Abstract
During an essay to rear in aquarium some colonies of the Mediterranean erect bryozoan Myriapora truncata (Gymnolaemata, Cheilostomatida, Ascophorina, Myriaporidae) it was possible to study its larval and ancestrular morphology by scanning electron microscope (SEM) and by light microscope. The different phases of metamorphosis, giving origin to the preancestrula and the ancestrula, were also investigated. The larva of M. truncata is short‐lived, lecithotrophic, and of the coronate type, with expanded corona and small pallial sinus (AEO/ps type, according to Zimmer & Woollacott; VB type, according to d'Hondt). Its morphology, life and metamorphosis appear very similar to those of other well described cheilostome larvae (Neocheilostomida sensu d'Hondt). But, unlike in the other known species of this group, the ancestrula derived from metamorphosis is an ‘ancestrular twin’, in which two zooids simultaneously develop; these can form in two possible reciprocal orientations (same—ipsolateral—or opposite—contralateral—in the direction of growth).
Introduction
Myriapora truncata (Pallas, 1766) is a showy Mediterranean bryozoan species (Gymnolaemata, Cheilostomatida, Ascophorina, Myriaporidae), rather common in sciaphilous infralittoral and circumlittoral rocky environments from the surface to –60 m (Gautier Citation1962; Zabala Citation1986). It forms calcareous erect colonies, vinculariiform, more or less branched, with truncated extremities and with a vivid red colour: owing to its shape and colour this species is commonly named ‘false coral’. No heterozooids are present except for the ovicells, which are large, globular, and with a porous frontal wall, and which protrude only slightly from the zoarial surface (Figure ). The ovicellate zooids have openings larger than those of sterile autozooids.
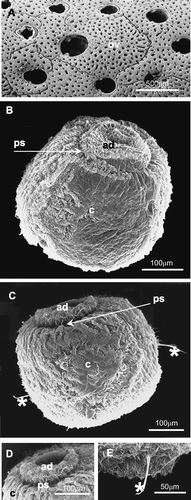
Figure 1 Scanning electron microscopy photographs.A, surface view of a portion of an adult colony of Myriapora truncata, showing some normal (male or sterile) autozooids and an ovicellate one, with a larger opercular opening; B, latero‐aboral view of a larva of M. truncata; C, larva with two ciliary tufts (asterisks) in the coronal region; D, detail of the aboral region of the larva; at the centre of the apical disc is an invagination that corresponds to the neural plate. The metachronal waves of the coronal cilia are organised in a radial pattern; E, detail of a ciliary tuft (asterisk) in the coronal region. ad, apical disc; c, ciliated corona; ov, ovicell; ps, pallial sinus.
In natural conditions embryos and larvae are produced in March (Gautier Citation1962), whereas in aquaria adult colonies release the swimming larvae during the whole year, with higher intensity from April to September (personal observation).
M. truncata is seriously threatened by human activities (divers, anchors, nets and other fishing tools); this is of particular concern considering the slow growth rate of its colonies, as observed in a field study (Gristina & Balduzzi Citation1999). Rearing experiments of this species in aquarium conditions allowed observations on larval release, morphology, and settling mechanisms and on the first phases of zoarial development (Balduzzi et al. Citation1999). Up to now, no other data on the larval and ancestrular morphology of any Myriaporidae species have been presented in the literature.
Materials and methods
Samples of M. truncata were collected by SCUBA diving at depths from 10 to 20 m on the rocky bottoms around the Gallinaria Island (Ligurian Sea, Italy), from October 1997 to July 2000. Colonies of M. truncata were carried alive to the Genoa Aquarium and maintained in vessels containing filtered seawater at 15–16°C (Mediterranean System). The culture water was periodically enriched using the microalgae Tetraselmis suecica as foodstuff.
After a period of adaptation to the new conditions, some colonies were exposed to temperatures of 19–20°C and to high light conditions in isolated vessels with filtered seawater but without food. Under these conditions the colonies began to release free‐swimming larvae from ovicells after about 1 h. Free‐swimming larvae were fixed for both a few minutes and 24 h after release from the adult colony. Ancestrulae used for SEM study were settled on natural substrata (the coralline alga Lithophyllum stictaeforme or the bryozoan Diplosolen obelia); those for histological study were settled on glass cover slides.
For light microscopy, larvae and ancestrulae were fixed in either Bouin's solution or 1% paraformaldehyde in artificial seawater (ASW) as a buffer (pH = 7.6) for 2 h at 4°C. Fixed specimens were rinsed in filtered ASW, completely dehydrated through a graded series of ethanols and embedded in Technovit 8100 or 7100 (Kulzer). Sections were cut at 3 µm, collected on slides and stained with toluidine blue, haematoxylin–eosin and Schiff Periodic Acid reagent (PAS). Slides were mounted in resin (Eukitt) and observed using a Leitz Wetzlar Dialux 20 EB light microscope. Drawings of larval, preancestrular and ancestrular morphology were made using the same light microscope with a camera lucida. Photomicrographs were taken using a Leitz Diaplan microscope (Wild MPS 55 automatic system).
For scanning electron microscopy (SEM) samples were fixed in 2.5% glutaraldehyde in ASW for 1 h at 4°C. After dehydration with a graded ethanol series, samples were critical‐point dried using a Pabisch CPD 750 system, coated with gold using a Balzers SCD 004 evaporator, and examined and photographed using a Philips EM 515 electron microscope.
Results
Larval behaviour
Observations taken in aquarium suggest that larval release is enhanced in conditions of high light and the absence of water current. In still water, the released larvae swim actively after liberation from the ovicells, showing an initial photopositive reaction; then they become photonegative (behavioural type I according to Ryland Citation1960, Citation1974) and tend to attach themselves onto the aquarium walls to start the metamorphosis. This generally occurs within few hours, but we observed several free swimming larvae as long as 24 h after larval release.
When the larvae contact the substratum they begin to crawl over the surface with the apical disc forward. To find a suitable location for the metamorphosis, larvae explore the substratum with a ciliary tuft (probably the pyriform complex vibratile plume). Occasionally, during crawling, larvae stop moving and undergo an unexplained contraction event.
Larval morphology and histology
The living larva of M. truncata has a subspherical shape and a vivid red colour. Larvae measure about 460 µm in the oro‐aboral axis and about 390 µm in the antero‐posterior axis. The morphology is typical of a lecithotrophic larva. The larval locomotory organ, the ciliated corona, is very large and covers most of the larval surface except the two polar fields that are also ciliated. M. truncata has a quite large aboral field (Figure ) whereas the oral field is little and not very distinct from the corona.
At the aboral field there is a convex, ciliated apical disc that has under the ciliary layer, a series of ridges and grooves disposed radially, probably indicating radially disposed series of cells (Figure ). At the central area of this apical disc there is an unciliated depressed area, corresponding to the neural plate with sensory function (Figure ). The apical disc is separated from the corona by an unciliated pallial sinus, evident but not deep (Figure ).
The apical disc is formed by a ciliated epithelium constituted by one layer of cuboidal cells (Figure ). Its central zone constitutes the neural plate, that appears unciliated (Figure ). The neural plate is presumably connected to the internal sac, corona and pyriform complex by a neuromuscular cord as in Bugula (Woollacott & Zimmer Citation1971, Zimmer & Wollacott 1977a). In our frontal sections this cord appears to be paired, but only its first tract below the neural plate is visible (Figure ).
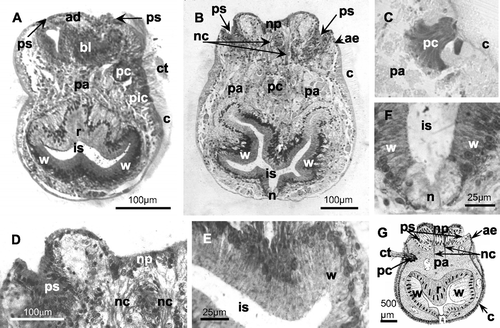
Figure 2 Histological sections of the larvae ofM. truncata (A, B, D–F: toluidine blue staining; C: PAS staining). A, sagittal section of a larva fixed immediately after its release from the colony; B, frontal section of a larva fixed 24 h after the release; C, sagittal section of the pyriform complex (200×); D, detail of the apical disc; E, F, details of the internal sac; G, schematic reconstruction of the larva based on several samples diversely oriented: in particular the pyriform complex is located as in sagittal sections and the neuromuscular cords are drawn as in frontal sections. ad, apical disc; ae, aboral epithelium; bl, blastema; c, ciliated corona; ct, ciliary tuft; is, internal sac (n, neck, r, roof, w, wall); nc, neuromuscular cord; np, neural plate; pa, parenchyma; pc, pyriform complex; pic, pigmented cells (possible photoreceptor? see text); ps, pallial sinus.
The pallial sinus, forming a shallow groove at the margin of the apical disc, is constituted of a columnar epithelium (Figure ). The aboral margin of the corona and the oral margin of the pallial epithelium are connected by a narrow unciliated epithelium (Figure ), probably corresponding to the vesicular aboral epithelium described in similar larvae (Zimmer & Woollacott Citation1977a).
In the corona, tufts of longer cilia can be seen (Figure ). In some images two lateral ciliary tufts, directed toward the oral field, are visible (Figure ). It seems to be excluded that these paired ciliary tufts are related to the pyriform complex (see below). They could concern sensory organs, as those described in several bryozoan larvae by Woollacott and Zimmer (Citation1972) and Hughes and Woollacott (Citation1980), or could correspond to the balancers, arising from infracoronal cells in larvae of Watersipora arcuata and other ctenostome and cheilostome species, already described by Barrois (Citation1877) and Zimmer and Woollacott (Citation1989a,Citationb). In M. truncata, which has a corona larger than that of W. arcuata, these long tufts seem to be located in an intercoronal rather than infracoronal position (Figure ), but a confirmation of their topological relationships needs further investigation.
A pyriform complex is visible in the upper half of the larval height. It is formed by unicellular glands (PAS+) (Figure ) and associated with a ciliary tuft or vibratile plume (Figure ). Its location identifies the anterior larval region (according to Calvet Citation1900 and d'Hondt Citation1977a). The glandular complex visible in Figure probably corresponds to the superior glandular field, described by several authors (see Zimmer & Woollacott Citation1977a), while the inferior glandular field is not clearly visible. Furthermore in our samples it was not possible to detect the ciliated groove that, according to Zimmer & Woollacott (Citation1977a), should lie in the midline below this organ.
A group of pigmented cells is situated under the corona near the larval equator (Figure ). These cells probably can be interpreted as photoreceptors as described by Hughes and Woollacott (Citation1980) for Cellularioidea, but their unusual location (below the pyriform complex towards the oral region) raises doubts on this interpretation.
In the aboral field, there is one mass of columnar cells (Figure ) probably corresponding to the mesodermal blastema of Bugula neritina (Woollacott & Zimmer Citation1971; Zimmer & Woollacott Citation1977a); in our samples no evidence of two distinct layers (epidermal and mesodermal blastemas, as in Bugula) was detectable. In B. neritina the blastemas surround the neuromuscular cords (Zimmer & Wollacott Citation1977a), whereas in our sections (Figure ) this evidence is not clearly proved.
At the oral field the internal sac is an invagination of the oral epithelium. The large internal sac is formed by three regions called the neck, wall and roof (Figure ). The roof and wall consist of columnar epithelia containing numerous flask‐shaped cells rich in vacuoles. The neck epithelium is formed by shorter cells rich in unstained droplets, and with nuclei located near their base.
The lecithotrophic larva of M. truncata lacks a digestive tract and the internal parenchyma cells of its body are filled with large yolk deposits and lipid droplets (Figure ). These nutrient reserves are initially very abundant (Figure ) and then are gradually reduced in abundance during the planktonic existence (Figure ).
Metamorphosis of the larva
Few direct observations have been carried out on the initial phases of metamorphosis, when a larva settles on the substratum and its external structures involute, due to the difficulties in fixing larvae during this rapid event. Only one image shows the eversion of the internal sac and the first stages of involution of ciliated corona (Figure ). Also in a histological section we can observe an initial eversion of the internal sac roof region through an opening at the level of the neck region (Figure ).
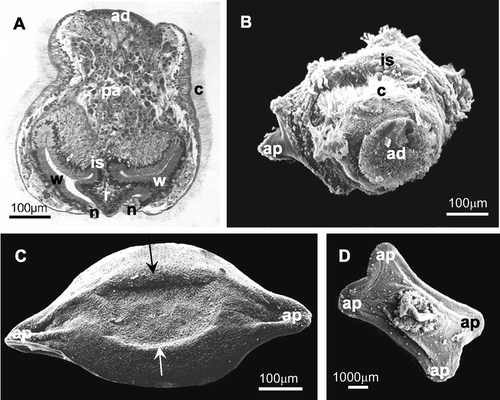
Figure 3 Metamorphosis of the larva: eversion of the internal sac and the first stages of involution of the ciliated corona. A, histological section that shows an opening at the level of the oral field (neck region) with a beginning of the eversion of the internal sac (roof region); B, SEM photograph in which the internal sac begins to cover the ciliated corona and the first adhesion process to the substratum is visible; C, D, preancestrulae. C, preancestrula with subelliptical shape with two external adhesion processes to the substratum. The calcification of the lateral walls occurs centripetally from the edges of the encrusting base (arrows); D, subrectangular shape with four external adhesion processes to the substratum. ad, apical disc; ap, adhesion process to the substratum; c, ciliated corona; is, internal sac (n, neck, r, roof, w, wall); pa, parenchyma.
Morphology and histology of preancestrula and ancestrula
At the end of the phase of eversion of the internal sac, an incompletely developed preancestrula is formed (4–24 h after larval release). Initially, a preancestrula of M. truncata can attach to the substratum by means of either two (Figure ) or four external adhesion processes (Figure ), and consequently it assumes a different shape, being subelliptical (660–665 µm long and 330–335 µm wide) or subrectangular (890–960 µm long and 465–600 µm wide), respectively. The calcification of the lateral walls occurs centripetally from the edges of the attached base (Figure ).
The following phase of the metamorphosis, during which an ancestrula differentiates from the preancestrula, is much longer than required for preancestrular formation. In vivo we observed completed ancestrulae, with active polypides, about 10 days after attachment to the substratum.
We examined preancestrular and ancestrular morphology in samples fixed 5, 14, 34 and 40 days after settling (Figures ).
Figure 4 Five‐day preancestrula. A, B, SEM photographs. A, specimen with two opercular outlines situated in opposite (contralateral) positions (visible under the external cuticle). The calcified trabecular structure of the frontal wall is somewhere perceivable under the external cuticle (arrow); B, specimen with two opercular outlines situated near each other (ipsolateral); C–E, histological sections. C, preancestrula in toto; D, larval tissues in degeneration; E, vesicle that will form the polypide structures in the next stages; F, schematic drawing of the preancestrular structure. ct, external cuticle; ep, epidermis; ltd, larval tissues in degeneration; op, opercular outlines; ve, vesicle (polypide primordium).
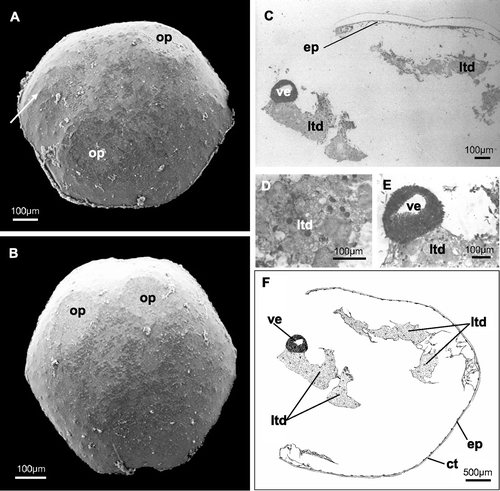
Figure 5 Fourteen‐day ancestrula. A, SEM photograph of a specimen with the two opercula completely formed (in contralateral position); B–F, histological sections. B, horizontal section showing the two completely formed polypides; C‐D, different sections of ciliated tentacles; E, section of the digestive tract (probably pylorus and rectum); F, particular of the walls of the presumed pylorus (simple columnar ciliated epithelium) and rectum (simple not ciliated epithelium); G, schematic drawing. ct, external cuticle; ep, epidermis; mf, muscle fibres; py, presumed pylorus; pol, polypide; op, operculum; re, presumed rectum; te, tentacles; ts, tentacle sheath.
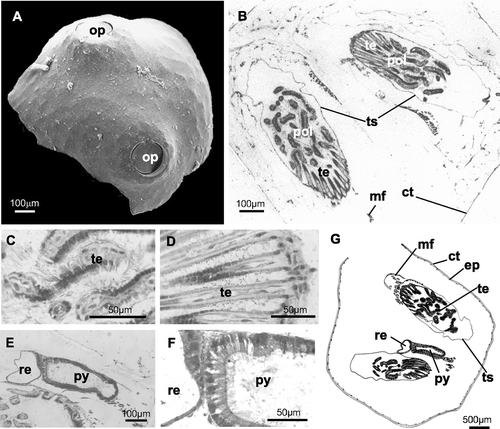
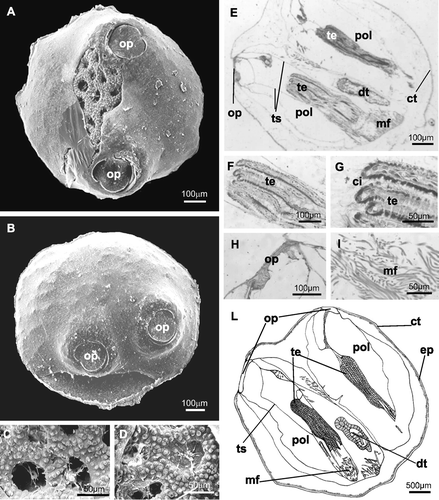
Figure 6 A–D, SEM photographs. A, 34‐day ancestrula with the opercula in contralateral position. The trabecular structure of the skeleton is visible through a fracture of the external cuticle; B, 40‐day ancestrula with the opercula in ipsolateral position; C, trabecular structure in a 34‐day ancestrula; D, trabecular structure in a 40‐day ancestrula that is very similar to the previous. E–I, histological sections of a 40‐day ancestrula. E, horizontal section of an ancestrula with the two polypides orientated in the same direction (ipsolateral); one operculum is visible; F, G, longitudinal section of ciliated tentacles, at different magnifications; H, longitudinal section of an operculum; I, retractor muscle fibres at the base of the polypide structure; L, schematic drawing of a 40‐day ancestrula. ci, tentacle cilia; ct, cuticle; dt, digestive tract; ep, epidermis; mf, muscle fibres; pol, polypide; op, operculum; te, tentacles; ts, tentacle sheath.
The completely calcified preancestrula and the ancestrula are subovoidal in shape, measuring 680–960 µm in maximum diameter. Two zooids form contemporarily in the preancestrula: 5 days after settling two opercular outlines are usually visible under the outer cuticle (Figure ). In some cases these outlines are still not evident at this time. The opercula can form in contralateral (Figure ) or ipsolateral positions (Figure ). Under the external cuticle the calcified trabecular structure of the frontal wall is perceivable (Figure ).
In the ancestrula fixed after 14 days the opercula are completely formed and the zooids are completely developed, as confirmed by observations in vivo. The ancestrular orifices (Figure ) have the typical morphology of those in the adult Myriapora (Figure ), with a deep and rounded vanna and a subcircular porta divided by two condyles. The external morphology of the ancestrula remains the same 34 and 40 days after the settlement (Figure ). In samples where the external cuticle was fractured during the fixation and mounting phases, a perforated wall with finely tuberculated trabeculae is clearly visible (Figure ). Also in completely formed ancestrulae, the opercula can be situated in contralateral (Figure ) or ipsolateral positions (Figure ).
Study of the histology of the preancestrula and ancestrula confirms our observations from SEMs. The 5‐day preancestrula has an external cuticle with an epidermis below (Figure ). The interior of the preancestrula is still partially occupied by disintegrating larval tissues (Figure ), but a primordium of a polypide, in the form of a thick‐walled vesicle, is already visible (Figure ). The vesicle wall is actually two‐layered, as observed by direct microscope examination, although this feature is not clearly showed by the photographs. In this particular section neither the second forming polypide nor the opercular outlines are visible. In the 14‐day ancestrula, below the external thin cuticle and epidermis, the polypides are completely formed (Figure ): hollow ciliated tentacles and tentacle sheath (Figure ), digestive tract (Figure ) and muscle fibres (probably the retractor muscles of the polypide) (Figure ) are visible. Sections of the digestive tract show a region delimited by a simple columnar ciliated epithelium that we interpreted as the pylorus and another one, delimited by a simple unciliated epithelium, that probably corresponds to the rectum (Figure ).
The 34‐ (Figure ) and 40‐day ancestrulae (Figure ) are not significantly different from 14‐day‐old ones. In sections there is a very thin cuticle external to the epidermis (Figure ), hollow ciliated tentacles (Figure ) encircled by the tentacle sheath (Figure ), retractor muscle fibres at the base of the lophophore (Figure ), digestive tract (Figure ) and the opercula (Figure ).
Discussion
The larva of Myriapora truncata, like in most cheilostomes (Zimmer & Woollacott Citation1977a), is lecithotrophic with a short pelagic existence, after a brooding phase in the ovicells. According to its morphological and histological features it belongs to type AEO/ps of the classification by Zimmer & Woollacott (Citation1977a): ‘coronate larvae with expanded coronas that are aboral, equatorial and oral (AEO) in position and with small pallial sinuses (ps)’. It also corresponds to the larval type VB, described for Cheilostomatida Ascophorina, in the classification by d'Hondt (Citation1977b): ‘larves de Cheilostomes de type columniforme, à sillon palléal symétrique et superficiel’. This larval type is one of the diagnostic characters of the Neocheilostomida. This taxon was defined as a suborder by d'Hondt (Citation1985) on ontogenetic criteria and more recently has been confirmed as an order of the subclass Cheilostomona (d'Hondt Citation2001). It includes all the cheilostomes except the Malacostegida (sensu stricto), the Scrupariida and the Inovicellatida, and therefore contains the genus Myriapora (included in the suborder Ascophorina, infraorder Lepraliomorpha).
Morphologically and anatomically, the larvae of Neocheilostomida are very similar; intrageneric and intraspecific variabilities have been well described and illustrated by various authors, particularly Barrois (Citation1877), Calvet (Citation1900) and more recently Humphries (Citation1975, Citation1977). Usually Neocheilostomida species that are characterized by an encrusting zoarium have larvae that are wider than high, while those with erect zoarium (as Myriapora) have larvae that are higher than wide (d'Hondt Citation1977b).
The morphological and anatomical structure of M. truncata larvae strictly corresponds to that of the anascan Bugula neritina (L.) (Neocheilostomida, Flustrina, Cellulariomorpha, Buguloidea) described by Woollacott and Zimmer (Citation1971). Also the observations made during the first steps of metamorphosis suggest that this mechanism is very similar in both species: after a period of free swimming, a larva settles and everts the internal sac to adhere to the substratum by means of its roof region (Figure ). The wall region of internal sac begins to extend over the ciliated corona (Figure ) following a mechanism probably corresponding to those described by Woollacott and Zimmer (Citation1971) for B. neritina and Ikezawa et al. (Citation1994) for three Celleporina species (Ascophorina). At the end of a complex phase involving the degeneration of most of larval organs, the wall region of the internal sac covers the entire preancestrular body and differentiates into its epidermis, then secreting an external cuticle. Meanwhile the invagination of the apical organ gives rise to the polypide rudiment (from the blastema); the tentacle sheath will form from the pallial epithelium (according to Zimmer & Woollacott Citation1977b) or from suprapallial epidermal cells (according to d'Hondt Citation1976b, Citation1979).
The two observed shapes of the preancestrula, subelliptical or subrectangular, depending on the number (two or four) of the adhesion points to the substratum, were described and figured similarly by d'Hondt (Citation1976a) for Microporella ciliata.
At the end of the second phase of metamorphosis (from the preancestrula to the ancestrula), M. truncata produces a composite ancestrula as in several bryozoan species, in particular an ‘ancestrular twin’ as in the anascan genus Membranipora membranacea (Malacostegina) that instead has cyphonautes larvae (Zimmer & Woollacott Citation1977b). But, whereas multiple zooid buds are already present at the end of the metamorphosis in Membranipora, the ancestrula of M. truncata is composed of only two functional zooids, without any sign of further budding. Long‐term observations in aquarium conditions (up to two years: S. Repetto, personal communication) revealed no case of colony development in still living ancestrulae. This may be due to a very slow growing rate of this species, as already observed even in natural conditions (Gristina & Balduzzi Citation1999).
Another peculiarity of the ancestrula of Myriapora, in respect to other known cases of compound ancestrulae (Zimmer & Woollacott Citation1977b), is the lack of a rigid pattern in the orientation of the two ancestrular zooids. Up to now it is impossible to say if the arrangement of the two opercula, opposite (contralateral) or near each other (ipsolateral), is due to environmental (substratum morphology or microhabitat conditions) or genetic factors. D'Hondt (personal communication) supposes a possible hormonal determinism for this phenomenon.
Acknowledgements
The authors would like to thank Laura Castellano (Genoa Aquarium, Italy) for her assistance in maintaining colonies in the Mediterranean system holding pools, Sabrina Mozzone (Piemonte Orientale University, Italy) for help and suggestions in histological stainings and mounts, Sabrina Repetto (Le Navi Sea Park, Cattolica, Italy) for help and information of her observations during her stay at the Genoa Aquarium. We also would like to thank Jean‐Loup d'Hondt (MNHN, Paris, France) and Russel L. Zimmer (USC, Los Angeles, USA) for critically reviewing this manuscript.
References
- Balduzzi , A. , Castellano , L. , Ferretti , C. , Repetto , S. and Magnino , G. 1999 . “ Morfologia delle prime fasi di sviluppo di Myriapora truncata (Bryozoa, Gymnolaemata, Cheilostomatida). ” . In 60° Congresso Nazionale Unione Zoologica Italiana, Pavia Riassunti dei contributi scientifici. Modena: Poligrafico Mucci. pp. 54
- Barrois , J. 1877 . Recherches sur l'embryogénie des Bryozoaires. . Travaux de la Station Zoologique de Wimereux , 1 : 1 – 305 .
- Calvet , L. 1900 . Contribution à l'histoire naturelle des Bryozoaires Ectoproctes marins. . Travaux de l'Institut de Zoologie de l'Université de Montpellier NS , 8 : 1 – 458 .
- Gautier , Y. V. 1962 . Recherches écologiques sur les Bryozoaires Chilostomes en Méditerranée Occidentale. . Recueil des Travaux de la Station Marine d'Endoume , 38 : 1 – 434 .
- Gristina , M. and Balduzzi , A. 1999 . Prime osservazioni sull'accrescimento di Myriapora truncata sui fondali di Ustica (Bryozoa: Cheilostomatida). . Biologia Marina Mediterranea , 6 : 256 – 258 .
- Hondt , J. L d'. 1976a . Les larves, la métamorphose larvaire et la morphogenèse post‐larvaire chez les Bryozoaires Gymnolaemates (Etude anatomique et ultrastructurale) [dissertation]. Thèse de Doctorat d'Etat. , 325 Paris : Université Pierre et Marie Curie .
- Hondt , J. L d'. 1976b . Evolution des lignées cellulaires larvaires des Bryozoaires Gymnolaemates au cours de la métamorphose et de l'organogenèse ancestrulaire. . Bulletin de la Société Zoologique de France , 101 (Suppl) : 41 – 47 .
- Hondt , J. L d'. 1977a . Structure larvaire et histogenèse post‐larvaire chez Bowerbankia imbricata (Adams, 1798), Bryozoaire Cténostome (Vésicularines). . Archives de Zoologie Expérimentale et Générale , 118 : 211 – 243 .
- Hondt , J. L d'. 1977b . Valeur systématique de la structure larvaire et des particularités de la morphogenèse post‐larvaire chez les Bryozoaires Gymnolaemates. . Gegenbaurs Morphology Jahrbuch , 123 : 463 – 483 .
- Hondt , J. L d'. 1979 . “ Ultrastructural characteristics of the various larval cell categories of the Gymnolaematous Bryozoa. ” . In Advances in bryozoology. , Edited by: Larwood , G. P and Abbott , M. B . 47 – 58 . London : Academic Press .
- Hondt , J. L d'. 1985 . Contribution à la systématique des Bryozoaires Eurystomes. Apports récents et nouvelles propositions. . Annales des Sciences Naturelles‐Zoologie 13e Sér , 7 : 1 – 12 .
- Hondt , J. L d'. 2001 . Flustrina versus Neocheilostomina (Byozoaires). Remarques sur la biosystématique aux niveaux supraspécifiques. . Bulletin de la Société zoologique de France , 126 : 391 – 406 .
- Hughes , R. L. and Woollacott , R. M. 1980 . Photoreceptors of bryozoan larvae (Cheilostomata, Cellularioidea). . Zoologica Scripta , 9 : 129 – 138 .
- Humphries , E. 1975 . A new approach to resolving the question of speciation in smittinid bryozoans (Bryozoa: Cheilostomata). . Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon, Hors Série , 3 : 19 – 35 .
- Humphries , E. 1977 . Larval behaviour and post‐larval development in Parasmittina nitida morphotype B (Bryozoa: Cheilostomata). . American Zoologist , 17 : 5 – 20 .
- Ikezawa , H. , Nodasaka , Y. and Mawatari , S. F. 1994 . “ Larval morphology and preancestrula formation of three Celleporina species (Bryozoa: Cheilostomatida) from Hokkaido, Japan. ” . In Biology and paleobiology of bryozoans. , Edited by: Hayward , P. J , Ryland , J. S and Taylor , P. D . 87 – 92 . Fredensborg : Olsen & Olsen .
- Ryland , J. S. 1960 . Experiments on the influence of light on the behaviour of polyzoan larvae. . Journal of Experimental Biology , 37 : 783 – 800 .
- Ryland , J. S. 1974 . Behaviour, settlement and metamorphosis of bryozoan larvae: A review. . Thalassia Jugoslavica , 10 : 239 – 262 .
- Woollacott , R. M. and Zimmer , R. L. 1971 . Attachment and metamorphosis of cheilo‐ctenostome bryozoan Bugula neritina (Linné). . Journal of Morphology , 134 : 351 – 382 .
- Woollacott , R. M. and Zimmer , R. L. 1972 . Fine structure of a potential photoreceptor organ in the larva of Bugula neritina (Bryozoa). . Zeitschrift für Zellforschung und mikroskopische Anatomie , 123 : 458 – 469 .
- Zabala , M. 1986 . Fauna dels Briozous dels Països Catalans. . Institut d'Estudis Catalans, Arxius de la Secció de Ciències , 84 : 582 – 583 .
- Zimmer , R. L. and Woollacott , R. M. 1977a . “ Structure and classification of gymnolaemate larvae. ” . In Biology of bryozoans. , Edited by: Woollacott , R. M and Zimmer , R. L . 57 – 89 . New York : Academic Press .
- Zimmer , R. L. and Woollacott , R. M. 1977b . “ Metamorphosis, ancestrulae, and coloniality in bryozoan life cycles. ” . In Biology of bryozoans. , Edited by: Woollacott , R. M and Zimmer , R. L . 91 – 142 . New York : Academic Press .
- Zimmer , R. L. and Woollacott , R. M. 1989a . Larval morphology of the bryozoan Watersipora arcuata (Cheilostomata: Ascophora). . Journal of Morphology , 199 : 125 – 150 .
- Zimmer , R. L. and Woollacott , R. M. 1989b . Intercoronal cell complex of larvae of the bryozoan Watersipora arcuata (Cheilostomata: Ascophora). . Journal of Morphology , 199 : 151 – 164 .