Abstract
In the present paper we report the results of our study aimed to the morphological and cytochemical identification of dissociated Petrosia ficiformis cells, a sponge species common on the rocky shores of the Mediterranean Sea. Indeed, in most species classification of sponge cells is difficult for their high plasticity and totipotency, beside the absence of organs and tissues. In a 30‐year‐old classification, four main classes of sponge cells were identified: covering cells, scleroblast‐like cells, i.e. cells secreting the skeleton, contractile cells and archeocytes. After mechanical dissociation of the specimens collected in the Adriatic Sea, we identified three main cell types, with a diameter ranging from 2 µm to 20 µm: (i) choanocytes, very small cells (5 µm in diameter), (ii) archeocytes, intermediate size cells (from 5 to 10 µm in diameter), (iii) spherulous cells, very heterogeneous elements (8–20 µm in diameter). Indeed, the cytoplasm of these latter cells is filled by a variable number of granules, which confer peculiar cytochemical staining patterns. In addition, we also identified the relevant presence of symbiotic microorganisms, namely cyanobateria, preferentially distributed inside the ectosome.
Keywords:
Introduction
Petrosia ficiformis is a sponge species common on the rocky shores of the Mediterranean Sea. It represents one of the approximately 15,000 species of Porifera, most of which live in the marine environment. This phylum is characterised by a very simple structure and pseudo‐tissue organisation. Indeed, desmosomes, maculae adherentes or gap junctions were not reported (Harrison & De Vos Citation1991). Transient intercellular connections occur with rapidly formed intercellular communicating channels (Green & Bergquist Citation1979). The majority of the cells are organised to form the external (pinacoderm) and internal (choanoderm) covering layers. Pinacoderm is composed by flattened, rhomboid pinacocytes overlapping each other, whereas choanoderm is formed by choanocytes or flagellated collar cells, which line specialised chambers, and are responsible for the water current (Müller 1982). In the mesohyl, the gelatinous layer, a certain number of morphologically and functionally different cells are present, whose general names are spherulous cells and amoebocytes, also called wandering cells (Harrison & De Vos Citation1991). The mesohyl seems also to be responsible for distant intercellular communications, permitting the diffusion of hormone‐like substances (Van de Vyver Citation1981).
A peculiarity of most sponge cells is their ability to radically change shape and function (Bonasoro et al. Citation2001), thus impairing a well‐defined cell classification (Harrison et al. Citation1974; De Vos Citation1977; Donadey Citation1979; Gaino et al. Citation1985). In fact, in spite of the presumptive sponge structural simplicity, very few data are available about the specific morphological features of their cells. Until recently, only few cell types were reported in Porifera (Brusca & Brusca Citation2003). In 1978, Bergquist proposed a classification of sponge cells identifying three main classes (covering cells, skeleton‐secreting cells, contractile cells) besides other cells with a high differentiation potential i.e. archeocytes; (Koziol et al. Citation1998; Müller et al. Citation2001). Recently, molecular genetic methods have been developed to support the morphological observation and the in vitro cell identification of Axinella corrugate (Lopez et al. Citation2002). These molecular methods have also been used to identify cultured in vitro dissociated cells of Dysidea avara in order to avoid confusion with unicellular microbial organisms hosted by a large number of Porifera species (Wilkinson Citation1987; Sipkema et al. Citation2003). Microorganisms and sponges association was described in most species, included P. ficiformis (Vacelet & Donadey Citation1977; Rutzler Citation1990; Bigliardi et al. Citation1993). Microorganisms included heterotrophic bacteria, autotrophic cyanobacteria, zoochlorellae, and zooxanthellae and were observed free among cells in the mesohyl matrix as well as inside cell vacuoles, in most cases inside specialised cells (Sarà Citation1971; Sarà & Vacelet, Citation1973; Wilkinson Citation1987; Rosell & Uriz Citation1992; Bigliardi et al. Citation1993; Frost et al. Citation1997; Friedrich et al. Citation1999; Scalera Liaci et al. Citation1999).
Taking into account that sponges can represent a potentially valuable experimental model to study important biological processes like cell–cell interactions and cell differentiation, their cell types identification, in terms of specific morphological and cytochemical features is a prerequisite to gain deeper insight on cell function and tissue histology (Buscema et al. Citation1980). In this paper morphological characterisation of dissociated cells of P. ficiformis have been reported. Cytochemical properties of dissociated cells, and the presence and distribution of symbiotic microorganisms were analysed by light and electron microscopy.
Materials and methods
Sponges
Specimens of marine sponge Petrosia ficiformis (Porifera, Demospongiae) were collected by SCUBA divers in the Adriatic sea (Otranto, Italy), then kept in aerated aquaria and fed with Liquifry Marine (Liquifry Co., Dorking, UK) at 16°C and 16 h of light a day until use.
Cell dissociation
Dissociated sponge cells were obtained by mechanical dissociation. Briefly, sponges were rinsed in filtered seawater (FSW) and cut into little pieces, which were pressed through a nylon mesh (pore size of 100 µm) to obtain a suspension of dissociated cells. The suspension was centrifuged at 550 g at room temperature for 10 min; after two rinses in FSW, the pellet was re‐suspended in the same medium to obtain the final concentration of 107 cells/ml.
Bright field and fluorescence microscopy
Freshly dissociated cells were observed either under a light microscope (Nikon Eclipse E1000 microscope) for morphological analysis or under a fluorescent microscope (filter Ex/Em wavelength: 510/560 nm) for the detection of symbionts. Cells stained with different cytochemical dyes were analysed under the same light microscope. Images were taken by a Nikon digital camera DXM1200F.
Confocal laser microscopy
Living sponges, maintained in FSW, were cut with a scalpel in thin sections of about 0.1 mm, mounted between two coverslip glasses in the same medium and immediately examined under a conventional light and confocal laser scanning microscope (Nikon PCM 2000 laser scanning head based on a Nikon Eclipse 600 microscope, equipped with a Krypton–Argon laser). The sections were scanned sequentially with the 488, 543 nm lines. Sequential scans at a series of optical planes separated by 7.0 µm were performed with a 10× Plan fluor objective lens through the sponge in order to reveal presence and distribution of auto‐fluorescent bacteria.
Cytochemical assay
Dissociated cells were fixed for 30 min at 4°C in glutaraldeyde 2.5% + sucrose 1% in FSW, then washed in phosphate‐buffered saline (PBS: 1.37M NaCl, 0.03M KCl, 0.015M KH2PO4, 0.065M Na2HPO4, pH 7.2) and stained according to the cytochemical methods reported below.
-
Haemathoxylin/eosin staining: fixed cells were rapidly washed in distilled water and maintained in Carazzi's haemathoxylin solution for 8 min. Cells were then washed in distilled water, stained with Eosin for 2 min and washed in tap water.
-
Giemsa's dye: cells stained for 10 min in a 10% Giemsa solution in FSW, were then washed in distilled water.
-
PAS (Periodic acid Schiff) reaction: fixed cells were treated with 1% periodic acid for 10 min, rinsed in tap water and stained with Schiff's reagent for 30 min at 37°C to reveal sites rich in polysaccharides (red colour).
-
Toluidine blue: fixed cells were stained with 0.1% Toluidine blue in ethanol for 7 min at 37°C to evidence acid and sulphated mucopolysaccharides (MPS) (blue colour).
-
Alcian blue: fixed cells were treated with 1% Alcian blue in 0.1 N HCl for 15 min to reveal sulphated MPS (azure colour).
-
Sudan Black: to highlight lipids, fixed cells were dipped in 70% ethanol for 30 s and stained with a saturated solution of Sudan Black in 70% ethanol for 15 min at 70°C. Cells were rinsed in 70% ethanol and washed in distilled water (black colour).
-
Neutral red dye: 60 µl of cells suspension (107 cells/ml FSW) were placed in a culture chamber of 15 mm in diameter and 1mm thick. A coverslip, smeared with vaseline, was gently pressed down to touch the cell suspension; the culture chamber was then kept upside‐down for 30 min at room temperature to allow cells to adhere to coverslip. FSW in the culture chamber was replaced with 60 µl of Neutral Red solution (8 mg/L FSW). Living adhering cells were observed (red colour).
Transmission Electron microscopy (TEM)
Dissociated cells from P. ficiformis after fixation in 2.5% glutaraldehyde in FSW for 2 h on ice, were washed three times in FSW. Post‐fixation was performed with 1% OsO4 in 0.1 M cacodylate buffer (pH 7.2) for 1 h. Samples were washed several times in FSW, dehydrated with ethanol series at room temperature, and embedded in Spurr resin. Semithin sections (1 mm) were stained with Toluidine blue and observed under a Nikon Eclipse E1000 light microscope. Ultra‐thin sections (70 nm) were stained with uranyl acetate and lead citrate before their examination under a Philips CM10 TEM.
Results
The major sponge cell types, obtained after mechanical dissociation of P. ficiformis specimens, were identified and characterised in living and fixed cell suspensions. After dissociation, isolated cells became roundish, their diameter ranging from 2 µm to 20 µm. According to their morphological features, three main cell types could be recognised in living samples: i.e. choanocytes, archaeocytes and spherulous cells. The most abundant cell population was represented by spherulous cells, followed by choanocytes and then by archeocytes (cell population ratio 4:2:1, respectively). Each cell type could be recognised by cell size, nucleus and nucleolus shape and by the presence and amount of granules. Indeed, choanocytes (Figure ) were the smallest cells (maximum diameter of 5 µm), easily distinguished also for the flagella; archaeocytes (Figure ), characterised by an intermediate size (diameter ranging from 5 to 10 µm), were recognised by the presence of a large nucleolate nucleus and by the absence of granules in the cytoplasm; spherulous cells (Figure ), showing the highest variability of cellular size (diameter range between 8 and 20 µm) were recognised for the presence of granules in the cytoplasm, whose amount is highly variable. Conversely, according to granules amount and distribution and to the presence of flagella, isolated cells could be divided in five different types (Figure ). The following cell types could be recognised: (i) cells with flagella, (ii) cells without granules, (iii) cells with very small granules, (iv) cells with few granules and (v) with many granules. Moreover, granules showed peculiar characteristic size, distribution in the cytoplasm and staining properties. Small granules were randomly distributed inside the cytoplasm, whereas cells with few granules showed a polarised appearance due to their uneven distribution in the cytoplasm. The presence of many granules filled almost completely the cytoplasm and could hide the nucleus. The five different cell types showed also different cytochemical properties due to the different granules content. Data are summarised in Table . They showed homogeneous cytoplasm, often colourless (Figure , asterisks). In cells with small granules, no lipids or mucopolysaccharides were detected, while a slight positive PAS reaction was observed (Figure , arrows). On the other hand, cells bearing few granules resulted strongly positive to PAS (Figure , arrowheads). These cells were strongly positive to Sudan black staining and they were also positive to Alcian blue (Figure , arrowhead) and Toluidine blue staining. The presence of acid compartments in most of these cells was also evidenced by positivity to Neutral red reaction (Figure , arrowhead).
Table I. Cytochemical characterisation of dissociated spherulous cells from P. ficiformis.
Figure 1 Light micrographs of cells isolated fromP. ficiformis. Unstained freshly isolated cells: a, choanocytes with their flagellum (arrows); b, archeocyte; c, spherulous cells. Bright field microscopy of fixed cells stained with Giemsa (d). Different cell types can be observed: cells without granules (arrows); cells with small granules (arrowheads); cells with few granules (asterisks); cells with granules (>6) (double arrows).
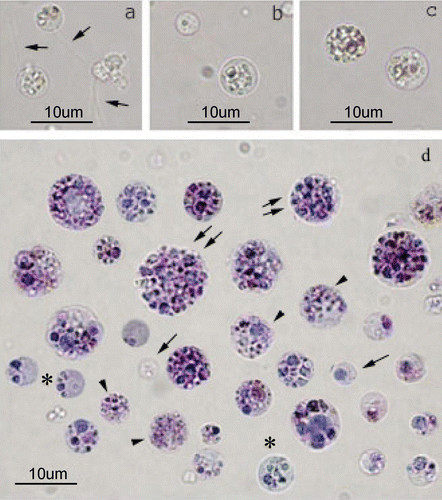
Figure 2 Cytochemistry staining of isolatedP. ficiformis cells; a, PAS reaction; b, Alcian blue; c, Sudan black; d, Neutral red. Cells without granules (asterisks) and cells with small granules (arrows) are generally less positive with all the methods used; than cells with few (arrowheads) or numerous granules (double arrows). Bars = 10 µm.
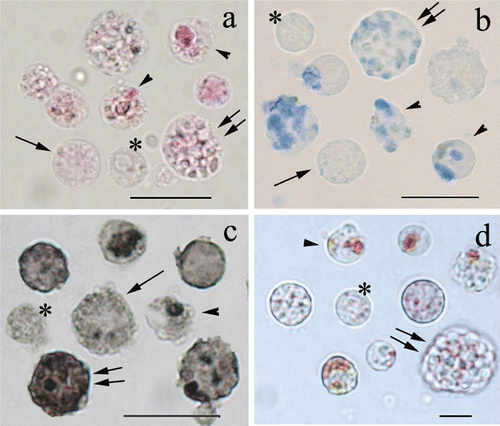
The majority of cells with many granules showed a faint positivity to PAS reaction, whereas they were positive to Alcian blue (Figure , double arrows), Toluidine blue as well as Sudan black (Figure , double arrows). Neutral red staining emphasised few little acid compartments (Figure , double arrows). Interestingly, in PAS‐positive cells, the reaction product (visible as pink/red colour) was observed in the cytoplasm among the granules (Figure , double arrows).
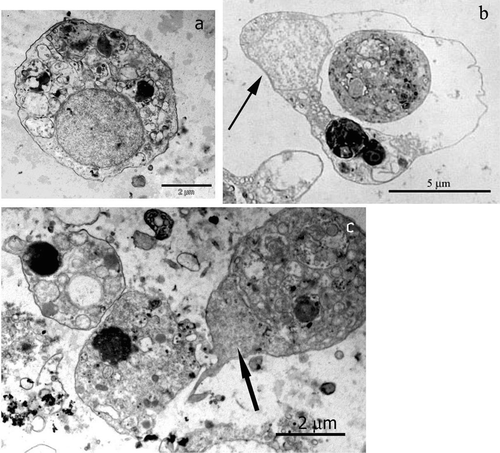
Sponge samples, freshly observed, showed the typical purple colour of P. ficiformis, due to the abundant presence of symbiotic autotrophic microorganisms accumulated in ectosome (the outer layer of sponge body wall). In freshly dissociated cell samples, heterotrophic or autotrophic microorganisms (Figure ) were observed in the cells as well as free in the medium. Autotrophic microorganisms (recognised by the red autofluorescence and the presence of tilacoids at the TEM observation) had both rod and coccus shape (Figure ) and they were always autofluorescent, inside as well as outside the cells (Figure ). The preferential localisation of symbionts in the external part of the sponge inside specialised cells (bacteriocytes) (Figure ), was further confirmed by TEM (Figure , black arrow) and confocal microscopy observations (Figure ). Serial optical sections of sponge pieces showed the red colour in the external part, whereas the inner zone of most of the observed sample was green.
Figure 3 Symbiontic microorganisms inP. ficiformis observed at light (conventional and confocal) and electron microscope. a, autofluorescence (i.e. yellow, green or pink/purple) of symbiontic microorganisms inside the cells (arrows); b,c, phase contrast microscopy and b’,c’, fluorescence microscopy (512/560 nm filter) of microorganisms free in the culture medium; d, light microscope observation of the ectosome–bacteriocytes are present; e, TEM micrographs of a cyanobacteria with a typical tilacoid inside a bacteriocyte; f, confocal images of P. ficiformis. In the optical sections the red colour represents the fluorescence of the autotrophic microorganisms mainly localised in the external part of the sponge (arrow). The green colour represents the fluorescence of eterotrophic microorganisms mainly localised in the inner part of the sponge (asterisk); g, TEM micrographs of isolated P. ficiformis bacteriocytes containing microorganisms inside the vacuole (black arrows) and free in the cytoplasm (white arrow); h, archeocytes with electron‐dense material and a peripheral nucleus (arrow). For a colour version of this figure, please go to the journal's website: http://www.informaworld.com/TIZO.
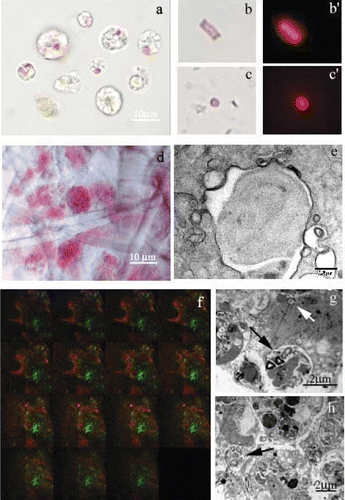
Autotrophic microorganisms were also found inside phagocytic vacuoles of spherulous cells, mixed with electron‐dense material (Figure , black arrow; Figure arrow; Figure ). Often in these cells the nucleus is confined at the cell periphery due to the abundant presence of electron‐dense material and large vacuole (Figure , white arrow; Figure , arrow).
Discussion
In living samples of dissociated cells from P. ficiformis, we identified three main cell types, whose sizes range about from 2 to 20 µm in diameter: choanocytes, archeocytes and spherulous cells. The significant variability in cell size is a common features among Porifera species; in particular, the size of P. ficiformis cells are intermediate between those of Stylotella agminata (Zhang et al. Citation2003) (diameter ranging from 5 to 10 µm), and Suberites domuncula that are generally larger (diameter range 15–60 µm) (Müller et al. Citation1999). However, the identification of dissociated cells from P. ficiformis is strictly related to the investigation method used. Indeed, the number of cell type is different when living or fixed dissociated cells are observed under light microscope. In fixed samples of dissociated cells from P. ficiformis, five different cell types could be identified according not only to cell size, but also to granules amount and distribution and to the presence of flagella.
Population of dissociated spherulous cells from P. ficiformis were those with the major differences in terms of staining properties (indicating a wide range of different molecules) and presence of granules, whose amount and size allowed us to classify at least three different subpopulations (i.e. cells with small granules, cells with few granules and cells with many granules). Very pronounced staining differences were observed by comparing cells with different amount of granules. For example, cells with few granules often showed a strongly positive reaction to the polysaccharide present in cell compartments located near the cell membrane. Polysaccharide storage in well defined compartments was indicated by PAS‐positive granules, whereas positivity to Sudan black, Alcian blue and Toluidine blue staining indicated the presence of lipids and acid and sulphated mucopolysaccharides.
The presence of different molecules inside the granules could be due to both secretion or degradation pathways as already reported in the literature. Vacelet (Citation1967) suggested that in Verongia ( = Aplysina), spherulous cells inclusions represented waste substance, while Donadey (Citation1978, Citation1979) suggested a part in secretion for Plakina trilopha spherulous cells. A possible storage function of the granules could also not be excluded due to the relevant presence of mucopolysaccharides and lipids (detected by the Alcian blue, Toluidin blue and Sudan black staining, respectively). The involvement of spherulous cells also in the digestive activities of sponges is supported by the positive reaction to Neutral red staining and by the presence of numerous large vacuole with electron‐dense material (TEM observations). This variability supports the general idea that spherulous cells could accumulate and discharge granules in the meshoyl. In addition spherulous cells can easily change morphology and function thus impairing a well‐defined sponge cell classification.
P. ficiformis, as well as other species (Sarà Citation1971; Friedrich et al. Citation1999; Scalera Liaci et al. Citation1999), hosts a number of microorganisms that, according to Vacelet and Donadey (Citation1977) are inside special cells, called bacteriocytes. Our ultrastructural study of symbiosis between bacteria and P. ficiformis cells, showing that symbionts are surrounded by an envelope and closely adhere to vacuole membrane, are in agreement with the work of Bigliardi et al. (Citation1993). Indeed, since bacteria can be found in digestive vacuoles, it is likely that they could have a part in digestion processes of foreign material. However, the role of microorganisms observed free in the cytoplasm needs to be investigated. Microorganisms found in the culture medium are probably derived by bacteriocyte ruptures during cell dissociation procedures. The fact that the ectosome showed a red fluorescence indicates a massive presence of cyanobacteria containing chlorophyll A. Cyanobacteria are known to be strong producer of secondary metabolites, that are important molecules used in the defence of sponge, thus explaining their massive localisation in the ectosome. Indeed, preliminary experiments of microorganism identification (data not shown) suggest that the majority of the autotrophic symbionts are cyanobacteria. The identification of the species of cyanobacteria will clarify if the two different shapes (rod and coccus) can indicate dimorphism inside the same species or different species. With experiments that are currently carried out in the laboratory, we have identified at least eight different species of cyanobacteria (personal communication). Many other species of microorganisms, autothrophic as well as heterotrophic, are hosted by P. ficiformis, according to the strong green fluorescence of the inner zone of the samples.
In summary, the morphological and cytochemical properties of dissociated cells from P. ficiformis, which have been described in the present work in order to identify and classify the sponge cells, have shown the complexity of the cell population. In spite of the apparent simplicity of the structure, the classification of the sponge cells is a very difficult goal. It is worth mentioning that the different cell types described are due not only to the high plasticity and totypotency of the sponge cells but also to the use of living or fixed cells during the morphological, i.e. light microscopy, electron microscopy, cytochemistry, investigations.
References
- Bergquist , P. R. 1978 . Sponges , Berkeley and Los Angeles : University of California Press .
- Bigliardi , E. , Sciscioli , M. and Lepore , E. 1993 . Interactions between prokaryotic and eukaryotic cells in a sponge. . Endocytobiosis and Cell Research , 9 : 215 – 221 .
- Bonasoro , F. , Wilkie , I. C. , Bavestrello , G. , Cerrano , C. and Carnevali , M. D. C. 2001 . Dynamic structure of the mesohyl in the sponge Chondrosia reniformis (Porifera, Demospongiae). . Zoomorphology , 121 : 109 – 121 .
- Brusca , R. C. and Brusca , G. J. 2003 . Invertebrates. , Sunderland, MA : Sinauer Associates Inc .
- Buscema , M. , De Sutter , D. and Van De Vyver , G. 1980 . Ultrastructural study of differentiation processes during aggregation of purified sponge archaeocyte. . Wilhelm Roux's Archives of Developmental Biology , 188 : 45 – 53 .
- De Vos , L. 1977 . Etude au microscope électronique à balayage des cellules de l'éponge Ephydatia fluviatilis. . Archives of Biology , 88 : 1 – 14 .
- Donadey , C. 1978 . Origine choanocytarie des cellules à inclusions de l'Eponge Plakina trilopha Schulze (Demosponge Homosclerophoride). . Comptes Rendus de l'Académie des Sciences, Paris , 286 : 519 – 521 .
- Donadey , C. 1979 . “ Contribution à l'étude cytologique de deux Démosponges Homosclerophorides: Oscarella lobularis (Schmidt) et Plakina trilopha Schulze. ” . In Biologie des Spongiaires. , Edited by: Lévi , C and Boury‐Esnault , N . 165 – 172 . Paris : Editions du CNRS .
- Friedrich , A. B. , Merkert , H. , Fendert , T. , Hacker , J. , Proksch , P. and Hentschel , U. 1999 . Microbial diversity in the marine sponge Aplysina cavernicola (formerly Verongia cavernicola) analyzed by fluorescence in situ hybridization (FISH). . Marine Biology , 134 : 461 – 470 .
- Frost , T. M. , Graham , L. E. , Elias , J. E. , Haase , M. J. , Kretchmer , D. W. and Franzfelder , J. A. 1997 . A yellow–green algal symbiont in the freshwater sponge, Corvomeyenia everetti: convergent evolution of symbiotic associations. . Freshwater Biology , 28 : 395 – 399 .
- Gaino , E. , Burlando , B. , Sabatini , M. A. and Buffa , P. 1985 . Cytoskeleton and morphology of dissociated sponge cells. A whole‐mount and scanning electron microscopic study. . European Journal of Cell Biology , 39a : 328 – 332 .
- Green , C. R. and Bergquist , P. R. 1979 . “ Cell membrane specialization in the Porifera. ” . In Biologie de spongiaires , Edited by: Levi , C and Boury‐Esnault , N . 233 – 237 . Paris : Colloques Int CNRS .
- Harrison , F. W. , Dunkelberger , D. and Watanabe , N. 1974 . Cytological definition of the poriferan stylocyte: A cell type characterized by an intranuclear crystal. . Journal of Morphology , 142 : 265 – 276 .
- Harrison , F. W. and De Vos , L. 1991 . “ Porifera. ” . In Microscopic anatomy of invertebrates Vol. 2 Placozoa, Porifera, Cnidaria and Ctenophora. , Edited by: Harrison , F. W and Westfall , J. A . 29 – 89 . New York : Wiley‐Liss, Inc .
- Koziol , C. , Borojevic , R. , Steffen , R. and Muller , W. E. 1998 . Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: The telomerase activity in somatic cells. . Mechanisms of Ageing and Development , 100 : 107 – 120 .
- Lopez , J. V. , Peterson , C. L. , Willoughby , R. , Wright , A. E. , Enright , E. , Zoladz , S. , Reed , J. K. and Pomponi , S. A. 2002 . Characterization of genetic markers for in vitro cell line identification of the marine sponge Axinella corrugata. . Journal of Heredity , 93 : 27 – 36 .
- Müller , W. E. 1982 . Cell membranes in sponges. . International Review of Cytolology , 77 : 129 – 181 .
- Müller , W. E. , Perovic , S. , Wilkesman , J. , Kruse , M. , Muller , I. M. and Batel , R. 1999 . Increased gene expression of a cytokine‐related molecule and profilin after activation of Suberites domuncula cells with xenogeneic sponge molecule(s). . DNA Cell Biology , 18 : 885 – 893 .
- Müller , W. E. G. , Steffen , R. , Lorenz , B. , Batel , R. , Kruse , M. , Krasko , A. , Muller , I. M. and Schroder , H. C. 2001 . Suppression of allograft rejection in the sponge Suberites domuncula by FK506 and expression of genes encoding FK506‐binding proteins in allografts. . Journal of Experimental Biology , 204 : 2197 – 207 .
- Rosell , D. and Uriz , M. J. 1992 . Do associated zooxanthellae and the nature of the substratum affect survival, attachment and growth of Cliona viridis (Porifera: Hadromerida)? An experimental approach. . Marine Biology , 114 : 503 – 507 .
- Rutzler , R. 1990. . New perspectives in sponge biology. , Edited by: Rutzler , K . 455 – 466 . Washington DC : Smithsonian Institution Press .
- Sarà , M. 1971 . Ultrastructural aspects of the symbiosis between two species of the genus Aphanocapsa (Cyanophyceae) and Ircinia variabilis (Demospongiae). . Marine Biology , 11 : 214 – 221 .
- Sarà , M. and Vacelet , J. 1973 . “ Ecologie des demosponges. ” . In Traite de Zoologie. Anatomie, systematiques, biologie. Tome XVI Spongiaries. , Edited by: Grassè , P. P . 462 – 576 . Paris : Masson .
- Scalera Liaci , L. , Sciscioli , M. , Lepore , E. and Gaino , E. 1999 . Symbiotic zooxanthellae in Cinachyra tarentina, a non‐boring demosponge. . Endocytobiosis and Cell Research , 13 : 105 – 114 .
- Sipkema , D. , Heilig , H. G. H. J. , Akkermans , A. D. L. , Osinga , R. , Onji , J. and Wijffels , R. H. 2003 . Sponge‐cell culture? A molecular identification method for sponge cells. . Marine Biotechnology , 5 : 443 – 449 .
- Vacelet , J. 1967 . Le cellules à inclusions de l'éponge corneé, Verongia cavernicola. . Journal of Microscopy , 6 : 237 – 240 .
- Vacelet , J. and Donadey , C. 1977 . Electron microscope study of the association between some sponges and bacteria. . Journal of Experimental Marine Biology and Ecology , 30 : 301 – 304 .
- Van de Vyver , G. 1981 . “ Organisms without special circulatory systems. ” . In Invertebrate blood cells, Vol 1. , Edited by: Ratcliffe , N. A and Rowelet , A. F . 19 – 32 . New York : Academic Press .
- Wilkinson , C. R. 1987 . Significance of microbial symbionts in sponge evolution and ecology. . Symbiosis , 4 : 135 – 146 .
- Zhang , X. , Cao , X. , Zhang , W. , Yu , X. and Jin , M. 2003 . Primmorphs from archaeocytes‐dominant cell population of the sponge Hymeniacidon perleve: Improved cell proliferation and spiculogenesis. . Biotechnology and Bioengineering , 84 : 583 – 590 .