Abstract
Balancer morphology and ultrastructure, with emphasis on changes occurring during development, are described for Triturus italicus, using stereomicroscopy and light microscopy, as well as scanning and transmission electron microscopy. Morphological differentiation of balancers involves elongation, swelling of the distal region of the organ and appearance of secretory cones. The extensive granular endoplasmic reticulum and the well‐developed Golgi apparatus characterize the inner cell layer of the mature balancer. Other characteristics include the presence of numerous rounded lipid‐like granules and extensive innervation. In the cytoplasm, numerous bundles of tonofilaments can be found, and their amount increases as development progresses. As already stated in previous studies, the general morphology of balancers shows great constancy.
Introduction
Balancers are a pair of larval organs typical of some species belonging to three caudate families: Hynobiidae, Salamandridae, and Ambystomatidae (Peters Citation1964). Their phylogenetic and evolutionary positions have recently been reviewed by Crawford and Wake (Citation1998). Each balancer is a rod‐like structure projecting from the side of the head just behind the eye. This ephemeral organ always disappears well before metamorphosis.
Most of the available literature regarding balancers refers to taxonomic diversity, while knowledge about their morphology and ultrastructure is still inadequate; in fact, anatomical studies have been carried out on few species only: Ambystoma opacum and Ambystoma jeffersonianum by Anderson and Kollros (Citation1962) and Pleurodeles waltl by Fox (Citation1985).
In this article, the functions of balancers are widely discussed and five nonexclusive functions, recently summarized by Crawford and Wake (Citation1998), are proposed.
Harrison (Citation1925) studied balancer development on Ambystoma maculatum; other embryological data are available in the literature for different species (e.g. Schreckenberg & Jacobson Citation1975; Utsunomiya & Utsunomiya Citation1977; Iwasawa & Kera Citation1980; Obara Citation1984), whereas little is known about interspecific and intraspecific variation of balancer development, as outlined by Crawford and Wake (Citation1998). Oyama (Citation1929) performed one of few comparative studies on balancer development in Cynops pyrrhogaster and Hynobius nebulosus.
Our study shows the anatomical modifications occurring during balancer development in Triturus italicus (Peracca 1898) and also provides an ultrastructural analysis of cells during balancer development and regression.
Materials and methods
All embryos and larvae used for this study originated from T. italicus adults (6 male, 5 female) collected from a natural pond close to Cosenza (southern Italy) during the breeding period. The animals were kept in 50‐l aquaria filled with water from the original pond, and allowed to mate in the laboratory, where they were kept at room temperature and under a natural light/dark cycle. After deposition, the eggs were gathered daily, sampled in test tubes, incubated for the initial stage of development in a constant‐temperature incubator, and examined regularly under a Leica MZ APO binocular microscope. The development stage was determined by using the special developmental chronological tables (Gallien & Bidaud Citation1959; Tripepi et al. Citation1998). Animal manipulation was performed according to the recommendations of the Ethical Committee and under supervision of authorized investigators.
The paired balancers were removed from anesthetized animals at different stages and fixed for 2 h in a solution containing 3% glutaraldehyde in 0.05 M phosphate buffer, pH 7.5, and postfixed in 1% osmium tetroxide in the same buffer. The samples used for TEM observation, after dehydration in ethanol at increasing concentrations, were enclosed in epon/araldite. Semithin sections were stained with Grimley's dyes (toluidine blue method, malachite green, acid fuchsine), observed and photographed at the LEITZ Dialux EB 20 light microscope.
Ultrathin sections (600–900 Å) were stained with uranyl acetate and lead citrate and observed with the transmission electron microscope ZEISS EM 900.
The specimens for the scanning electron microscopy, already dehydrated, were dried up by hexamethyldisilazane (HMDS), according to the methods reported by Nation (Citation1983), coated with gold in an Emitech K550 ion sputter unit and then examined under a Zeiss DSM 940 scanning electron microscope.
Results
Balancers appear as slightly protruding buds, located between the optic vesicles and gill buds, of the mandibular arch at stage 29 when the embryo is about 5.5 mm in length. At stage 30, the balancer buds are conically shaped (Figure ); as development progresses, they change from a conical–cylindrical shape (stage 33, Figure ) to a claviform one (stage 35, Figure ). Their length can be compared to that of gill filaments. Stage 35 is the last embryonic stage.
Figure 1. Triturus italicus embryo and larvae as seen under the binocular microscope. A, embryo at stage 30 of Gallien and Bidaud: the balancer buds (arrow) are conically shaped (bar = 0.5 mm). B, embryo (stage 33 of Gallien and Bidaud) showing a conical–cylindrical balancer (arrow) (bar = 0.5 mm). C, embryo (stage 35 of Gallien and Bidaud); the length of the claviform balancer (arrow) can be compared to that of gill filaments (gf) (bar = 0.2 mm). D, larva (stage 38 of Gallien and Bidaud). After hatching, the balancer (arrow) length increases (gf = gill filaments; bar = 0.1 mm). E, larva (stage 40 of Gallien and Bidaud); the balancers (arrow) reach their largest size (bar = 0.08 mm). F, larva (stage 42 of Gallien and Bidaud); the balancers have completely disappeared (bar = 0.07 mm).
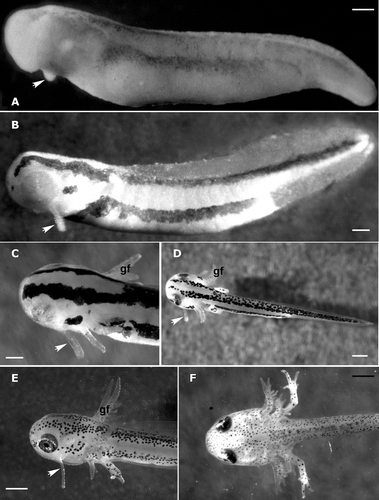
Hatching occurs at stage 36. The growth of balancers continues and at stage 38 they appear lengthened (Figure ). At stage 40, they reach their greatest size, becoming long and slim (Figure ); this stage also marks the beginning of their regression, which is complete at stage 42 (Figure ).
SEM observations
During development, balancer morphology undergoes several modifications in shape; as the lengthening progresses, the balancer loses the conical arrangement that characterised its earlier stages of development. Observed with a scanning electron microscope at stage 32 (Figure ), the epithelial surface appears to be formed by irregular polygonal cells (pavement cells) with rather clear margins characterized by the presence of numerous microridges (Figure ). The pavement cells are rounded in the distal portion of the balancers and elongated in the proximal one (Figure ).
Figure 2. Triturus italicus balancers as seen under the SEM. A, balancer at stage 32 of Gallien and Bidaud; the epithelial surface is formed by polygonal pavement cells (bar = 50 µm). B, balancer at stage 38 of Gallien and Bidaud; note the epithelial surface differentiation (PR = proximal region; MR = median region; DR = distal region; bar = 40 µm). C, a higher magnification showing the rather clear margins of pavement cells and the presence of numerous microridges (bar = 5 µm). D, a higher magnification of the median region: the pavement cells appear to be covered by mucus (*) in the central portion of the cells (bar = 4 µm). E, a higher magnification of the apical portion: numerous secretory cones can be recognized (bar = 10 µm). F, secretory cone; each secretory cone appears to have originated from a single pavement cell (bar = 3 µm).
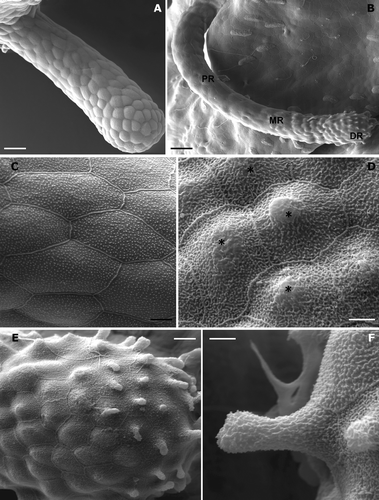
At stage 38 (Figure ), the distal portion widens and the free external surface is differentiated. Three regions can be distinguished: a proximal portion, a short median (subapical) portion and a peripheral (apical) one. The proximal portion extends from the base to about 3/4 of the balancer length, while the pavement cells maintain their own organization. In the subapical region, the pavement cells appear to be covered by mucus that lines the microridge system in the central portion of the cells (Figure ).
In the apical bulbous portion, numerous secretory cones can be recognized (Figure ). With further enlargement, each secretory cone appears to have originated from a single pavement cell (Figure ).
Light and transmission microscopy
The light microscope observations show that the balancer epithelium (stage 34) is formed by 2–3 cell layers (Figure ). The external layer is made up of pavement cells always covered by a thick mucous coat, while the inner layer consists of columnar basal cells. Several rounded lipid‐like granules are easily recognizable in the inner epithelial layers. In the apical portion of the balancer, digitiform protrusions, the secretory cones, originate from the central region of the PVCs (pavement cells; Figure ).
Figure 3. Triturus italicus balancers: LM and TEM observations. A, light‐microscope micrograph through the subapical portion of the balancers (stage 34 of Gallien and Bidaud); the pavement cells lie on the inner layer of columnar basal cells (PVC = pavement cells; BC = basal cell; bar = 25 µm). B, light‐microscope micrograph through the apical portion of balancers (stage 34 of Gallien and Bidaud); note that the secretory cones (arrow) originate from the central region of the PVCs (* = lipid‐like granules; bar = 25 µm). C, stage 34 of Gallien and Bidaud: a transmission electron micrograph of PVC. Note the numerous elongated or rounded secretion granules (g), which are placed under the apical plasmalemma (bar = 4 µm). D, the ultrastructural features of PVC in the apical region of a well‐developed balancer (stage 38 of Gallien and Bidaud). The whole cytoplasm is filled by granules (g) and bundles of tonofilaments (arrowheads) (bar = 2 µm). E, a higher magnification showing the microridges embedded in the fuzzy coat of mucus (*). The secretory granules (g) are arranged in 5–7 layers (stage 38 of Gallien and Bidaud; bar = 500 nm). F, well‐developed rough endoplasmic reticulum (RER) of the inner layer cells (stage 38 of Gallien and Bidaud; n = nucleus; bar = 1 µm).
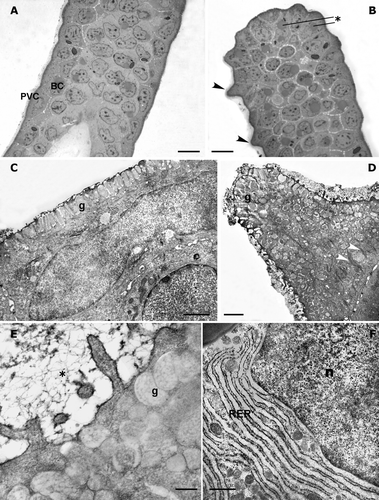
The pavement cell observed with the electron microscope shows numerous elongated or rounded secretion granules, located under the apical plasmalemma; the large nucleus has an elongated shape (Figure ). These ultrastructural features characterize all PVCs of early stage embryos and are also found in the proximal region of embryos and larvae until stage 34. Basal cells can be recognized below the PVC.
In the apical bulbous portion of a well‐developed balancer (stage 38), the PVC ultrastructural organization is completely lost; almost the whole cytoplasm is filled by numerous electronuclear granules and bundles of tonofilaments, which form a network between them. The external surface is covered by a dense mucous layer; the connection with underlying cells is performed by desmosomes (Figure ).
The PVC microridges, both in the subapical and peripheral regions, are embedded in the fuzzy coat of the mucus; in the apical cytoplasm, the electronuclear secretory granules are arranged in 5–7 layers (Figure ).
At stage 38, the cells of the inner layers show the typical features of cells involved in active protein synthesis. The rough endoplasmic reticulum is well developed and lies beside the nuclear surface (Figure ); Golgi apparatuses are found throughout the cytoplasm (Figure ).
Figure 4. Triturus italicus balancers: TEM observations. A, Golgi apparatus (G) surface in the inner epithelial layer cell (stage 38 of Gallien and Bidaud; bar = 200 nm). B, a large lipid‐like granule (lg) is placed near the nuclear surface in the inner epithelial layer cell; n = nucleus; (stage 38 of Gallien and Bidaud; bar = 1 µm). C, numerous bundles of tonofilaments (*) can be observed in the epithelial cells: their amount increases as the development proceeds (stage 38 of Gallien and Bidaud; bar = 330 nm). D, a transmission electron micrograph of unmyelinated neuritis present among the epithelial cells (stage 38 of Gallien and Bidaud; bar = 1µm). E, a transmission electron micrograph of neurites, enveloped by a Schwann cell, in the basal layer of a well‐innervated epithelium (stage 38 of Gallien and Bidaud; bar = 330 nm). F, a transmission electron micrograph of a degenerating pavement cell in the balancers of a Triturus italicus larva at stage 40 of Gallien and Bidaud. Note the large cytoplasmic lacunae (*) (stage 40 of Gallien and Bidaud; bar = 2 µm).
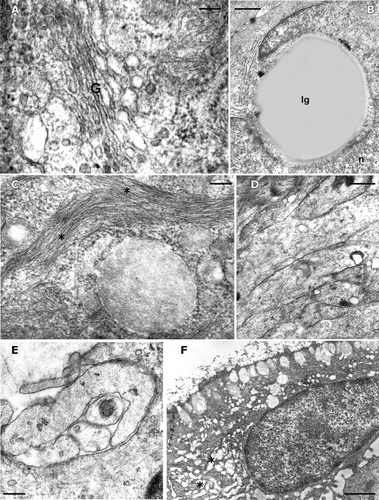
Large rounded lipid‐like granules are frequently located near the nuclear surface (Figure ). In the cytoplasm of all epithelial cells, numerous bundles of tonofilaments can be observed and their amount increases as development progresses (Figure ). Among the epithelial cells, well‐developed unmyelinated neurites are found (Figure ); they are often enveloped by neighboring cells. In some instances, neuritis, enveloped by a Schwann cell, can be found in the basal layer of a well‐innervated epithelium (Figure ).
Balancer regression starts at stage 40 and cells show features of degeneration, with large cytoplasmic lacunae occurring; most of the organelles are highly degenerated, although some mitochondria are still recognizable (Figure ).
Discussion
The present study was carried out to obtain data on the morphology and ultrastructure of balancers from the Italian newt T. italicus; it is the first study to examine such parameters systematically during development by means of stereomicroscopy and light microscopy, as well as scanning and transmission electron microscopy.
Descriptions of balancers in various species of Urodela indicate remarkable constancy in the anatomical organisation of this organ. However, as pointed out by Crawford and Wake (Citation1998), little is known about interspecific and intraspecific variations of balancers; Oyama (Citation1929) carried out one of the few comparative studies on balancer development and showed that in Cynops pyrrhogaster the balancers are reduced in size after hatching, whereas in Hynobius nebulosus they are lengthened and found for a longer period. Clarke (Citation1880) observed a great variation in the progress of balancer development within Ambystoma maculatum, but he provided no data to support this finding. In the Italian newt, balancer length increases throughout development and peaks after hatching; we always observed a great consistency of this pattern.
The role of balancers has been widely discussed, and the different functions proposed for this organ were recently reviewed by Crawford and Wake (Citation1998). The involvement of balancers in structural support has often been reported and they have been considered each time as a support to keep the larva upright on the substrate until limb development (Egert Citation1913) and then to facilitate gill respiration (Clarke Citation1880; Latta Citation1919; Duellman & Trueb Citation1986; Pough et al. Citation1998). They also offer a way to reduce the substrate interfering with the heart beat from Clarke (Citation1880).
Immunohistochemical studies of balancers from Ambystoma gracile and Taricha torosa (Crawford & Wake Citation1998) revealed the presence of a thick collagen layer (type II) that may contribute to a skeletal function of balancers; however, their major function seems to be adhesion (Bell Citation1907; Latta Citation1919; Harrison Citation1925; Fox Citation1985; Crawford & Wake Citation1998).
Our ultrastructural observations, in agreement with those of Fox (Citation1985), confirm this hypothesis; the presence of numerous secretory granules and of a well‐developed RER (rough endoplasmic reticulum) supplies evidence for a more extensive secretory activity than that found in other epidermal cells of larval urodeles (Lavker Citation1972; Shield & Bentley Citation1973; Warburg & Lewison Citation1977; Greven Citation1980; Warburg et al. Citation1994).
Fox (Citation1985) pointed to the external epithelial cells as being a major site of active protein synthesis; in contrast, our observations show the presence of an extensive RER and well‐developed Golgi in the inner layer of the epithelium. The external cells seem to be intended for granule storage and excretion.
The PVC secrete the mucous substances by exocytosis; when the secretory cones release the mucus, new ones originate by accumulation of granules in the cytoplasm.
The presence of unmyelinated neurites and Schwann cells, which are widely distributed in the basal layers of the epithelium, confirms the possible function of balancers as a chemo‐sensory or mechano‐sensory organ, as previously reported by Fox (Citation1985) and Crawford and Wake (Citation1998).
In summary, to date, the accumulated evidence proves the Urodela balancer to be a multi‐function organ rather than an organ for mechanical support or chemo‐sensory function only.
Acknowledgements
We thank Enrico Perrotta for technical assistance. This research was supported by the MemoBioamar MURST Project, and carried out with the approval of the “Ministero dell'Ambiente e della Tutela del Territorio (Direzione per la Protezione della Natura)” permit number 2004/30911.
References
- Anderson , B. and Kollros , J. J. 1962 . The ultrastructure and development of balancers in Ambystoma embryos with special reference to the basement membrane. . Journal of Ultrastructure Research , 6 : 35 – 56 .
- Bell , E. T. 1907 . On regeneration and transplantation of the balancers of embryos of Diemyctylus (with a note on the external gill). . Anatomischer Anzeiger , 31 : 283 – 291 .
- Clarke , S. F. 1880 . The development of Ambystoma punctatum, Baird, part I, external. . Johns Hopkins University Studies from Biological Laboratory , 11 : 105 – 125 .
- Crawford , J. A. and Wake , B. D. 1998 . Phylogenetic and evolutionary perspectives on an enigmatic organ: the balancer of larval caudate amphibians. . Zoology , 101 : 107 – 123 .
- Duellman , W. E. and Trueb , L. 1986 . Biology of amphibians , New York : McGraw‐Hill .
- Egert , F. 1913 . Die Kopfanhänge der Amphibien. . Zoologischer Anzeiger , 42 : 283 – 291 .
- Fox , H. 1985 . The tentacles of Ichthyophis (Amphibia: Caecilia) with special reference to the skin. . Journal of Zoology, London , 205 : 223 – 234 .
- Gallien , L. and Bidaud , O. 1959 . Table cronologique du développement chez Triturus helveticus Razoumowsky. . Bulletin de la Societe Zoologique de France , 84 : 22 – 32 .
- Greven , H. 1980 . Ultrastructural investigations of the epidermis and the gill epithelium in the intrauterine larvae of Salamandra salamandra (L.) (Amphibia, Urodela). . Zeitschrift für Mikroskopisch–Anatomsiche Forschung Leipzig , 94 : 196 – 208 .
- Harrison , R. G. 1925 . The development of the balancer in Ambystoma, studied by the method of transplantation and in relation to the connective‐tissue problem. . Journal of Experimental Zoology , 85 : 33 – 52 .
- Iwasawa , H. and Kera , Y. 1980 . Normal stages of development of the Japanese lungless salamander, Onychodactylus japonicus (Houttuyn). . Japanese Journal of Herpetology , 8 : 73 – 89 .
- Latta , J. S. 1919 . The morphology of the so‐called balancers in certain species of Ambystoma. . Anatomical Record , 17 : 63 – 71 .
- Lavker , R. M. 1972 . Fine structure of the newt epidermis. . Tissue & Cell , 4 : 663 – 675 .
- Nation , J. L. 1983 . A new method using hexamethyl‐disilazane for preparation of soft insect tissues for scanning electron microscopy. . Stain Technology , 58 : 347 – 351 .
- Obara , K. 1984 . Development of the salamander, Hynobius (Hynobius) naevius naevius (Schlegel). . Saishu to Shiiku (Collecting and Breeding) , 46 : 300 – 301 .
- Oyama , J. 1929 . Balancer in Diemictylus pyrrhogaster and in Hynobius nebulosus. . Copeia , 1929 : 103 – 106 .
- Peters , J. 1964 . Dictionary of herpetology , New York : Hafner .
- Pough , F. H. , Andrews , R. M. , Cadle , J. E. , Crump , M. L. , Savitzky , A. H. and Wells , K. D. 1998 . Herpetology , Upper Saddle River, NJ : Prentice Hall .
- Schreckenberg , G. M. and Jacobson , A. G. 1975 . Normal stages of development of the Axolotl, Ambystoma mexicanum. . Developmental Biology , 42 : 391 – 400 .
- Shield , J. W. and Bentley , P. J. 1973 . Respiration of some Urodele and Anuran Amphibia in water: Role of the skin and gills. . Comparative Biochemistry and Physiology , 46A : 17 – 28 .
- Tripepi , S. , Rossi , F. and Peluso , G. 1998 . Embryonic development of the newt Triturus italicus in relation to temperature. . Amphibia–Reptilia , 19 : 345 – 355 .
- Utsunomiya , Y. and Utsunomiy , T. A. 1977 . On the development of Tylototriton andersoni. . Journal of Faculty of Fisheries and Animal Husbandry, Hiroshima University , 16 : 65 – 76 .
- Warburg , M. R. , Lewison , D. and Rosenberg , M. 1994 . Structure and function of Salamandra skin and gills. . Mertensiella , 4 : 423 – 452 .
- Warburg , M. R. and Lewison , D. 1977 . Ultrastructure of epidermis of Salamandra salamandra followed throughout ontogenesis. . Cell and Tissue Research , : 369 – 393 .