Abstract
In an Alpine area, scat analysis and marking activity were used to assess the feeding habits, habitat preference and the degree of overlap of trophic niche and habitat use in sympatric carnivores: the red fox (n = 133 faecal samples), the badger (n = 177), the pine and the stone marten (Martes sp., n = 382). Fruits were the main trophic resource for all species. The diet of martens differed from those of the red fox and badger by means of a higher consumption of garbage and non‐Rosaceae fruits. The red fox preyed on more lagomorphs and roe deer and relied almost exclusively on two fruit species, rose‐hips and whitebeam berries. Badgers ate few invertebrates, with cultivated fruits and pine seeds forming the bulk of their diet. On the whole, trophic niche overlap was kept low by the exploitation of different species of berries and mammals and, secondly, by seasonal differences in the use of same items by the three carnivores. Badger trophic niche overlapped to a wider extent than those of the other two predators in summer, when fruit availability is higher. Foxes used all habitats according to their availability, except for villages, which were avoided. Badgers used mainly open habitats, particularly Alpine meadows, avoiding villages and mixed woods. Martens selected the habitats avoided by the other two predators and avoided all the others. The narrow range of habitat used by martens and diet evidence suggest that, within the context of interspecific competition, they could play the role of sub‐ordinate species, segregating in fox‐free urban environments.
Introduction
The role played by interspecific competition in structuring communities has been recognized since the pioneering works of Volterra (Citation1926) and Lotka (Citation1932). The use of a limited resource (exploitation) or its non‐consumptive pre‐emption (interference) by one species is expected to reduce the resource availabilty to the other species (Wiens Citation1989), implying resource partitioning and niche differentiation in sympatric species occupying the same trophic level (Pianka Citation1969; Schoener Citation1974, Citation1982; Pimm & Rosenzweig Citation1981; Begon et al. Citation1986; Ricklefs, Citation1990).
Food, habitat and time have been suggested to be the most important niche dimensions in resource partitioning between species (Pianka Citation1969; Schoener Citation1986): coexisting species should reduce competition shifting to different diets, selecting different habitats, carrying out different patterns of activity or, more probably, showing a specific combination of the former three modes imposed by local environmental conditions and their intra‐ and inter‐specific densities (Guthrie & Moorhead Citation2002).
A further and lesser investigated (Palomares & Caro Citation1999) layer of complexity is introduced by interspecific killing between potential competitors (“intraguild predation”; Polis et al. Citation1989), inducing weaker species to seek for habitats avoided by their competitors (“refuges”) so as to escape being killed (Durant Citation1998).
The red fox Vulpes vulpes, the badger Meles meles, the stone marten Martes foina and the pine marten M. martes are medium‐sized generalist carnivores widespread in the Italian Alps (Spagnesi & De Marinis Citation2002).
Foxes are considered to be prototypical generalists, feeding on a wide variety of food resources according to their local and seasonal availability (Ables Citation1975; Lloyd Citation1975, Citation1980; Macdonald Citation1977; Doncaster et al. Citation1990). In the Alps the fox shows carnivorous feeding habits relying on mammals, mainly ungulates and rodents, and, only secondly, on fruits and invertebrates (Leinati et al. Citation1960; Cantini Citation1991; Lucherini & Crema Citation1994; Cagnacci et al. Citation2003).
The badger is considered a “forager” (Neal Citation1986) rather than a predator. In NW Europe Lumbricidae comprise the main item of badger diet, such that badgers have been considered as earthworm specialists (Kruuk & Parish Citation1981; Kruuk Citation1989). Nonethe‐less, within its wide distribution range, the badger is better described as an opportunistic food generalist relying mainly on cereals, fruits and invertebrates (Roper Citation1994; Neal & Cheeseman Citation1996; Revilla & Palomares Citation2002; Balestrieri et al. Citation2004; Virgós et al. Citation2004; Rosalino et al. Citation2005). In the Alps, invertebrates—Coleoptera, Orthoptera and earthworms—form the bulk of badgers' diet (Rinetti Citation1987; Lucherini & Crema Citation1995), followed by rodents and carrion. Occasionally, fruits are intensively used (Kruuk & de Kock Citation1981).
Martens show much flexibility in their diet (stone marten: Marchesi et al. Citation1989; Libois & Waechter Citation1991; Romanowski & Lesinski Citation1991; Genovesi et al. Citation1996; Rödel et al. Citation1998; Padial et al. Citation2002; Lanszki Citation2003; pine marten: Marchesi & Mermod Citation1989; De Marinis & Massetti Citation1995; Russel & Storch Citation2004). Where the two species occur sympatrically, the pine marten is associated primarily with coniferous and mixed wood forest habitats, whilst the stone marten selects rocky open areas and urban areas (Frenchkop Citation1959; Novikov Citation1962; Delibes Citation1983). Differential habitat use has repercussions on the diet of martens, pine martens relying mainly on forest‐dwelling trophic sources and stone martens widely using food associated with human activity (Marchesi & Mermod, Citation1989; Marchesi et al. Citation1989; Lanszki Citation2003). Their feeding habits in their Italian Alpine range are poorly known. Fruits and rodents form the bulk of Martes diet in the central (Cantini Citation1991) and western Italian Alps (Prigioni et al. 1998, unpublished report), whilst a high frequency of occurrence of insects has been occasionally reported (Lucherini & Crema Citation1993; Pedrini et al. Citation1995a).
Available information about direct interactions between pairs of these carnivores has been reviewed by Palomares and Caro (Citation1999). Interspecific killing occurs between foxes and badgers, each one being able to kill only non‐adult individuals of the other species, but rates of killing are likely to be quite low if we consider that they can share the same burrows (Neal & Cheeseman Citation1996). Diet data from several Italian studies support this hypothesis (reviewed by Remonti et al. Citation2005). Foxes may kill adult martens (American martens Martes americana and pine martens), even sharply limiting their population density (Thompson Citation1994; Lindström et al. Citation1995), as suggested by pine marten recovery following an epidemic of scabies among red foxes (Lindström et al. Citation1995; Smedshaug et al. Citation1999). On the contrary, no attacks are known between badgers and martens, predatory habits probably being a factor influencing interspecific killing at least as much as the relative body size of the interacting species (Donadio & Buskirk Citation2006).
We investigated the feeding habits of the above mentioned carnivores in an Alpine area of NE Italy with the aim of estimating (i) the relative importance of the different food items and their seasonal variation, (ii) the degree of interspecific diet overlap, (iii) carnivore preference for different Alpine habitats and (iv) the degree of interspecific overlap in habitat use.
We hypothesized that niche overlap would be larger between martens and foxes (badgers preying mostly on earthworms and other invertebrates), and that foxes, having a body weight ratio between foxes and martens about 3:1, would behave as the dominant species, exploiting a broader range of habitats and, consequently, a wider range of food resources. Also, if martens were victims of attacks by foxes, they would be expected to reduce the chances of encountering competitors using, as much as possible, different habitats for hunting and/or resting, this habitat selection determining qualitative and/or quantitative differences in food items exploitation.
Materials and methods
Study area
The Fiemme Valley is a wide east–west‐oriented valley, located in the eastern Italian Alps (NE Trentino region). The study area (23.77 km2) covers its central part, i.e. the surroundings of the village named Cavalese (about 3200 inhabitants), between 840 m a.s.l. (River Avisio) and 1550 m a.s.l. The climate is typically alpine‐continental, with annual rainfall averaging 828 mm (with a wet period in mid‐summer and a dry one in winter) and annual temperature 7.5°C at 900 m a.s.l. Vegetation consists of four main types:
-
mixed woods (13.3% of the study area), dominated by beech Fagus sylvatica, hazel Corylus avellana, alders (Alnus viridis, A. incana, A. glutinosa) and spruce fir Picea excelsa;
-
coniferous forest (60.6%), consisting in a mosaic of several stands of coeval spruce firs and larches (Larix decidua) as a consequence of timber harvesting activities;
-
shrubs (2.8%), occurring mainly at the wood–Alpine prairie transition, with Rosa canina, Berberis vulgaris, Ligustrum vulgare and Corylus avellana;
-
Alpine meadows (8.1%), probably originated by tree‐cutting in the first half of the 20th century.
Regularly mowed grasslands and orchards (10%) lie next to villages (5.2%).
Three fox dens and as many badger setts were found active during the study period in the area. In the urban area of Cavalese, Prigioni and Sommariva (Citation1997) assessed the presence of 52 stone martens by radiotelemetry. No sound information was available about pine martens, which have been reported as the least common mustelid of the western Trentino region (Pedrini et al. Citation1995b).
Diet analysis
Faeces were collected monthly from June 1994 to June 1996 along four transects crossing the main habitats of the study area (Table ). Badger scats were collected from typical latrines. Shape and dimensions (martens scats diameter <10 mm, foxes scats diameter >15 mm; Bang & Dahlström Citation1974) were considered when distinguishing fox faeces from those of martens. The scats of pine martens are not distinguishable by eye from those of stone martens; anyway, pine marten scats are mainly found on branches or tree bases beneath arboreal dens (Kleef Citation1997), and at low densities pine martens may not defecate on trails and paths (Balharry et al. Citation1996). As a consequence, a quite higher proportion of stone marten scats was probably collected. None the less, scats were cautionary classified as Martes sp.
Table I. Habitat composition (%) and total length of the four transects used to collect faecal samples in the study area.
A total of 692 faecal samples (fox: 133; badger: 177; Martes sp.: 382) was stored in polythene bags and refrigerated until processing.
Scat analysis was performed according to Kruuk and Parish (Citation1981). Samples were washed with three sieves of 1.5, 0.3 and 0.1 mm mesh and food remains were inspected to count or estimate the total numbers of each item.
Mammal hairs were compared at 20× and 40× magnification with the keys provided by Debrot et al. (Citation1982) and Teerink (Citation1991), while reptiles and amphibians were detected by the keys of Di Palma and Massa (Citation1981). Bird feathers were identified with reference to Day (Citation1966). The undigested remains of insects (wings, legs and cuticle parts) and wild or cultivated fruits (seeds) were identified using personal collections. Sediment remained in the sieve with the thinnest mesh was examined under a binocular microscope to detect earthworm chetae. Food remains of human origin—generally including packing paper, tin foil, string, etc.—were recorded as “garbage”.
The level of prey identification affects food‐niche relationships among sympatric predators (Greene & Jacsíc Citation1983). According to Krebs (Citation1989), prey were categorized to the lowest possible systematic level, attaining 34 items (see Tables and ). Results were expressed as per cent frequency of occurrence (F% = number of faecal samples containing a specific food items/total number of faecal samples×100), per cent volume (V% = total estimated volume of each food item “as ingested”/number of faecal samples containing that item) and per cent mean volume (Vm% = F%×V%/100) which, combining frequency and volume information, reflects the proportional contribution of each food item to the overall diet. The F% of main food items was plotted against their V%, connecting points with equal Vm% values by isopleths (Kruuk & Parish Citation1981).
Table II. Diet composition for the three carnivores in the study area (N = number of analysed faeces; I = overall number of items found; F% = per cent frequency of occurrence; V% = per cent volume).
Table III. Seasonal variation (Vm%) in the diet of the three carnivores (RF: red fox, B: badger, M: Martes sp.; N: number of analysed faeces; in bold: values considered for PCAs).
Data were pooled seasonally (winter: I–III; spring: IV–VI; summer: VII–IX; autumn: X–XII) in order to investigate seasonal variations in carnivores diet.
A Principal Components Analysis (PCA) was used to describe the main sources of variation in the seasonal diet (Vm%) of the three species. PCA was performed on an arcsine transformed 3×N matrix, where N was, for each season, the number of items scoring Vm%>5% for at least one carnivore (i.e. those items scoring Vm%<5% for all carnivores simultaneously were considered unable to distinguish their diets).
Trophic niche breadth was estimated by Levins' B index (Feinsinger et al. Citation1981), using the proportions of occurrence (pi) of food categories in terms of Vm%. Trophic niche overlap between pairs of the three species was assessed by Pianka's O index (Citation1973), p ij and p ik being the per cent mean volume (Vm%) in the diet of the species j and k. The same 34 food categories used for the PCA were used for processing the two indexes.
Habitat selection
Marking ratio (M%)—i.e. the ratio between the number of faeces found in each habitat and the overall number of faeces found×100—was considered an index of habitat utilization. This method, an adaptation of that carried out for otter surveys (Lenton et al. Citation1980) has been widely used for assessing the abundance and habitat preferences of many terrestrial mammals (reviews by Putnam Citation1984; Kohn & Wayne Citation1997; Gese Citation2001). Its reliability has been disputed (e.g. Kruuk et al. Citation1986; Messenger & Birks Citation2000), the survey interpretation involving several assumptions about marking activity and droppings identification (see Sadlier et al. Citation2004). None the less, scat counts still represent an effective and low‐cost method to derive an index of carnivore relative abundance at different times or habitat of a same region (Sadlier et al. Citation2004).
To assess habitat selection M% was compared to the per cent availability of habitat types by the χ2 test, using the sequential Bonferroni's technique to determine the level of significance (Rice Citation1989). Expected frequencies were calculated considering the overall relative length of transects covered in each habitat (Table ).
Pianka's O index (Citation1973) was used to assess the overlap in habitat use, p ij and p ik being the proportions of use (M%) of habitat i by the species j and k.
Results
Diet analysis
Fruits were the main trophic resource for all the investigated species, reaching 49% (Vm%) in the diet of the red fox (almost exclusively Rosaceae), 73.2% for the badger (53% Rosaceae, 20.2% other fruits) and 57.9% for martens (Rosaceae and other fruits in similar proportions; Figure ). Insects, mainly ground‐living Coleoptera, and roe deer (Capreolus capreolus) were a secondary food source for foxes and badgers, the foxes relying also on hares (Lepus europaeus and L. timidus). Food of human origin (“garbage”) formed a relatively large part of martens' diet (19.3%) and was also exploited by badgers. Rodents were rarely preyed upon by foxes (F% = 8.3) and martens (F% = 10.5) and apparently avoided by badgers (Table ).
Figure 1 Estimated volume(V%) of the main food categories, whenever eaten, vs. their frequency of occurrence (F%) for the overall diet of the three species. Isopleths connect points of equal overall volume in the diet (Vm%, see methods).
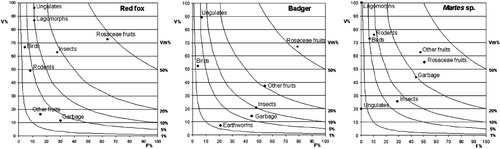
Seasonal variation in the diet of all the three species was large. The bulk of fox diet consisted of Rosaceae fruits in autumn (Vm% = 77.9) and winter (50.1%), and of insects in spring (35.3%) and summer (48.4%). Mammals were more important in winter (42.9%) except for roe deer which predominated in summer (27.0%), whilst birds were preyed on in spring (11.2%). Rosaceae, mainly cultivated fruits, were the main food item for badgers both in summer (60.8%) and autumn (68%), partially substituted by pine seeds (Pinus cembra) in spring (40.3%). Earthworms occurred almost exclusively in spring, amounting to only 4.65% of badger diet in that season. In winter no badger faeces was found, this being a period of heavy snow cover and least badger activity. Martens relied on wild Rosaceae in spring (29.3%) and summer (48.1%) and on other fruits in autumn (51.1%) and winter (40.7%); garbage was an important resource all year long (Vm% ranging between 13.5 and 29.2), whilst insects predominated in spring (14.7%).
Wider seasonal differences emerged when considering food items at species level, as shown by PCAs (Figure ; Table ). Foxes ate almost exclusively rowan berries (Sorbus aucuparia), widespread in mixed woods, and rose‐hips (Rosa sp.) and preyed more on medium‐sized mammals, i.e. roe deer in spring and summer and hares in autumn and winter. Cultivated fruits—apples (Malus sylvestris), plums (Prunus domestica) and pears (Pirus communis)—and raspberries (Rubus idaeus) marked badger diet in summer and autumn, whilst a higher consumption of pine seeds characterized its diet in spring. Foxes relied on Coleoptera and Orthoptera in spring and summer, whilst Orthoptera were exploited by badgers in autumn. Strawberries (Fragaria sp.) peaked in marten diet in spring, cherries (Prunus avium) in summer, cranberries (Vaccinium myrtillus and V. vitis‐idaea) in summer and autumn and privet fruits (Ligustrum vulgare) from autumn to spring. Garbage was significant in the diet of martens throughout the year.
Figure 2 Plot of carnivore seasonal diet in relation to the first two Principal Components extracted from Vm% data of 34 food categories, excluding a priori, for each season, those items scoring Vm%<5% simultaneously for all carnivores. Items are represented by lines that approximately point towards the direction of maximum variation of each factor. The length of each line is proportional to the importance of the item in the assemblage arrangement. In brackets are the per cent explained variance of each Principal Component; coordinates of the carnivores species on PCs axes not in scale.

Trophic niche breadth showed little seasonal variation for foxes, a bimodal pattern for martens and a peak in summer for badgers, whose overall yearly (three seasons) diet was the most diversified (B = 0.36; Table ). Fox trophic niche width was the narrowest, as a consequence of the small number of fruit species exploited and of a more carnivorous diet (mammals+birds Vm%: fox = 29.9%, martens = 15.3%, badger = 8.5%).
Table IV. Annual and seasonal trophic niche breath (B) and overlap (O) indices for the three carnivores.
Overall food overlap values were the greatest between badgers and martens (O = 0.40) and the smallest between foxes and martens (O = 0.20; Table ); in summer, badger trophic niche overlapped those of the other two predators to a wider extent (Table ), fruits and insects being intensively used by all species.
Habitat selection
Foxes avoided villages, using all other habitats according to their availability. Badgers used mainly open habitats (herbaceous areas and coniferous woods, whose undergrowth is scarce), showing a sharp preference for meadows, whilst avoiding villages and mixed woods. Martens showed an opposite pattern, selecting urban areas and mixed woods and avoiding open areas (Table ). The overlap in habitat use was maximal between badgers and foxes and minimal between badgers and martens. In summer all overlap indices between pairs were higher than in the other seasons (Table ).
Table V. Seasonal and overall habitat selection for the three carnivores based on the proportion of faeces encountered in each habitat (M%). Expected frequencies: villages = 11.2%; grasslands and orchards = 14.5%; meadows = 18.6%; coniferous woods = 34.3%; mixed woods = 21.4%. Selected habitats in bold, avoided ones in italic‐bold; * P<0.05; ** P<0.01; *** P<0.001.
Table VI. Annual and seasonal overlap in habitat use between pairs of the three carnivores.
Discussion
The unusually scarce use of invertebrates, particularly earthworms, shown by badgers was unexpected. The local combination of rainfall (on average 828 mm/year for the period 1953–1980), temperature (7.5°C) and snow cover probably limits earthworm availability for badgers. Moreover, Lumbricus terrestris, the only species of the study area foraging on the surface in substantial numbers, represents only a small (17%) fraction of the earthworm fauna (Cavada Citation1997). According to their foraging habits, badgers shifted to other sources “lying on the ground”, their diet turning decidedly frugivorous. Fruits seem to play an important role as alternative resources for Alpine carnivores, as already stressed for “rodent‐specialist” mustelids, such as the stoat Mustela erminea and the weasel M. nivalis (Martinoli et al. Citation2001; Remonti et al. Citation2007).
As a consequence of diet shift, badger trophic niche was the widest and overlapped those of the other two carnivores to a wider extent than hypothesized at the outset of the research.
In accordance with competition theory (Schoener Citation1982), which predicts a convergence of the diet of coexisting consumers when resources are abundant, diet (and habitat) overlap was higher in summer, when most fruits ripen.
On the whole, diet overlap between species was lower than that reported by other authors (Serafini & Lovari Citation1993; Fedriani et al. Citation1999; Baltrûnaitë Citation2001; Padial et al. Citation2002), even if the number of items used for the calculation of Pianka's index is likely to influence the result and thus to invalidate comparisons between different studies (Prigioni Citation1991).
Overlap was limited by the use, at species level, of different items, whilst temporal differences, i.e. the use of the same resources in different seasons (“sequential use”; Barrientos & Virgos Citation2006), seemed to play a secondary role, even though the small seasonal samples available for foxes and badgers could have underestimated temporal shifts.
Foxes preyed on larger mammalian species than martens, as can be expected according to the positive correlation between predator and prey body size observed for a number of communities of predators (Jaksic & Braker Citation1983; Jedrzejewski et al. Citation1989).
As predicted, foxes exploited the widest range of habitats, avoiding only urban areas, in accordance to radio‐tracking data from the Swiss Jura Mountains (Weber & Meia Citation1996). Martens were the most selective carnivores with regard to habitat, preferring those avoided by both of the other species (villages) or at least one (mixed woods), and avoiding all the others. Martes selection for mixed woods was reported also for the central Italian Alps (Pedrini et al. Citation1995a), whilst the avoidance of open areas, where the risk of predation would be higher, was reported for Mediterranean areas (Pittiglio Citation1996; Rondinini & Boitani Citation2002).
In the absence of competitors and predators, both pine (De Marinis & Massetti Citation1993; Clevenger Citation1994) and stone (Delibes Citation1978; Libois & Waechter Citation1991) martens are habitat generalists, even if stone martens have often been associated to human settlements (Waechter Citation1975; Hermann Citation1994). Delibes (Citation1983) proposed that this preference could be a consequence of competition with the pine marten, but more recent studies have reported the two species as syntopic (Kruger Citation1990; Genovesi Citation1993; Pittiglio Citation1996). Then, the narrow range of habitat used by martens in our study area and their selection for competitor‐free habitats could suggest the existence of asymmetrical competition (Wiens Citation1989) between martens and foxes (or, to a minor extent, both foxes and badgers).
Supporting this hypothesis, fox diet included a larger proportion of vertebrates. The energy content being equal, a diet including fruits provides less proteins and lipids than a largely carnivorous one, determining body fat loss and energy deficiency in carnivores (Larivière et al. Citation2001). In the context of optimal foraging theory (Krebs & Davies Citation1993), the dominant competitor is expected to exploit the most profitable food resource. Large‐sized mammals like roe deer may be an important source of proteins for carnivores. About 40% (84% in summer) of the meat eaten by foxes consisted of roe deer, both fawns (in summer), which are exposed to fox predation (Lindstrom et al. Citation1994; Jarnemo Citation2004) and carrion (in winter/spring). To a lesser extent, roe deer were exploited also by badgers, probably exclusively as carrion, whilst martens' exploitation of this resource was negligible, as reported also by other studies comparing their diets in sympatry (Goszczynski Citation1986; Brangi Citation1995). More detailed research is needed to determine if the avoidance of dangerous interactions with larger competitors could be the cause of the lack of such a profitable resource from the diet of martens (DeVault et al. Citation2003; see also Cagnacci et al. Citation2003 about foxes in the western Italian Alps).
According to the hypothesis of asymmetrical competition, urban areas, avoided by both foxes and badgers, were selected by martens (probably almost exclusively M. foina; Prigioni & Sommariva Citation1997). Hermann (Citation1994), reviewing several reports, suggested that stone martens select urban areas because they offer safe and warm resting places and trophic resources. Pine martens are said to rest in shelters above ground to avoid foxes (Pulliainen Citation1981; Webster Citation2001) and most dens of both Martes species are made on natural or artificial elevated places; in the urban environment of Cavalese village, attics and roofs of old houses are selected as resting places (Prigioni & Sommariva Citation1997), as reported elsewhere (Lachat Feller Citation1993; Brown Citation2004; Toth‐Apathy & Szenczi Citation2004). Recent studies have suggested that food resources would play a minor role in stone martens' selection for urban habitats compared to the availability of fox‐free shelters (Le Lay and Lodé Citation2004). Accordingly, as fruits formed the bulk of the diet of martens, this could explain their high selection for mixed woods.
Nonetheless, towns also offer unexploited (except for stray cats) human waste food, which may represent, particularly in conditions of food shortage, an important trophic source. The high stone marten density reported for Cavalese (0.87 ind./ha) together with the habitat composition of the home‐ranges of three radio‐tracked females, which almost totally included the village (Prigioni & Sommariva, Citation1997), suggest that urban areas and surroundings can satisfy all their ecological requirements. Stone marten adaptability to urban areas could represent a key factor in Alpine areas, where the decline of ecosystem productivity and the shrinkage of food resources with altitude may increase competition among top predators.
Acknowledgements
We would like to thank the District Office of the Forests of Cavalese for the logistic support. We are indebted to Renato Fumagalli for his help in field research and to Christopher Mason for the English revision.
References
- Ables , E. D. 1975 . “ Ecology of the red fox in North America. ” . In The wild canids: Their systematics, behavourial ecology and evolution , Edited by: Fox , M. V . 216 – 236 . New York : Van Nostrand Reinhold Co .
- Balestrieri , A. , Remonti , L. and Prigioni , C. 2004 . The diet of the Eurasian badger (Meles meles) in an agricultural riverine habitat (NW Italy). . Hystrix, Italian Journal of Mammalogy , 15 : 3 – 12 .
- Balharry , E. A. , McGowan , G. M. , Kruuk , H. and Halliwel , E. 1996 . Distribution of pine martens in Scotland as determined by field survey and questionnaire. SNH Survey and Monitoring Report No. 48 , Edinburgh, , UK : Scottish Natural Heritage .
- Baltrûnaitë , L. 2001 . Feeding habits, food niche overlap of red fox (Vulpes vulpes L.) and pine marten (Martes martes L.) in hilly moraine highland, Lithuania. . Ekologija (Vilnius) , 2 : 27 – 32 .
- Bang , P. and Dahlström , P. 1974 . Animal tracks , London : Collins .
- Barrientos , R. and Virgós , E. 2006 . Reduction of potential food interference in two sympatric carnivores by sequential use of shared resources. . Acta Oecologica , 30 : 107 – 116 .
- Begon , M. , Harper , J. H. and Townsend , C. R. 1986 . Ecology: Individuals, populations and communities , Oxford : Blackwell Scientific Publ .
- Brangi , A. 1995 . Seasonal changes of trophic niche overlap in the stone marten and the red fox. . Hystrix (N.S.) , 7 : 113 – 118 .
- Brown , H. 2004 . “ Santos‐Reiss M, Birks J, Messenger J (Eds) Resolving conflicts generated by pine martens denning in buildings in Scotland. ” . 4th International Martes Symposium, Lisbon, Portugal, 20–24 July 2004, Plenary session abstracts, 35
- Cagnacci , F. , Lovari , S. and Meriggi , A. 2003 . Carrion dependence and food habits of the red fox in an alpine area. . Italian Journal of Zoology , 70 : 31 – 38 .
- Cantini , M. 1991 . Alimentazione della Volpe (Vulpes vulpes) in aree boscate delle Alpi Orobie. . Hystrix (N.S.) , 3 : 83 – 90 .
- Cavada , L. 1997 . “ Ecologia comportamentale di carnivori in ambiente forestale e suburbano della Valle di Fiemme. ” . Università degli Studi di Padova, Padova. Unpublished degree thesis
- Clevenger , A. P. 1994 . Habitat characteristics of Eurasian pine martens Martes martes in an insular Mediterranean environment. . Ecography , 17 : 257 – 263 .
- Day , M. G. 1966 . Identification of hair and feather remains in the gut and faeces of stoats and weasels. . Journal of Zoology (London) , 148 : 201 – 217 .
- Debrot , S. , Fival , G. , Mermod , C. and Weber , J. M. 1982 . Atlas des Poils des Mammifères d'Europe , 1 – 208 . Neuchâtel : Ed. Institut de Zoologie de l'Université de Neuchâtel .
- Delibes , M. 1978 . Feeding habits of Stone marten, Martes foina (Erxleben 1777) in northern Burgos, Spain. . Zeitschrift fu˝r Säugetierkunde , 43 : 282 – 288 .
- Delibes , M. 1983 . Interspecific competition and the habitat of the stone marten Martes foina (Erxleben, 1777) in Europe. . Acta Zoologica Fennica , 174 : 229 – 231 .
- De Marinis , A. M. and Massetti , M. 1993 . Distribution of the pine marten Martes martes L. 1758 (Mammalia, Carnivora) on the island of Elba, Northern Tyrrhenian Sea. . Supplemento Ricerche di Biologia della Selvaggina , 21 : 263 – 267 .
- De Marinis , A. M. and Masseti , M. 1995 . Feeding habits of the pine marten Martes martes L., 1758, in Europe: A review. . Hystrix (N.S.) , 7 : 143 – 150 .
- DeVault , T. L. , Rhodes , O. E. and Shivik , J. A. 2003 . Scavenging by vertebrates: Behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. . Oikos , 102 : 225 – 234 .
- Di Palma , M. G. and Massa , B. 1981 . “ Contributo metodologico per lo studio dell'alimentazione dei rapaci. ” . 69 – 76 . Atti I Convegno Italiano di Ornitologia, Museo di Storia Naturale della Lunigiana, Aulla
- Donadio , E. and Buskirk , S. W. 2006 . Diet, morphology, and interspecific killing in Carnivora. . The American Naturalist , 167 : 524 – 536 .
- Doncaster , C. P. , Dickman , C. R. and Macdonald , D. W. 1990 . The feeding ecology of red foxes (Vulpes vulpes) in the city of Oxford, England. . Journal of Mammalogy , 71 : 188 – 194 .
- Durant , S. M. 1998 . Competition refuges and coexistence: An example from Serengeti carnivores. . Journal of Animal Ecology , 67 : 370 – 386 .
- Fedriani , J. M. , Palomares , F. and Delibes , M. 1999 . Niche relations among three sympatric Mediterranean carnivores. . Oecologia , 121 : 138 – 148 .
- Feinsinger , P. , Spers , E. E. and Poole , R. W. 1981 . A simple measure of niche breadth. . Ecology , 62 : 27 – 32 .
- Frenchkop , S. 1959 . Notes sur les mammiféres. XLVI. De la coexistence de la Martre et de la Fouine en Belgique. . Bulletin Institut Royal Science Natural de Belgique , 35 : 1 – 16 .
- Genovesi , P. 1993 . “ Strategie di sfruttamento delle risorse e struttura sociale della faina (Martes foina Erxleben 1777) in ambiente forestale e rurale. ” . 1 – 93 . PhD Thesis, Università di Roma “La Sapienza”, Roma
- Genovesi , P. , Secchi , M. B. and Boitani , L. 1996 . Diet of stone martens: An example of ecological flexibility. . Journal of Zoology (London) , 238 : 545 – 555 .
- Gese , E. M. 2001 . “ Monitoring of terrestrial carnivore populations. ” . In Carnivore conservation , Edited by: Gittleman , J. L , Funk , S. M , Macdonald , D. W and Wayne , R. K . 372 – 396 . Ithaca, NY : Cambridge University Press .
- Goszczynski , J. 1986 . Diet of foxes and martens in Central Poland. . Acta Theriologica , 36 : 491 – 506 .
- Greene , H. W. and Jaksíc , F. M. 1983 . Food–niche relationships among sympatric predators: effects of level of prey identification. . Oikos , 40 : 151 – 154 .
- Guthrie , C. G. and Moorhead , D. L. 2002 . Density‐dependent habitat selection: Evaluating isoleg theory with a Lotka–Volterra model. . Oikos , 97 : 184 – 194 .
- Hermann , M. 1994 . “ Habitat use and spatial organisation by the stone marten. ” . In The biology and conservation of martens, sables and fishers , Edited by: Buskirk , S. W , Harestad , A. S , Raphael , M. G and Powell , R. A . 122 – 136 . Ithaca, NY : Cornell University Press .
- Jaksic , F. M. and Braker , H. E. 1983 . Food–niche relationships and guild structure of diurnal birds of prey: Competition versus opportunism. . Canadian Journal of Zoology , 61 : 2230 – 2241 .
- Jarnemo , A. 2004 . Predation processes: Behavioural interactions between red fox and roe deer during the fawning season. . Journal of Ethology , 22 : 167 – 173 .
- Jedrzejewski , W. , Jedrzejewska , B. and Szymura , A. 1989 . Food niche overlaps in a winter community of predators in the Bialowieza Primeval Forest, Poland. . Acta Theriologica , 34 : 487 – 496 .
- Kleef , H. L. 1997 . “ Boommarterinventarisatie in Nederland: aanpak en resultaten, toegespitst op Noord‐Nederland. ” . In Wat Doen We Met de Boommarter , Edited by: Canters , K. J and Wijsman , H. J. W . 11 – 22 . Utrecht, , Netherlands : Werkgroep Boommarter Nederland .
- Kohn , M. H. and Wayne , R. K. 1997 . Facts from feces revisited. . Trends in Ecology and Evolution , 12 : 223 – 227 .
- Krebs , C. J. 1989 . Ecological methodology , New York : Harper and Row .
- Krebs , J. R. and Davies , N. B. 1993 . An introduction to behavioural ecology, 3rd edn , London : Blackwell Scientific Publications .
- Kruger , H. H. 1990 . “ Home ranges and patterns of distribution of stone and pine martens. ” . In Transactions 19th International Congress of game biologists , Edited by: Myberget , S . 348 – 349 . Norwegian Institute for Nature Research, Trondheim : Norway .
- Kruuk , H. 1989 . The social badger , Oxford : Oxford University Press .
- Kruuk , H. , Conroy , J. W. H. , Glimmerveen , U. and Ouwerkerk , E. J. 1986 . The use of spraints to survey populations of otters Lutra lutra. . Biological Conservation , 35 : 87 – 94 .
- Kruuk , H. and de Kock , L. 1981 . Food and habit of badgers (Meles meles L.) on Monte Baldo, northern Italy. . Sonderdruck aus Zeitschrift für Saugetierkunde , 46 : 295 – 301 .
- Kruuk , H. and Parish , T. 1981 . Feeding specialization of the European badger (Meles meles) in Scotland. . Journal of Animal Ecology , 50 : 773 – 788 .
- Lachat Feller , N. 1993 . “ Eco‐ethologie de la fouine (Martes foina Erxleben, 1777) dans le Jura suisse. ” . Faculté des Sciences de l'Université de Neuchâtel, Dégrée thesis
- Lanszki , J. 2003 . Feeding habits of stone martens in a Hungarian village and its surroundings. . Folia Zoologica , 52 : 367 – 377 .
- Larivière , S. , Crête , M. , Huot , J. , Patenaude , R. , Price , C. and Thomas , D. W. 2001 . Influence of food shortage during the summer on body composition and reproductive hormones in the red fox, Vulpes vulpes. . Canadian Journal of Zoology , 79 : 471 – 477 .
- Le Lay , G. and Lodé , T. 2004 . “ Santos‐Reiss M, Birks J, Messenger J (Eds) Stone marten's urban habitat: do red fox activities explain their distribution? ” . 4th International Martes Symposium, Lisbon, Portugal, 20–24 July 2004
- Leinati , L. , Mandelli , G. , Videsott , R. and Grimaldi , G. 1960 . Indagini sulle abitudini alimentari della volpe Vulpes vulpes del Parco Nazionale del Gran Paradiso. . La Clinica Veterinaria , 83 : 305 – 328 .
- Lenton , E. J. , Chanin , P. R. F. and Jefferies , D. J. 1980 . Otter survey of England 1977–79 , London : Nature Conservancy Council .
- Libois , R. and Waechter , A. 1991 . “ La fouine (Martes foina Erxl, 1777). ” . In Encyclopédie des Carnivores de France, vol. 10 , 1 – 53 . Paris : Société Francaise pour l'Etude et la Protection des Mammiféres .
- Lindström , E. R. , Andren , H. , Angelstam , P. , Cederlund , G. , Hornfeldt , B. , Jaderberg , L. , Lemnell , P. , Martinsson , B. , Skold , K. and Swenson , J. E. 1994 . Disease reveals the predator: Sarcoptic mange, red fox predation, and prey populations. . Ecology , 75 : 1042 – 1049 .
- Lindström , E. R. , Brainerd , S. M. , Helldin , J. O. and Overskaug , K. 1995 . Pine marten–red fox interactions: A case of intraguild predation? . Annales Zoologici Fennici , 32 : 123 – 130 .
- Lloyd , H. G. 1975 . “ The red fox in Britain. ” . In The wild canids: Their systematics, behavioural ecology and evolution , Edited by: Fox , M. W . 207 – 215 . New York : Van Nostrand Reinhold Co .
- Lloyd , H. G. 1980 . The red fox , London : B.T. Batsford Ltd .
- Lotka , A. J. 1932 . The growth of mixed populations: Two species competing for a common food supply. . Journal of the Washington Academy of Sciences , 22 : 461 – 469 .
- Lucherini , M. and Crema , G. 1993 . Diet of urban stone martens in Italy. . Mammalia , 57 : 274 – 277 .
- Lucherini , M. and Crema , G. 1994 . Seasonal variation in diet and trophic niche of the red fox in an alpine habitat. . Zeitschrift für Säugetierkunde , 59 : 1 – 8 .
- Lucherini , M. and Crema , G. 1995 . Seasonal variation in the food habits of badgers in an alpine valley. . Hystrix (N.S.) , 7 : 165 – 172 .
- Macdonald , D. W. 1977 . On food preference in the red fox. . Mammal Review , 7 : 7 – 23 .
- Marchesi , P. , Lachat , N. , Lienhard , R. , Debieve , Ph. and Mermod , C. 1989 . Comparaison des régimes alimentaires de la fouine (Martes foina Erxl.) et de la martre (Martes martes L.) dans une région du Jura suisse. . Revue Suisse de Zoologie , 96 : 281 – 296 .
- Marchesi , P. and Mermod , C. 1989 . Régime alimentaire de la martre (Martes martes, L.) dans le Jura suisse (Mammalia: Mustelidae). . Revue Suisse de Zoologie , 96 : 127 – 146 .
- Martinoli , A. , Preatoni , D. G. , Chiarenzi , B. , Wauters , L. A. and Tosi , G. 2001 . Diet of stoats (Mustela erminea) in an Alpine habitat: The importance of fruit consumption in summer. . Acta Oecologica , 22 : 45 – 53 .
- Messenger , J. E. and Birks , J. D. S. 2000 . “ Monitoring the very rare: Pine marten populations in England and Wales. ” . In Mustelids in a modern world. Management and conservation aspects of small carnivore–human interactions , Edited by: Griffiths , H. I . 217 – 230 . Leiden, , Netherlands : Backhuys .
- Neal , E. 1986 . The natural history of badgers , London & Sydney : Croom Helm .
- Neal , E. and Cheeseman , C. 1996 . Badgers , London : T & A D Poyser .
- Novikov , G. A. 1962 . Carnivorous mammals of the fauna of the U.S.S.R , Jerusalem : Monson .
- Padial , J. M. , Avila , E. and Gil‐Sanchez , J. M. 2002 . Feeding habits and overlap among red fox (Vulpes vulpes) and stone marten (Martes foina) in two Mediterranean mountain habitats. . Mammalian Biology , 67 : 137 – 146 .
- Palomares , F. and Caro , T. M. 1999 . Interspecific killing among mammalian carnivores. . The American Naturalist , 153 : 492 – 508 .
- Pedrini , P. , Prigioni , C. and Volcan , G. 1995a . Use of trophic resources and forest habitats by the genus Martes in Adamello‐Brenta Park (Central Italian Alps). . Hystrix (N.S.) , 7 : 127 – 136 .
- Pedrini , P. , Prigioni , C. and Volcan , G. 1995b . Distribution of mustelids in Adamello‐Brenta Park and surrounding areas (Central Italian Alps). . Hystrix (N.S.) , 7 : 39 – 44 .
- Pianka , E. R. 1969 . Sympatry of desert lizards (Ctenotus) in western Australia. . Ecology , 50 : 1012 – 1030 .
- Pianka , E. R. 1973 . The structure of lizard communities. . Annual Review of Ecology and Systematics , 4 : 53 – 74 .
- Pimm , S. L. and Rosenzweig , M. L. 1981 . Competition and habitat use. . Oikos , 37 : 1 – 6 .
- Pittiglio , C. 1996 . “ Analisi comparativa di uso e selezione dell'habitat della faina e della martora in condizioni di simpatria. Università di Roma “La Sapienza”, Roma. ” . Unpublished degree thesis
- Polis , G. A. , Myers , C. A. and Holt , R. D. 1989 . The ecology and evolution of intraguild predation: Potential competitors that eat each other. . Annual Review of Ecology and Systematics , 20 : 297 – 330 .
- Prigioni , C. 1991 . Lo studio della dieta della Volpe (Vulpes vulpes). . Hystrix (N.S.) , 3 : 51 – 62 .
- Prigioni , C. and Sommariva , A. 1997 . Ecology of the stone marten (Martes foina) in the urban habitat of Cavalese (Trento, Italy). . Centro di Ecologia Alpina, Report , 11 : 1 – 26 .
- Pulliainen , E. 1981 . “ Winter habitat selection, home‐range, and movements of the pine marten (Martes martes) in Finnish Lapland forest. ” . In Proceedings of “Worldwide Furbearer Conference”. Falls Church, R.R. Donnelly and Sons Co. Frostburg, Maryland, USA, II Edited by: Chapman , J. A. and Pursley , D. 1068 – 1087 .
- Putman , R. J. 1984 . Facts from faeces. . Mammal Review , 14 : 79 – 97 .
- Remonti , L. , Balestrieri , A. , Domenis , L. , Banchi , C. , Lo Valvo , T. , Robetto , S. and Orusa , R. 2005 . Red fox (Vulpes vulpes) cannibalistic behaviour and the prevalence of Trichinella britovi in NW Italian Alps. . Parasitology Research , 97 : 431 – 435 .
- Remonti , L. , Balestrieri , A. and Prigioni , C. 2007 . Role of fruits in the diet of small mustelids (Mustela sp.) from the western Italian Alps. . European Journal of Wildlife Research , 53 : 35 – 39 .
- Revilla , E. and Palomares , F. 2002 . Spatial organization, group living and ecological correlates in low‐density populations of Eurasian badgers, Meles meles. . Journal of Animal Ecology , 71 : 497 – 512 .
- Rice , W. R. 1989 . Analysing tables of statistical tests. . Evolution , 43 : 223 – 225 .
- Ricklefs , R. E. 1990 . Ecology. 3rd edn , New York : WH Freeman .
- Rinetti , L. 1987 . L'alimentazione estiva del tasso europeo Meles meles L. nel Parco Nazionale del Gran Paradiso. . Atti Società italiana di Scienze naturali Museo civico di Storia Naturale Milano , 128 : 261 – 264 .
- Romanowski , J. and Lesinski , G. 1991 . A note on the diet of stone marten in southeastern Romania. . Acta Theriologica , 36 : 201 – 204 .
- Rödel , H. G. , Ebersbach , H. and Stubbe , M. 1998 . Feeding ecology of the stone marten (Martes foina): The importance of mammalian prey. . Beiträge zur Jagdund Wildforschung , 23 : 219 – 230 .
- Rondinini , C. and Boitani , L. 2002 . Habitat use by beech martens in a fragmented habitat. . Ecography , 25 : 257 – 264 .
- Roper , T. J. 1994 . The European badger Meles meles: Food specialist or generalist? . Journal of Zoology (London) , 234 : 437 – 452 .
- Rosalino , L. M. , Loureiro , F. , Macdonald , D. W. and Santos‐Reis , M. 2005 . Dietary shifts of the badger Meles meles in Mediterranean woodlands: An opportunistic forager with seasonal specialisms. . Mammalian Biology , 70 : 12 – 23 .
- Russel , A. J. M. and Storch , I. 2004 . Summer food of sympatric red fox and pine marten in the German Alps. . European Journal of Wildlife Reseach , 50 : 53 – 58 .
- Sadlier , L. M. J. , Webbon , C. C. , Baker , P. J. and Harris , S. 2004 . Methods of monitoring red foxes Vulpes vulpes and badgers Meles meles: Are field signs the answer? . Mammal Review , 34 : 75 – 98 .
- Schoener , T. W. 1974 . Resource partitioning in ecological communities. . Science , 185 : 27 – 39 .
- Schoener , T. W. 1982 . The controversy over interspecific competition. . American Scientist , 70 : 586 – 595 .
- Schoener , T. W. 1986 . “ Patterns in terrestrial vertebrate versus arthropod communities: Do systematic differences in regularity exist? ” . In Community ecology , Edited by: Diamond , J and Case , T. J . 556 – 586 . New York : Harper & Row .
- Serafini , P. and Lovari , S. 1993 . Food habits and trophic niche overlap of the fox and the stone marten in a Mediterranean rural area. . Acta Theriologica , 38 : 233 – 244 .
- Smedshaug , C. A. , Selas , V. , Lund , S. E. and Sonerud , G. A. 1999 . The effect of a natural reduction of red fox Vulpes vulpes on small game hunting bags in Norway. . Wildlife Biology , 5 : 157 – 166 .
- Spagnesi , M. and De Marinis , A. M. 2002 . Mammiferi d'Italia. . Quaderni di Conservazione della Natura , 14 : 1 – 309 .
- Teerink , B. J. 1991 . Hair of west European mammals. Atlas and identification key , Cambridge, , UK : Cambridge University Press .
- Thompson , I. D. 1994 . Marten population in uncut and logged boreal forests in Ontario. . Journal of Wildlife Management , 58 : 272 – 280 .
- Toth‐Apathy , M. and Szenczi , P. 2004 . “ Santos‐Reiss M, Birks J, Messenger J (Eds) The stone marten and the city. ” . 25 4th International Martes Symposium, Lisbon, Portugal, Plenary session abstracts
- Virgós , E. , Mangas , J. G. , Blanco‐Aguiar , J. A. , Garrote , G. , Almagro , N. and Viso , R. P. 2004 . Food habits of European badgers (Meles meles) along an altitudinal gradient of Mediterranean environments: A field test of the earthworm specialization hypothesis. . Canadian Journal of Zoology , 82 : 41 – 51 .
- Volterra , V. 1926 . Variations and fluctuations of the numbers of individuals in animal species living together. Reprinted in RN Chapman, Animal ecology , New York : McGraw‐Hill .
- Waechter , A. 1975 . Ecologie de la Fouine en Alsace. . Revue d'Ecologie, Terre et Vie , 29 : 399 – 457 .
- Weber , J. M. and Meia , J. S. 1996 . Habitat use by the red fox Vulpes vulpes in a mountainous area. . Ethology Ecology and Evolution , 8 : 223 – 232 .
- Webster , J. A. 2001 . A review of the historical evidence of the habitat of the pine marten in Cumbria. . Mammal Review , 31 : 17 – 31 .
- Wiens , J. A. 1989 . The ecology of bird communities: Processes and variations, vol. 2 , Cambridge, , UK : Cambridge University Press .