Abstract
A new species of land planarian from Colombia, Gigantea maupoi sp. nov., is described herein. The penis papilla of the new species is provided with numerous accessory genital organs, or mgo. These mgo are of a unique morphology, consisting of a very strong barrel‐shaped circular muscle coat and an underlying longitudinal one, both derived from the subepithelial musculature of the penis papilla. The central mass of each mgo contains secretions and 8–15 canalicula that open to the male atrium on the central region of the organ. Differences in structures constituting the mgo and details of the muscular systems and of the reproductive organs among the 11 Gigantea species from literature and from type material of G. bistriata (Hyman, Citation1962), G. chiriquii (Hyman, Citation1962), and G. sandersoni (Prudhoe, Citation1949) reexamined here show that the genus is heterogeneous.
Introduction
The subfamily Geoplaninae Stimpson, 1857 (Platyhelminthes: Tricladida: Terricola), neotropical in distribution, includes geoplanid species with dorsal testes and numerous eyes. This subfamily is composed presently of 16 genera—in addition to the collective group Pseudogeoplana Ogren and Kawakatsu, Citation1990 for species inquerendae and nomina dubia—including Gigantea Ogren and Kawakatsu, Citation1990. The distribution of the genus is mainly Caribbean and North Andean since it comprises species from Costa Rica, Panama, Colombia, Trinidad and Tobago, Venezuela and Peru. The genus was erected by a combination of characteristics based on original descriptions, and is the only one within Geoplaninae with male accessory genital organs (glandular ridges as in the definition of the genus) in the penis papilla.
In recent years, a number of additional morphological characteristics have provided more detailed descriptions of species of Geoplaninae. However, the morphological knowledge within the genus Gigantea is relatively poor, hindering progress in the systematics within the subfamily. From this perspective a new species of Gigantea is described herein and a taxonomic revision of the genus is presented. The male accessory genital organs, mgo, of the new species are compared with those known in Geoplaninae from the literature and from new observations conducted by the author in the type material of Gigantea bistriata (Hyman, Citation1962), G. sandersoni (Prudhoe, Citation1949), and G. chiriquii (Hyman, Citation1962).
Material and methods
The holotype was collected from the grass in the type locality, in the city of Medellín, Antioquia, Colombia. Some observations of the live animal were made and then it was killed with boiling water, fixed with FAA and preserved in alcohol. Tissue blocks from different regions of the body were dehydrated in a graded series of ethanol, treated with isopropyl alcohol and embedded in paraffin. Sagittal, transverse or horizontal 7–8 µm serial sections of the anterior region, the pharynx, the pre‐pharyngeal region or the copulatory apparatus, were stained with Mallory/Cason method. When not specified, drawings were prepared using a camera lucida. Additionally, the type material of Gigantea bistriata, G. sandersoni, and G. chiriquii, were also analyzed.
Figure abbreviations
a, anterior end of the body; b, barrel‐shaped muscle coat; c, canaliculus of the male accessory genital organ ; cm, common muscle coat; co, common glandular ovovitelline duct; cr, creeping sole; dd, diagonal mesenchymal muscles; dv, dorso‐ventral mesenchymal muscles; e, eyes; ed, ejaculatory duct; ef, efferent duct; er, erythrophilic secretion; es, esophagus; fa, female genital atrium; go, gonopore; i, intestine; il, ill cyanophilic mass; in, sunk nucleus of the epithelium of the penis papilla; lm, longitudinal subepithelial muscles; ls, longitudinal subepidermic muscles; m, muscles; ma, male genital atrium; mgo, male accessory genital organ; mo, mouth; n, cell nucleus; ov, ovovitelline duct; p, pigment ; ph, pharyngeal pocket; pp, penis papilla; pv, prostatic vesicle; sb, sub‐intestinal transverse mesenchymal muscles; se, sub‐muscular peripheral nerve net; sg, shell glands; sp, supra‐intestinal transverse mesenchymal muscles; t, testes; v, vagina; vi, vitellaria; vn, ventral nerve plate.
Taxonomic accounts
SERIATA Bresslau, 1928–33
TERRICOLA Hallez, 1892
Geoplaninae Stimpson, 1857
Gigantea Ogren and Kawakatsu, Citation1990
Gigantea maupoi sp. nov.
Diagnosis
Flattened Gigantea ca. 6 cm long. Dorsum olive green in life. Sub‐neural mesenchymal muscle layer only present in the cephalic region. Pharynx cylindrical to bell‐shaped. Esophagus present. Inner musculature of the pharynx and of the esophagus composed of a one‐fiber‐thick longitudinal subepithelial layer followed by a circular layer with some interspersed longitudinal fibers. Genital apparatus long. Male atrium as long as the female one. Penis papilla obliquely emerging from the dorsal wall of the male atrium; each mgo of the penis papilla presents typically with several canalicula.
Distribution
Only known from the type locality.
Type material
Holotype. MZUSP PL. 220. Crawling on the grass at 10:00 am in the type locality. 8/XII/2002, Mauricio Andrés Muñoz Restrepo, leg. Region 1, from anterior tip to 8.5 mm behind: transverse sections on 19 slides (Figure ); region 2: sagittal sections on 16 slides; region 3: horizontal sections on 7 slides; pre‐pharyngeal region: transverse sections on 9 slides; pharynx and copulatory apparatus: sagittal sections on 51 slides. Region 4 and posterior end of the body are preserved in ethanol 80%.
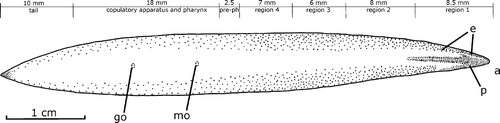
Figure 1. Gigantea maupoi sp. nov. Dorsal pigment pattern and distribution of the eyes of the fixed holotype. For clarity the eyes were not drawn in the first 2 mm of the right anterior side of the body. The regions into which the body was fragmented for histological preparation are also indicated. Free‐hand drawn. Scale bar: 1 cm.
Type locality
Near the road Las Palmas, km. 8 (6°13′10″N, 75°33′27″W), ca. 1900 m altitude, Municipality of Medellín, Antioquia State, Colombia. The area is occupied by buildings and grass‐covered gardens surrounded by conifer and eucalyptus forests. It was not possible to confirm if the species is native to the region.
Etymology
The specific epithet is a free combination of the collector's names, Mauricio Andrés Muñoz Restrepo, to whom the species name is dedicated.
External morphology
From collector's notes: The living specimen is 70 mm in length and 6 mm in width. The head is dorsally curled when crawling. The posterior end is very pointed. The appearance of the dorsum is olive green, maybe partially caused by the intestinal branches; the borders are light green except in the anterior region of the body, which is black in color. The middorsal region is black in the very anterior end of the body; posteriorly, it grows initially dark green and then lighter up to the region of the copulatory apparatus, where it has the appearance of a longitudinal light green discoloration 7 mm in length. The eyes are abundant externally up to the black middorsal stripe; towards the posterior end of the body they become scarcer. (Translated from original in Spanish.)
The preserved worm is 60 mm in length and 6 mm in width. The body is lanceolated and very flat (width: length ratio of 10%), with the maximum width in the pharynx region (Figure ). From this region to the extremities, the borders converge forming a curve; the anterior extremity is rounded, the posterior pointed. The ground color of the dorsum is yellowish, but the intestinal contents turn it dark brown. The margins of the body are brown in the first six millimeters. A pair of medial dark brown stripes course along the first 10 mm. The ventral side is pale yellow, with brown borders in the first 8 mm.
The eyes are monolobated, 30 µm in diameter, without halos, bordering the cephalic region in a single row in the first millimeter (Figure ). Behind this region, the eyes become biserial up to 4 mm. From 4 mm to 30 mm they become dorsal, occupying a band with one‐third of the body width. Posteriorly, the band with eyes narrows progressively up to the posterior end of the body. A total of 1910 eyes were counted. Sensory pits are simple invaginations 30 µm deep, ventrally surrounding the anterior region of the body (equivalent to 14% of body length) in a single row. The mouth is at a distance from anterior tip equivalent to 60% of the body length; the gonopore, 73%.
Epidermis and subepidermic secretions
The epithelium is ciliated only in the creeping sole, which has approximately 90% of body width at the pre‐pharyngeal region. The epithelium is 17 µm high in the medial region of the dorsal pre‐pharyngeal region, 25 µm in the lateral region and 17 µm ventrally. In the cephalic region, the epithelium is 6 µm high, dorsally and ventrally.
Two types of granulous secretory cells discharge onto the entire epidermis in the cephalic region: xanthophilic and indistinctly cyanophilic, respectively. A third, scarcer type is xanthophilic, with sparse granules and openings located between the creeping sole and the sensory border. A fourth type, erythrophilic, discharge onto the dorsal and ventral epithelium, being absent at the margins. The necks of most cells of this erythrophilic type run in bundles of parallel ducts, as previously reported by Froehlich (Citation1955) for several land planarians.
In the pre‐pharyngeal region, two types of secretory cells, granulous cyanophilic and rhabditogen cells, respectively, open onto the dorsal epithelium and scarcely onto the marginal one. Granulous acidophilic secretory cells open only dorsally. Scarce secretory cells of xanthophilic sparse granules open onto the body margins. The ventral epithelium and closed tissues of the pre‐pharyngeal region are artifactually ill‐stained and only granulous cyanophilic secretory cells were observed.
Subepidermic musculature
There are the typical three layers of Geoplaninae: a subepithelial circular layer is followed by a diagonal with decussate bundles, and then a longitudinal layer also arranged in bundles (Table ). The subepidermic musculature thickness relative to body height ( = MCI, or mc:h) is 9.1% in the pre‐pharyngeal region. The same arrangement of the fibers occurs in the cephalic region.
Table I. Thickness of subepidermic musculature in the pre‐pharyngeal region of Gigantea maupoi sp. nov.
Mesenchymal musculature
In the pre‐pharyngeal region, the mesenchymal muscle fibers are arranged in various directions, while the dorso‐ventral are the most abundant. Other mesenchymal fibers gather in three layers: a dorsal diagonal layer with decussate fibers (37 µm thick) located under the dorsal sub‐muscular peripheral nerve net, and two transverse ones, supra‐intestinal (125 µm), and sub‐intestinal (100 µm), respectively (Figure ). The dorsal diagonal layer is constituted by bundles of 1–4 fibers each. The other two layers do not present bundles. The fibers of the supra‐intestinal layer are somewhat more sparsely distributed than those of the sub‐intestinal layer.
Figure 2. Gigantea maupoi sp. nov. Diagrammatic transverse section of half of the pre‐pharyngeal region of the holotype. Scale bar: 1 mm.
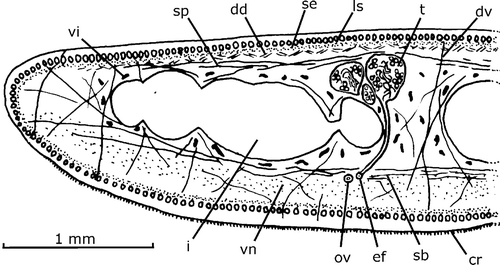
In the cephalic region, there is a fourth layer, sub‐neural ( = below the ventral nerve plate, 25 µm) constituted by transverse fibers. It occurs in the first ca. 1.5 mm. In this region the supra‐ and sub‐intestinal fibers become more intensely scarce than the others, so that the dorso‐ventral fibers and the dorsal diagonal layer are more apparent than the former. Close to the anterior apex of the body, the fibers diminish in number and become extinct.
Digestive system
The mouth is located in the middle of pharyngeal pocket. The pharynx is 3.9 mm long, cylindrical to bell‐shaped (Figure ), occupying three‐quarters of the pharyngeal pocket. There is a short esophagus: 0.4 mm long.
Figure 3. Gigantea maupoi sp. nov. Diagrammatic reconstruction of the pharynx in lateral view of the holotype. Scale bar: 1 mm.
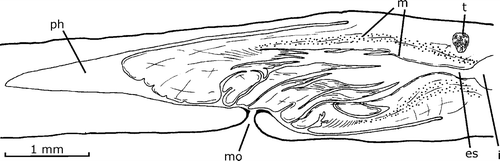
The lining epithelium of the pharyngeal pocket is squamous, non‐ciliated, columnar around the mouth; its musculature is composed of a one‐fiber‐thick longitudinal subepithelial layer. The outer pharyngeal epithelium is cuboidal, ciliated in the ruffled free border of the pharynx. This region is crossed by two types of abundant secretory cells, with cyanophilic and erythrophilic granules, respectively, the bodies of which are located outside the pharyngeal stroma. The outer musculature is composed of a one‐fiber‐thick longitudinal layer followed by a two‐fiber‐thick circular one. The epithelium of the esophagus is cuboidal, ciliated, the apical surface of the cells being irregular, and crossed by two types of granulous secretory cells, erythrophilic and cyanophilic, respectively. The inner epithelium of the pharynx is proximally similar to that of the esophagus, and squamous distally. The same two types of granulous secretory cells found in the esophagus are associated with this epithelium. The inner musculature of the pharynx and of the esophagus consists of a one‐fiber‐thick longitudinal subepithelial layer followed by a circular layer (170 µm) with some interspersed longitudinal fibers. Longitudinally and radially oriented muscle fibers run in the stroma of the pharynx
Male reproductive system
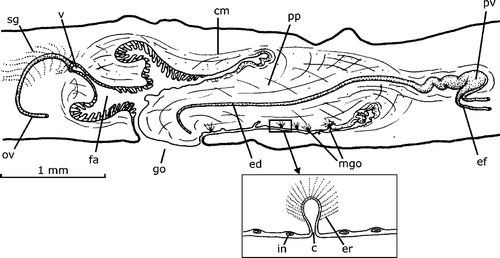
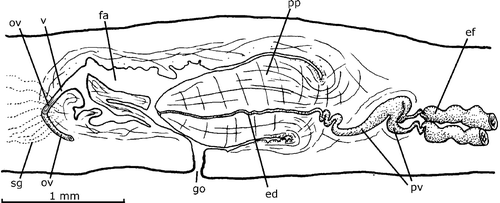
The testes are mature, with spermatozoa in their lumen. Their shape is rounded, 250–300 µm in diameter, dorsal in position, between supra‐intestinal muscle layer and intestine, partially between its diverticles (Figure ). They are arranged in a single‐to‐triple row on each side of the body between the ovaries and the root of the pharynx (Figure ). Most anterior and posterior testes are located, in relation to the anterior end, at 25.4% and 55.5% of the body length, respectively. A ductule (rarely two) communicates dorso‐ventrally or obliquely each testis with the efferent duct of the same body side. The efferent ducts run between fibers of the transversal sub‐intestinal muscle layer (Figure ); at the prostatic vesicle level, they narrow and bend slightly ventrally. Their distal portions are dorso‐anteriorly oriented and communicate with the proximal part of the paired branches of the prostatic vesicle (Figure ). The prostatic vesicle is located outside the common muscle coat. Its paired branches are bent dorso‐anteriorly to the sagittal plane, which open laterally into the unpaired portion, which is rounded proximally and sinuous tubular distally. The vesicle penetrates the common muscle coat and continues as a sinuous ejaculatory duct inside the penis papilla. The penis papilla is large, with the dorsal insertion posterior to the ventral one. It shows a longitudinal fold probably caused during fixation. The papilla occupies most of the male atrial lumen.
Figure 4. Gigantea maupoi sp. nov. Diagrammatic reconstruction of copulatory apparatus in lateral view of the holotype. The double‐dashed lines indicate a fold of the penis papilla. The dashed line shows the limit between the efferent duct and the prostatic vesicle. Scale bar: 1 mm.
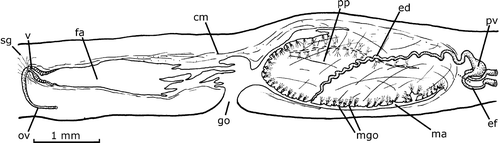
The efferent ducts are distally full of sperma. Its muscularis is composed of a one‐fiber‐thick circular layer. The prostatic vesicle is composed of cuboidal ciliated epithelium. Erythrophilic granulous secretory cells open into the whole prostatic vesicle, with the exception of its very proximal portion; the muscularis is composed of a layer (25 µm thick) of intermingled fibers. The ejaculatory duct has a columnar ciliated epithelium crossed by scarce erythrophilic granulous secretory cells. Its muscularis is composed of a circular layer (6 µm). The epithelium of the penis papilla is infra‐nucleated and crossed by two types of granulous secretory cells, cyanophilic and erythrophilic, respectively. The wall of the epithelial cells of the penis papilla was not examined. The muscularis of the penis papilla is composed of a subepithelial circular muscle layer (8 µm) followed by a longitudinal one (8 µm). The stroma of the penis papilla possesses abundant fibers radially and longitudinally arranged.
The surface of the penis papilla is provided with numerous male accessory genital organs, mgo, as conical protuberances, each ca. 140–180 µm in diameter and 100–130 µm in height (Figures – ). They are abundant, being visible a maximum of 20–35 per histological section. The most apparent structure of a mgo is a very strong barrel‐shaped circular muscle coat and an inner space filled with erythrophilic granules and with an ill cyanophilic mass. The muscle coat is composed of overlain layers of bundles with 2–8 fibers each. Some longitudinal and oblique fibers are interspersed with those of the circular muscle coat. Abundant muscle fibers, longitudinally arranged, cross under base of the circular muscle coat. The circular and longitudinal muscle fibers are most likely sunken fibers of the circular subepithelial layer of the penis papilla since this layer is inconspicuous under the epithelial surface of the mgo.
Figure 5. Gigantea maupoi sp. nov. Reconstruction of a longitudinal section of a male accessory genital organ of the penis papilla of the holotype. For clarity, the secretions represented are only of erythrophilic type crossing the organ. Scale bar: 50 µm.
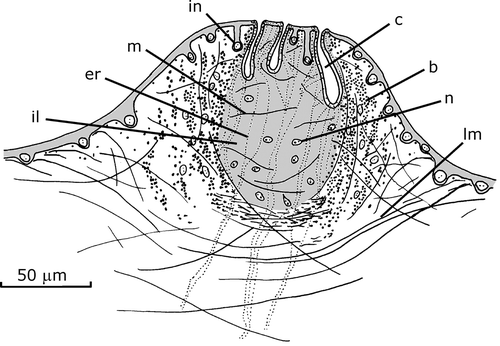
Figure 6. Gigantea maupoi sp. nov. Reconstruction of a transverse section of a male accessory genital organ of the penis papilla of the holotype. For clarity, the secretions represented are only of erythrophilic type crossing the organ. Scale bar: 50 µm.
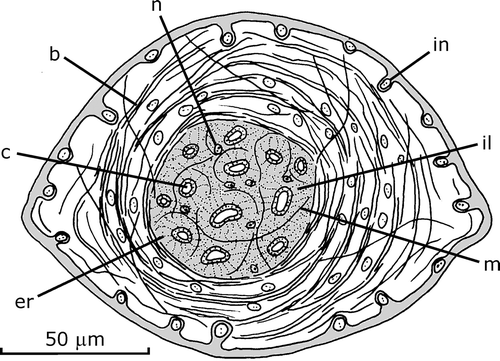
Figure 7. Gigantea maupoi sp. nov. Schematic representation of the proposed mode of action of a male accessory genital organ, before (on the left) and after muscle contraction (on the right).
An mgo contains 8–15 upright canalicula, 35–50 µm deep, slightly dilated in its basis. The canalicula open centrally into the surface of the conical protuberance. The epithelial surface of the papilla region is thinner centrally, and its nuclei are more deeply sunk into the penis papilla and do not possess musculature under it. Some muscle fibers run between the canalicula and around each canaliculus. Abundant secretory cells of erythrophilic granules, whose nuclei are located outside the mgo, penetrate the organ through its base and discharge into the cavity of the male atrium through the epithelial surface of the organ and also through the distal portion of the canalicula. Cell nuclei are also located inside the space delimited by the circular muscle coat and also between the muscle layers of the coat. They send out cyanophilic necks towards the canaliculi, but it was not possible to discern secretory material in them.
The male atrium has a cuboidal to columnar ciliated epithelium and receives openings of two types of granulous secretory cells, cyanophilic and erythrophilic, respectively. The muscularis is composed of a subepithelial circular layer (7 µm) followed by a longitudinal layer (7 µm).
Female reproductive system
Vitellaria are in incipient stage of maturation. The ovaries are elongated (1×0.1 mm) and ventral in position, between the sub‐intestinal muscle layer and the ventral nerve plate. They lay at a distance from the anterior end of the body equal to 25.4% of body length. The ovovitelline ducts arise from the first third of ovaries in its dorso‐lateral (external) region. The proximal portion of the ovovitelline ducts is slightly dilated and full of spermatozoa. They run posteriorly at the same height as the ovaries, slightly external to the efferent ducts. Behind the female atrium they become dilated and curve to the dorsum and the sagittal plane, and then anteriorly and join each other and enter the vagina (Figure ). There is no common glandular ovovitelline duct. The vagina is located inside the common muscle coat, and is ventrally forward oriented. It opens the proximal region of the female atrium. It is funnel‐shaped proximally and elongated in the rest of its extension, with the exception of some folds that narrow its lumen at the distal portion. The female atrium is as long as the male's.
The ovovitelline ducts have a tall cuboidal ciliated epithelium and openings of shell glands into the anteriorly curved portion; its muscularis is composed of a layer of intermingled fibers (6 µm thick). The epithelium of the female atrium is lacunar non‐ciliated, proximally tall columnar, and distally cuboidal to columnar, with the apical surface of each cell convex. The epithelium dorsal to the gonopore is similar to that of the adjacent epithelium of the female atrium. The lacunae are filled with cyanophilic granules. Scarce cells with erythrophilic amorphous secretions cross the epithelium of the female atrium. The muscularis of the atrium is constituted by a subepithelial circular layer (4 µm) followed by a longitudinal one.
Common muscular coat
It is constituted by a layer (25–70 µm thick) of longitudinal and diagonal fibers and envelops the male and female atria (Figure ).
Gigantea bistriata (Hyman, Citation1962)
This species is only known from three specimens on which the original description was based. The copulatory apparatus reconstructed in figure 13 in Hyman (Citation1962) is that of the paratype FMNH Nr. 3147, which is also outlined in detail in Figure . The male accessory genital organs in the penis papilla are located only in the ventral side of it, as seen in Figure with 1–4 organs in one histological section. An organ consists of a 10–12 µm deep pyriform invagination of the epithelium of the penis papilla (Figure , inset). The epithelium of the penis papilla is infranucleated. The wall of the pyriform invagination is thin, unnucleated and crossed in its inner half by the cell necks of secretory cells, the whole of which shows a spherical aspect. The nuclei of the secretory cells were not observed. No musculature was observed associated with the organ.
Figure 8. Gigantea bistriata (Hyman, Citation1962). Diagrammatic reconstruction of copulatory apparatus in lateral view of the paratype FMNH Nr. 3147. In the inset, a reconstruction of a longitudinal section of a male accessory genital organ of the penis papilla (free‐hand drawn). Scale bar: 1 mm.
Gigantea sandersoni (Prudhoe, Citation1949)
The only worm known from the species was re‐examined. The copulatory apparatus of the holotype reconstructed in Figure in Prudhoe (Citation1949) is seen in Figure . The description of the mgo is consistent with that by Prudhoe (Citation1949). The dorsal epithelium and the distal ventral half epithelium of the penis papilla present openings of the mgo. An mgo, called small pyriform organs by Prudhoe, consists of a pyriform‐to‐globose sheath of muscles apparently longitudinally orientated (inset in the Figure ). Part of these muscle fibers run joined in bundles inside the stroma of the penis papilla. Centered in the sheath and immediately beneath the epithelium of the penis papilla there is a pyriform cavity opened to the male atrium. The muscle sheath is approximately 100–120 µm in diameter; the pyriform cavity, 8–12 µm high. The space between the muscle sheath and the cavity is filled with a mass of granular secretion. In the mass, there are also necks of cells with granular secretions. They open into the cavity through the epithelium of the base of the cavity.
Figure 9. Gigantea sandersoni (Prudhoe, Citation1949). Diagrammatic reconstruction of copulatory apparatus in lateral view of the holotype. In the inset, a reconstruction of a longitudinal section of a male accessory genital organ of the penis papilla (free‐hand drawn). Scale bar: 1 mm.
Gigantea chiriquii (Hyman, Citation1962)
Remarks
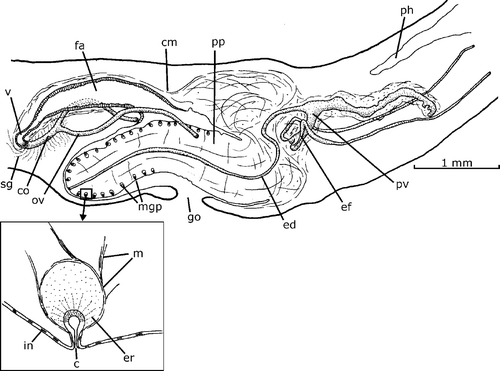
The copulatory apparatus of holotype, drawn by Hyman in figure 20 (Hyman, Citation1962) is the same examined and drawn herein (Figure ). The observations of the worm are consistent with the analysis those of Hyman (Citation1962). Male accessory genital organs were also not seen.
Figure 10. Gigantea chiriquii (Hyman, Citation1962). Diagrammatic reconstruction of copulatory apparatus in lateral view of the holotype. Scale bar: 1 mm.
Discussion
Taxonomic position of the new species
In addition to the collective group Pseudogeoplana, 5 of the 16 genera of Geoplaninae share with the new species a copulatory apparatus provided with a penis papilla and a female genital canal approaching horizontally or from below. They are Enterosyringa Ogren and Kawakatsu, Citation1990, Gigantea, Gusana E. M. Froehlich, Citation1978, Liana E.M. Froehlich, Citation1978, and Xerapoa Froehlich, Citation1955. Excepting Gigantea, all of these genera present characteristics absent in Gigantea maupoi sp. nov.: the sensory pits in Xerapoa are located at the tip of extensible papillae, which are visible to the naked eye on the epidermis of the anterior ventro‐lateral region of the body; Liana and Gusana possess the subepidermic longitudinal musculature partially sunk into the mesenchyme, and Enterosyringa presents a single subepidermic intestinal canal that opens to the ventral side near the copulatory complex.
The new species fits only into Gigantea, characterized by Ogren and Kawakatsu (Citation1990) as:
Geoplaninae of large, broad body (mc : h [ = MCI] value of G. idaia [du B.‐R.] Marcus, Citation1951, is 15%); penis papilla present, typically with glandular ridges on the penis papilla (but ridges may be absent as in G. chiriquii); female canal horizontal or approaching from below; female antrum dilated. Localities in Peru, Colombia, Costa Rica, Panama, and Trinidad.
The genus Gigantea was erected from the non‐formal G. gigantea‐group created by Froehlich (Citation1967). The group was proposed for species of Geoplana sensu Froehlich (Citation1955):
with the female canal horizontal or coming from below, most of the species provided with glandular ridges on the penis papilla, some also with a dilated female atrium. The group extends from Costa Rica to Peru and Trinidad.
Froehlich (Citation1955) included within the G. gigantea‐group:
G. gigantea, and G. chiriquii, both without glandular ridges on the penis papilla, and the following species provided with these structures, G. idaia (du B.‐R. Marcus, Citation1951), G. montana (Hyman, Citation1939), G. picadoi (Beauchamp, 1912), G. sandersoni, G. vongunteni (Fuhrmann, Citation1914), and, perhaps, G. cameliae (Fuhrmann, Citation1914).
Froehlich avoided providing a formal subgenus status to the Gigantea group because most species pass gradually to others in their morphological aspects or show minor differences between them (Froehlich Citation1967). Later, Ogren and Kawakatsu (Citation1990) added G. bistriata and G. unicolor (Hyman, 1955) to the G. gigantea group, from Panama and Peru, respectively, and raised the group to generic rank.
These species together with G. gouvernoni Jones and Sterrer, 2005 seem to constitute a heterogeneous group. For a comparative discussion of the new species, the genus is here subdivided into two groups. A first group includes Gigantea species, in which the penis is horizontal, and the ejaculatory duct horizontally crosses the penis papilla. G. chiriquii (Figure ), G. gigantea (Graff, 1899), G. montana, G. picadoi and G. unicolor belong to this group. In these species, the female genital canal (common ovovitelline duct + vagina) enters the proximal region of the female atrium running upwards and anteriorly.
A second group includes Gigantea species, which possess a penis obliquely emerging from the dorsal wall of the male atrium, and their ejaculatory duct runs postero‐downwards. G. bistriata, G. cameliae, G. gouvernoni, G. maupoi sp. nov., and G. vongunteni belong to this group. The species G. cameliae is included here with some caution, since its non‐dilated female atrium is not consistent with the definition of the genus, and the course of the ovovitelline ducts, an important taxonomic characteristic, is unknown. With the exception of G. gouvernoni, in all species of the group, the female genital canal enters the proximal region of the female atrium running downwards and anteriorly. Regarding the female genital canal G. gouvernoni fits into the first group.
Two species remain that do not fit clearly into any of the groups: G. idaia, and G. sandersoni. In Gigantea idaia the penis papilla, originally described as oblique in shape, is not a typical one, consisting of a fold of the whole wall of the male atrium, which in the median sections only displays its continuity with the proximal part as a typical penis papilla (Froehlich Citation1978). Consequently, E.M. Froehlich questioned the inclusion of the species into the G. gigantea group, and hence in the genus.
Gigantea sandersoni presents the female characteristics of the first group (the female genital canal enters the female atrium coming from the dorsal‐to‐horizontal aspect), and the male characteristics of the second group (penis obliquely emerging from the dorsal wall of the male atrium, and ejaculatory duct postero‐backwards oriented). Nevertheless, since the only worm known is strongly contracted and the penis papilla is partially everted, the interpretation of the anatomy of the male organs must be interpreted cautiously. The difficulty of gathering these species into any of the groups reinforces the heterogeneous status suggested for the genus.
Regarding the species of the second group, the new species is distinguished from G. cameliae, G. gouvernoni, and G. vongunteni by the relatively shorter length of the genital apparatus in these three species. Gigantea bistriata and G. maupoi sp. nov. which present a similarly long copulatory apparatus, differ from each other in histological details of the female atrium: in the new species the female atrium shows a lacunar epithelium underlain by a muscularis. The female atrium in G. bistriata is covered with a non lacunar epithelium and its muscularis is absent. Furthermore, the new species could be distinguished from G. bistriata and from all species of Geoplaninae solely on the basis of structure of the male accessory genital organs. A comparative account on the mgo in Geoplaninids species follows below.
Diversity of male accessory genital organs in Geoplaninae
There are eight geoplaninid species with mgo: G. bistriata, G. idaia, G. maupoi sp. nov., G. montana, G. picadoi, G. sandersoni, G. vongunteni, and Pasipha ercilla (Froehlich Citation1978). In the comparative discussion of Gigantea montana, Hyman (Citation1939) referred to G. cameliae as having mgo, which evidently was a misinterpretation following the original description by Fuhrmann (Citation1914) reporting that the species has no male accessory genital organs, neither of glandular, nor muscular nature.
Concerning the musculature, two types of mgo can by distinguished in the Geoplaninid: non muscularized mgo, present in G. bistriata, G. picadoi, G. vongunteni, and P. ercilla; and muscularized ones, seen in G. idaia, G. maupoi sp. nov., and G. sandersoni. The organs of the worms of P. ercilla species, which resemble those of G. vongunteni, are incompletely differentiated and do not present differentiated muscles, not even primordia of glands (Froehlich Citation1978). Gigantea montana was not included in this division because of the deviation from the common type of mgo:
epithelium [of the penis papilla] crossed at intervals by bundles of muscle fibers, which reach the surface of the penis, sometimes elevating this into a small papilla. Where these muscle bundles come to the surface, the regular epithelium appears to be modified. … The bundles seem to enclose some large cells, which may be gland cells (Hyman Citation1939).
The structures observed by Hyman in the histological sections of G. montana, which are inadequate to reveal details (Hyman Citation1939), might be of a glandular type instead of muscular one, since when comparing the organs of G. montana with other species Hyman attributed a muscular nature to the structures of G. vongunteni, described by Fuhrmann (Citation1914) as of glandular type (Hyman Citation1939). The position of muscle fibers for Turbellarians is entirely under the epidermal cells layer (Rieger et al. Citation1991) usually beneath the basement membrane; however, Hyman (Citation1951, p. 78) states that in turbellarians the fibers may reach the surface of the epithelium. The species should be re‐examined.
The mgo are not restricted to Geoplaninae. They were also reported in the families Bipaliidae and Rhynchodemidae (Terricola), and in the Paludicola and Maricola. Grant et al. (Citation2006) reported that the variation in morphology and position of mgos of adenodactyl type is possibly evidence of independent evolution several times within Tricladida.
The mgo in G. idaia, G. maupoi sp. nov., and G. sandersoni, differ in the position, and histological and cytological details: in G. idaia the mgo project from the penis papilla and from part of the atrium wall, their musculature is located immediately under the epithelium of the organ, and the wall of the canaliculi is of a lamellar nature. In contrast, in G. sandersoni and G. maupoi sp. nov., the mgo project from the penis papilla, the muscle sheath is sunk in the penis papilla, and the wall of the canaliculus is not of a lamellar nature. In addition to the fact that G. maupoi sp. nov. is the sole species having various canalicula in each mgo, the fibers of the mgo of this new species run across the base of the barrel‐shaped circular muscle coat and attach onto the basal membrane of the epithelium that surrounds each mgo, while in G. sandersoni muscle fibers of the organs continue more deeply into the penis papilla.
The differences in position and morphology of the mgo existing in Geoplaninae also suggest that they are not homologous.
Very little is known of the function of the mgo in geoplaninids, and few authors have considered it. After Prudhoe (Citation1949), the organs of G. sandersoni have been thought to probably be individual prostatic organs. Du Bois‐Reymond Marcus (Citation1951) did not suggest a function for G. idaia, but Winsor (Citation1998) related the organs of this species together with G. montana, G. picadoi, G. sandersoni, and G. vongunteni with a probable cocoon‐forming role.
More likely, the mgo in G. maupoi sp. nov. are associated with a copulatory function since they are entirely projected through the epithelium of the penis papilla. The mgo might stimulate the intercourse or help an individual to maintain their penis papilla interlocked with the female atrium of the partner during sperm transfer.
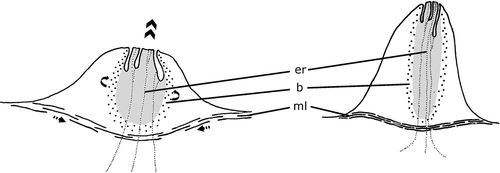
It is hypothesized that contraction of the fibers of the barrel‐shaped muscle coat of the mgo increases internal pressure in the organ and enlarging it as a long papilla (Figure ). Simultaneous shortening of the longitudinal subepithelial muscles underlying the muscle coat prevents the papilla from sinking into the mesenchym, and provides a basis for the protrusion of mgo as a papilla. Additional internal pressure would also narrow the lumen of the canalicula, which was increased from the shortening of the surrounding muscle fibers, and secretions of the cells would be driven out of the canalicula. Relaxation of these muscle systems would reduce the internal pressure, retracting the papillar mgo.
Albeit that there is evidence of heterogeneity in the genus, proposing any taxonomic change was avoided here while morphological knowledge of the species remains in a poor state and existing genera show no evidence of being natural. It is noteworthy that G. maupoi sp. nov. presents two uncommon morphological characteristics of taxonomic value: the presence of a sub‐neural transverse mesenchymal musculature restricted to the cephalic region, and the inverted organization of the muscle layers beneath the inner pharyngeal epithelium; that is, a thin longitudinal subepithelial layer followed by a circular one with some interspersed longitudinal fibers. Both characteristics are useful tools in comparative studies within Geoplaninae and Tricladida, and might contribute to hypothesizing phylogenetic relationships for the geoplaninid flatworms.
Acknowledgements
To G. Souza (PUCRS) for helping with the histological sections; E.A. Harris (NHM) and Dr. J. Gerber (FMNH) their uncomplicated aid in the loan of specimens in their care (G. sandersoni, and G. bistriata and G. chiriquii, respectively); Dr. E.M. Froehlich (IB‐USP) for the valuable comments and suggestions on an early version of the text; J. Hesson for his help with English. Two anonymous reviewers are also gratefully acknowledged. This work was partially supported by FAPESP (Proc. No. 06/04788‐8) and CNPq (150692/2003‐0).
References
- Du Bois‐Reymond Marcus , E. 1951 . On South American geoplanids. . Boletim de Faculdade de Filosofia Ciências e Letras, Universidade de Sao Paulo. Série Zoologia , 16 : 217 – 255 .
- Froehlich , C. G. 1955 . Sôbre morfologia e taxonomia das Geoplanidae. . Boletim de Faculdade de Filosofia Ciências e Letras, Universidade de Sao Paulo. Série Zoologia , 19 : 195 – 279 .
- Froehlich , C. G. 1967 . A contribution to the zoogeography of neotropical land planarians. . Acta Zoologica Lilloana , 23 : 153 – 162 .
- Froehlich , E. M. 1978 . On a collection of chilean landplanarians. . Boletim de Faculdade de Filosofia Ciências e Letras, Universidade de Sao Paulo, Série Zoologia , 3 : 7 – 80 .
- Fuhrmann , O. 1914 . Planaires terrestres de Colombie. . Mémoires de la Societe des Sciences Naturelles de Neuchatel , 5 : 748 – 792 .
- Grant , L. J. , Sluys , R. and Blair , D. 2006 . Biodiversity of Australian freshwater planarians (Platyhelminthes: Tricladida: Paludicola): New species and localities, and a review of paludicolan distribution in Australia. . Systematics and Biodiversity , 4 : 435 – 471 .
- Hyman , L. H. 1939 . New species of flatworms from North, Central, and South America. . Proceedings of the United States National Museum , 86 : 419 – 439 .
- Hyman , L. H. 1951 . The Invertebrates: Platyhelminthes and Rhynchocoela , New York : McGraw Hill . vol. 2
- Hyman , L. H. 1962 . Some land planarians from Caribbean countries. . American Museum Novitates , 2110 : 1 – 25 .
- Ogren , R. E. and Kawakatsu , M. 1990 . Index to the species of the family Geoplanidae (Turbellaria, Tricladida, Terricola) Part I: Geoplaninae. . Bulletin of Fuji Women's College , 28 : 79 – 166 .
- Prudhoe , S. 1949 . Some roundworms and flatworms from the West Indies and Surinam. IV land planarians. . Zoological Journal of the Linnean Society , 281 : 420 – 433 .
- Rieger , R. M. , Tyler , S. , Smith , J. P. S. and Rieger , G. E. 1991 . “ Platyhelminthes: Turbellaria. ” . In Microscopic anatomy of invertebrates, Vol. 3. Platyhelminthes and Nemertinea , Edited by: Harrison , F. W and Bogitsh , B. J . 7 – 140 . New York : Wiley‐Liss .
- Winsor , L. 1998 . Aspects of taxonomy and functional histology in terrestrial flatworms (Tricladida: Terricola). . Pedobiologia , 42 : 412 – 432 .