrisi (Beddard, 1892) and Metaphire californica (Kinberg, 1867) have also colonized ravines near cultivated plots, as in Vallehermoso. Similarly, Amynthas rodericensis (Grube, 1879) is spreading towards the National Park, constituting a risk to the native earthworm species there.
Ocnerodrilus occidentalis Eisen, 1878 is a semiaquatic species distributed near irrigation reservoirs and channels in Euphorbia communities and occasionally in banana groves. Instead, Dichogaster affinis Michaelsen, 1990, a narrow spectrum species, is restricted to 100‐year‐old avocado plantations at Playa de Santiago; similarly M. phosphoreus, was only found in cultivated zones below 300 m. M. dubius and P. litoralis are also scarce; the latter is a marine animal inhabiting the SW littoral and supralittoral zones, with 23 specimens collected from under stones at the ravine mouth in San Sebastián.
Discussion
The earthworm fauna of Gomera belongs mainly to the families Lumbricidae and Megascolecidae, which is in accordance with the literature for other islands in Macharonesia. The species Dendrobaena subrubicunda recorded by Bacallado and Talavera (Citation1980) is invalidated because of an earlier misidentification; other corrections have been made since the specimens collected in El Cedro, Rejo and Apartacaminos do not correspond to E. fetida but rather to E. andrei; lastly, the presence of E. rosea in the pine forest on Gomera is ruled out.
The results in Table also show that 17 species (almost 63%) live in the banana groves, this being the biotope that contains the greatest number of species; some of them, introduced recently with the banana plants transported presumably from Tenerife (e.g. Amynthas spp.), show a coherent distribution with their habitats of origin and with the results for the whole Canary Archipelago (Talavera Citation1990). In the laurel forests, only 13 species were found, since the Garajonay National Park has not yet been colonized by peregrine megascolecids. The Myrica–Erica and Euphorbia communities host fewer species, usually in ravine basins, near springs and around irrigation systems (occasionally in rubbish dumps). The pine forest is the biotope with the least biodiversity (six lumbricids), due to the drier soils and absence of understorey. No endemic Macharonesian species have been recorded, but the absence of the autochthonous semiaquatic Allolobophora moebii Michaelsen, 1895 is striking, since it is common elsewhere in the Canaries (Talavera Citation1987), Madeira (Talavera Citation1996), the Maghreb (Omodeo & Martinucci Citation1987; Omodeo et al. Citation2003), and the SW Iberian peninsula (Heitor Citation1960).
The richness of Palearctic elements in the laurisilva contrasts with the abundance of recently introduced species of the basal belt. The affinity between the fauna of Gomera and that of the nearby islands is evident in the following pattern: a dominance of lumbricids, with some megascolecids of southeastern Asian origin and a few tropical American species (Talavera Citation1987, Citation1990, Citation1992a, Citation1992b, Citation1996). Other faunistic affinities are to be found with the Maghreb, e.g. records of A. caliginosa and E. eiseni (in tree bark), as reported in Omodeo and Martinucci (Citation1987) and Omodeo et al. (Citation2003). There are probable connections with southern Portugal, as suggested by finds of D. lusitana (which is also abundant in the Maghreb), while D. cognettii and Octodrilus complanatus (Dugès, 1828) point to possible relationships with Mediterranean areas. Regarding the difficulty in finding reliable criteria to distinguish between native and introduced species, we agree with Abbott (Citation1982), who suggests overlaps in the choice of habitats, since both types sometimes appear in anthropized areas. These occurrences are also evident in the Canaries.
The origin of the Gomera earthworms is mainly linked to human action, as reported by Bouché (Citation1983), Easton (Citation1984) and Lee (Citation1981) for the fauna of other oceanic islands. The introduction of many peregrine species can be attributed to commercial and agricultural exchanges initiated in the last decades of the XV century. However, natural dispersion pathways must not be ruled out. Once on the island, P. litoralis became established in the supralittoral zone; it was formerly common in the Western Mediterranean in stranded Posidonia, and its introduction may be related to plant fragments transported long ago by marine currents. On the other hand, O. occidentalis was introduced long ago with rice culture in the Palearctic; the possibility that it comes from the Maghreb is still not confirmed: recent studies (Omodeo et al. Citation2003) only record the taxon Ocnerodrilus sp. in Saharian oases. The octochaetids and megascolecids brought in by man have colonized the tropical polyculture plantations and their adjacent zone, as in Tenerife (Talavera Citation1992a, Citation1992b).
D. cognettii, L. rubellus and O. lacteum belong to the oldest fauna of Gomera and their origin is perhaps related to introduction by pioneers. These three species gradually climbed to more inhospitable habitats in the laurel forest, as did A. chlorotica and E. tetraedra, which suggests quite long periods of colonization, as described by Omodeo (Citation1984) for Sardinia. The dispersion of E. eiseni is among the widest; it may have reached Gomera through the Maghreb.
Two historical facts deserve special mention, since they have favoured the introduction of species into Gomera. (1) The existence in the Tertiary era of large Maghrebian rivers that carried great quantities of vegetation and soil into the sea, part of which were then swept ashore by the Canary currents. (2) The arrival more than three millennia ago of the first Berber people from NW Africa transporting plants, livestock and agricultural products.
References
- Abbott , I. 1982 . The distribution of earthworms in the Perth Metropolitan Area. . Records Western Australian Museum , 10 : 11 – 34 .
- Bacallado , J. J. and Talavera , J. A. 1980 . Introducción al estudio de los oligoquetos terrícolas del Parque Nacional de Garajonay (Isla de la Gomera, Canarias). . Vieraea , 10 : 137 – 146 .
- Bouché , M. B. 1983 . “ The establishment of earthworm communities. ” . In Earthworm ecology from Darwin to vermiculture , Edited by: Satchell , J. E . 431 – 448 . London : Chapman and Hall .
- Easton , E. G. 1984 . Earthworms (Oligochaeta) from islands of the south‐western Pacific, and a note on two species from Papua New Guinea. . New Zealand Journal of Zoology , 11 : 111 – 128 .
- Heitor , F. P. C. 1960 . Lumbricidae de Portugal. I. Identifiçao e caracteristicas ecológicas de algunas espécies. . Agronomia Lusitana , 22 : 231 – 244 .
- Lee , K. E. 1981 . Earthworms (Annelida: Oligochaeta) of Vanua Tu (New Hebrides Islands). . Australian Journal of Zoology , 29 : 535 – 572 .
- May , W. 1912 . Gomera die Wandinsel der Kanaren. Reisetagebuth eines Zoologen, Oligochaeta. . Verhandlugen des Naturwissenschaftlichen Vereins in Karlsuhe , 24 : 170 – 171 .
- Omodeo , P. 1984 . The earthworm fauna of Sardinia. . Revue d'Ecologie et Biologie du Soil , 21 : 115 – 126 .
- Omodeo , P. and Martinucci , G. B. 1987 . “ Earthworms of Maghreb. ” . In Earthworms. Selected symposia and monographs UZI , Edited by: Bonvicini Pagliai , A. M and Omodeo , P . 235 – 250 . Modena : Mucchi . vol.2
- Omodeo , P. , Rota , E. and Baha , M. 2003 . The Megadrile fauna (Annelida: Oligochaeta) of Maghreb: a biogeographical and ecological characterization. . Pedobiologia , 47 : 458 – 465 .
- Talavera , J. A. 1987 . Lombrices de tierra presentes en la laurisilva de Tenerife (Islas Canarias). . Miscellània Zoològica , 11 : 93 – 103 .
- Talavera , J. A. 1990 . Peregrine earthworms from the Canary Archipelago (Oligochaeta, Megascolecidae). . Bollettino di Zoologia , 57 : 159 – 164 .
- Talavera , J. A. 1992a . Octochaetid earthworms of the Canary Islands. . Bonner zoologische Beiträge , 43 : 339 – 348 .
- Talavera , J. A. 1992b . Earthworms of the banana groves from Tenerife (Canary Islands). . Soil Biology & Biochemistry , 24 : 1369 – 1375 .
- Talavera , J. A. 1996 . Madeira earthworm fauna. . Italian Journal of Zoology , 63 : 81 – 86 .
Appendix I. List of the sampling localities presented in the study.
Basal belt: 1, Barranco de la Villa 28RBS9208. 2, Seimal 28RBS8908. 3, Chejelipes 28RBS8707. 4, Tanques de Sardina 28RBS8404. 5, Laguna de Santiago 28RBS8402. 6, Embalse Benchigigua 28RBS8206. 7, El Toscón 28RBS7903. 8, Alajeró 28RBS7906. 9, La Pedregosa 28RBS7507. 10, Borbalán 28RBS7110. 11, Valle Gran Rey 28RBS7010. 12, Los Granados 28RBS7213. 13, Alojera 28RBS7217. 14, Barranco del Mono 28RBS7117. 15, Lomo Gaguja 28RBS7250. 16, Barranco del Valle 28RBS7721. 17, Tamargada 28RBS7919. 18, Las Rosas 28RBS8119. 19, Cabo Verde 28RBS8420. 20, Costa Agulo 28RBS8620. 21, Agulo 28RBS8519. 22, LLano Campos 28RBS8517. 23, Hermigua 28RBS8417. 24, Taguluche 28RBS8716. 25, Barranco Juel 28RBS8916. 26, Casas de Aluce 28RBS9113. 27, El Molinito 28RBS9110. 28, San Antonio y Pilar 28RBS8910.
Montane zone: 29, Enchereda 28RBS8813. 30, El Rincón 28RBS8613. 31, Cañadas Casas Blancas 28RBS8610. 32, Vegaipala 28RBS8509. 33, Tejiade 28RBS8507. 34, La Laja 28RBS8411. 35, El Bailadero 28RBS8313. 36, Roque Agando 28RBS8011. 37, Barranco del Cedro 28RBS8112. 38, Monte el Cedro 28RBS8214. 39, El Rejo 28RBS8314. 40, Mériga 28RBS8016. 41, Fuensanta 28RBS7915. 42, Mora Gaspar 28RBS7814. 43, Laguna Grande 28RBS7813. 44, Argumame 28RBS7912. 45, Barranco Almagrero 28RBS7808. 46, Pinar Infantes 28RBS7613. 47, Jardín de las Creces 28RBS7514. 48, Fuente Vica 28RBS7511. 49, Las Cuadernas 8RBS7415. 50, Vega de Arure 28RBS7314. 51, Chorros de Epina 28RBS7417. 52, Montaña Blanca 28RBS7717. 53, El Palmar 28RBS7618. 54, Aceviños 28RBS8216. 55, La Meseta 28RBS7516. 56, Raso de Bruma 28RBS7515. 57, Apartacaminos 28RBS7416. 58, Agua de los Llanos 28RBS8015. 59, Cumbre del Carbonero 28RBS8413. 60, Ermita las Nieves 28RBS8510. 61, Barranco la Guancha 28RBS8309. 62, Embalse Arure 28RBS7215.