Abstract
Meiofaunal nematodes are among the most important components of the benthic environment. They have unusually high abundance and diversity. They are largely understudied in many parts of the world and explored very little from the Indian subcontinent, possibly due to lack of expertise. Meiofauna was investigated with emphasis on nematodes, which were the most dominant group and one species – Terschellingia longicaudata (De Man, 1907) – along the central west coast of India, stretching between Ratnagiri and Mangalore, during 2004. Maximum nematode diversity was found at the offshore location at the water depth of 35 m, while the minimum was found in the estuarine region. Nematode density was positively correlated with sediment organic matter (r= 0.73, p< 0.05). Among the 94 identified nematode species, T. longicaudata was one of the dominant species comprising >21% of nematodes and 15% of the total meiofaunal population. The species had high abundance at the stations mostly characterized by silty sediment. T. longicaudata has been hypothesized to have a global distribution and the present study, for the first time, adds to the inventory of its distribution along the central west coast of India.
Introduction
Meiobenthic nematodes are among the most diverse and numerically dominant metazoans in the marine habitat (Heip et al. Citation1982; De Ley & Blaxter Citation2001), with a global species estimate (Lambshead & Boucher Citation2003) between 105 and 108. Despite their remarkable diversity and their potential use as indicators, nematodes are among the least studied components of meiofauna (Heip et al. Citation1985). Nematodes play an important role in the benthic environment by (i) mechanical breakdown of the detritus, (ii) excretion of limiting nutrients to bacteria, (iii) producing microfilm conducive to bacterial growth and (iv) bioturbating sediment around detritus (Tietjen Citation1980). Nematode diversity has been well documented from the Atlantic and the Pacific Ocean (Heip et al. Citation1985). Ingole et al. (Citation1998, Citation2005, Citation2006) and Ingole and Koslow (Citation2005) have studied the meiofaunal communities from the deep and continental Indian Ocean, but very few studies are available on the nematode community dynamics (Ndaro & Olafsson Citation1999; Muthumbi et al. Citation2004; Raes et al. Citation2007). Meiofauna (Coull & Chandler Citation1992; Kennedy & Jacoby Citation1999) and nematode communities (Bongers et al. Citation1991) have been widely used in bio-monitoring programmes to assess the benthic environmental health and many species are good pollution indicators (Heip et al. Citation1985).
The central west coast of India has unique physical settings and dynamic biogeochemistry, with intense seasonality due to the influence of monsoon, coastal upwelling, seasonal anoxia and phytoplankton bloom (Naqvi et al. Citation2000). The main objective of this study was to investigate the meiofaunal community and nematode species diversity from the central west coast of India, which has no past account in any literature dealing with nematode community distribution. The aim was also to investigate the distribution and abundance of a nematode species Terschellingia longicaudata from this subtidal region, as it is hypothesized that T. longicaudata has a cosmopolitan distribution (Bhadury et al. Citation2005). This nematode species has gained importance due to its ability to thrive in low oxygen sediments (Sergeeva Citation1991) and its presence in polluted habitats (Liu et al. Citation2008).
Materials and methods
Study area
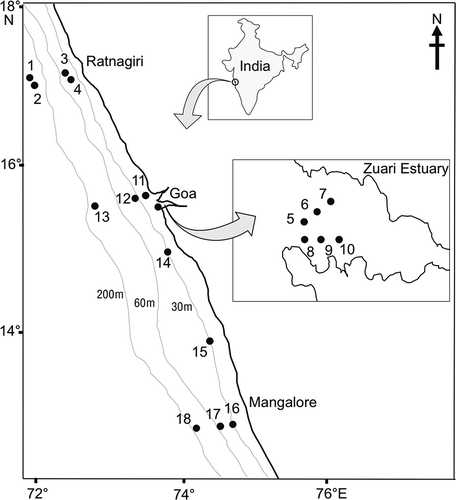
Sampling sites were located along the central west coast of India (). In total, 18 subtidal sites were selected randomly between Ratnagiri and Mangalore (). Sampling locations 1 and 2 were from the marginal region, locations 3, 4 and 5–10 were from Zuari river mouth, a shallow estuarine region and 11–18 were from the shelf region. In the north, the first two stations were taken in the deeper region (500 m). The river mouth sites (Stations 5–10) were in shallower depths between 7 and 15 m. The remaining sites were in 20–100 m water depths. All the stations had silty/muddy type of sediments.
Table I. Stations and parameters
The sediment samples from the deeper depths were collected on board CRV Sagar Sukti (SASU-60) and ORV Sagar Kanya (SK-211). The sampling in the shallower locations, particularly the harbour area (Zuari river mouth), was done with a country craft. Sediment samples were collected with a van Veen grab (0.11 m2) and by deploying a spade box corer (147.894 cm2). Separate samples were collected for sediment chlorophyll-a, organic carbon and granulometry, and immediately preserved in deep freeze. The sediment chlorophyll-a analysis was carried out by flurometric method (Holm-Hansen et al. Citation1965). The organic carbon of the sediment was estimated by wet oxidation method (El Wakeel & Riley Citation1957). For the analysis of sediment grain size, samples were dried, weighed and sieved with a 63-μm sieve to separate the sand fraction and pipette method was employed to determine the silt and the clay fraction (Folk Citation1968). For meiofaunal samples, an acrylic core (4.5 cm diameter) was used to sample the top 0–5 cm sediment layer. Duplicate cores were taken from each station. All samples were immediately preserved in 5% buffered seawater formalin solution with Rose Bengal as stain. The samples were sieved with 500 μm mesh and then by 45-μm sieve. Material retained on the 45-μm sieve was investigated for meiofauna. Meiofauna was sorted under binocular stereoscopic microscope and mounted in glycerol for taxonomic identification. Meiofaunal identification up to group level was done using the key by Higgins and Thiel (Citation1988) and the nematodes were identified up to the lowest possible taxa (genus/species) using a pictorial key by Platt and Warwick (Citation1983, Citation1988) and Warwick et al. (Citation1998). The meiofaunal abundance was converted to ind. 10 cm–2. The Bray–Curtis similarity using untransformed meiofaunal and nematode abundance was made by the multi-dimensional scaling (MDS) ordination using PRIMER 6.0 software.
Results and discussion
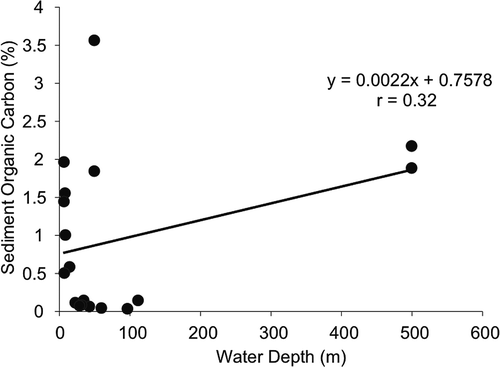
In the open ocean, light penetration limits the benthic primary production in deeper water, restricting the availability of chlorophyll in the sediment. On the other hand, organic matter in the sediment is accumulated over a time period both from the pelagic flux as well as contribution from riverine sources (Rao & Veerayya Citation2000; Ingole et al. Citation2001). In this study there was a positive correlation between sediment organic carbon and water depth (r = 0.32, ).
Meiofauna is an important link between the bacteria–detritus and the carnivore level (Chardy & Dauvin Citation1992).
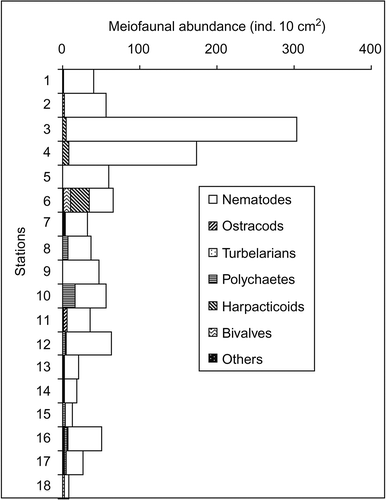
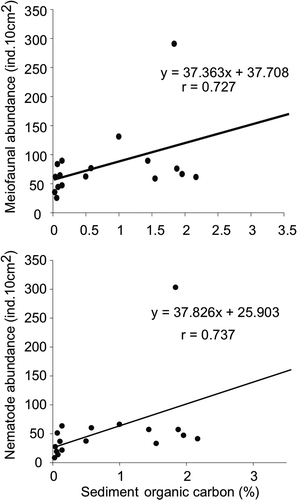
Among meiofauna; nematodes, ostracods, turbellarians, polychaetes, harpacticoid copepods, bivalves and oligochaetes were recorded from the sampling area besides hydroids, nauplii and gastropodes. The group with unidentified specimens was kept under others. The nematode density was highest at Station 3 (303 ind. 10 cm–2) and lowest at Station 18 (19 ind. 10 cm–2). Very high numbers of harpacticoid copepods were seen at Station 6 (35 ind. 10 cm–2) (). Maximum numbers of meiofaunal groups were recorded at Stations 12, 16 and 17 in the study area and the minimum were at Stations 6 and 8. There was positive correlation between the sediment organic carbon and meiofaunal density (r = 0.72, p < 0.05; ). Moreover, the MDS ordinates for meiofauna abundance revealed no clear distinction of the habitats (). The low densities of meiofauna differences were attributed to high hydrodynamic stress around the continental slope (Rao & Veeryya Citation2000) preventing phytoplankton from reaching the deeper sediments (Vanaverbeke et al. Citation2000). Moreover, higher current speed above the sediment increases the risk of the meiobenthos being eroded or suspended (Vanaverbeke et al. Citation2000). Low occurrence of meiofaunal groups and high percent dominance of nematodes suggests sensitivity of other meiofaunal groups to dynamic habitat compared to nematodes (Heip et al. Citation1985; Coull & Chandler Citation1992). Therefore, in-depth taxonomic resolution of the nematode community might give a better picture of the heterogeneous habitats.
Figure 5. Multi-dimensional scaling (MDS) ordination for untransformed meiofaunal (a) and nematode (b) abundance on a two-dimensional scale at each station location.
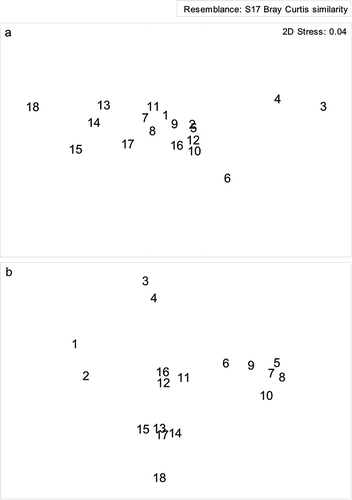
Nematodes were found at all stations and were the most dominant with mean abundance of 84%, followed by harpacticoids and polychaetes with 5% each (). The highest number of species (35) was found at Station 12 and lowest (07) was at Station 7 (). The total number of nematode species recorded from the study area was 94 (). The family Xyalidae was the most dominant and was represented by 13 out of 94 species (). The MDS ordinates for nematode species abundance shows a clear differentiation between the habitats where the estuarine stations show grouping (Stations 5–10) and the shelf community can be seen separated (Stations 11–18) and the deepest (500 m; Stations 1 and 2) are again well separated from the others (). Cluster analysis depicts that Stations 3 and 4 are part of the shelf community (Stations 11–18) while a very different estuarine community (Stations 5–10) is separated from the continental marginal (Stations 1 and 2) and shelf community (). As Stations 3 and 4 fall in the depth range of the shelf region and share similar hydrodynamic settings, the nematodes also reveal marked similarity with the shelf community. Habitat heterogeneity clearly separates the nematode community according to the habitats and the hydrodynamics of that particular location (Vanaverbeke et al. Citation2000; Schratzberger et al. Citation2006). The most widely distributed nematode was Desmoscolex sp., accounted from all the stations (). The species Polysigma sp. was most conspicuous in occurrence in terms of abundance (126 ind. 10 cm–2). Food source is also an important aspect for the distribution of nematode species (Moens et al. Citation1999). Organic matter plays an important role in structuring the nematode community (Pusceddu et al. Citation2009) and apparently nematode abundance shows positive correlation with sediment organic carbon (r= 0.73, p< 0.05; ). It may suggest the dependence of the nematode community on the bacterial biomass and the organic matter reaching the sediments (Meyer-Reil & Faubel Citation1980; Danovaro Citation1996).
Figure 6. Bray–Curtis similarity cluster analysis based on nematode species abundance at each station location.
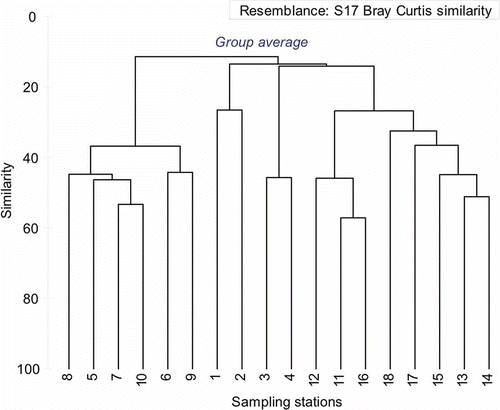
Table II. Occurrence of nematode species at the sampling stations
Table III. Details of nematode family and genera and percent occurrence and prevalence of T. longicaudata at various stations
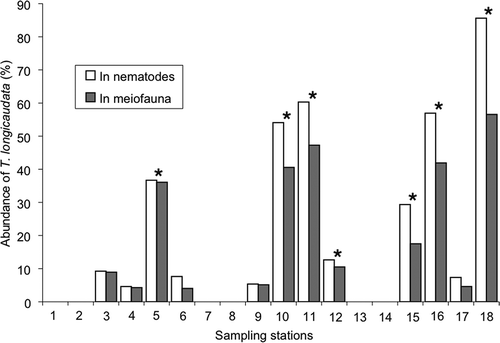
The percent dominance was calculated for mean abundance of T. longicaudata at all the stations. T. longicaudata was present at 12 out of the 18 sampled locations (). The highest percent dominance was observed at station 18 (86%) and it constituted about 21% of the nematode community and 15% of the meiofauna ().
Figure 7. Percent composition of Terschellingia longicaudata in nematodes and meiofauna (*stations with a silty type of sediment).
T. longicaudata is a selective deposit feeder (Wieser Citation1953), mainly feeding on heterotrophic bacteria and detritus with EPS (Rezeznik-Orignac et al. Citation2008). It has been reported from most of the world's oceans and estuaries and was typically the dominant species in soft sediments from inshore water, and is also considered as having a cosmopolitan distribution (Bhadury et al. Citation2005).
The presence of T. longicaudata in heterogeneous habitats proves its ubiquitous distribution in the marine sediments such as mangroves, mudflats (Hodda & Nicholas Citation1985), various subtidal habitats (Heip et al. Citation1985; Travizi & Vidakovic Citation1997; Tita et al. Citation2002; Schratzberger et al. Citation2004, Citation2006; Bhadury et al. Citation2005), seagrass bed (Novak Citation1989) and lagoons (Villano & Warwick Citation1995). The species is also known to excel in anthropogenically disturbed and polluted habitats (Lambshead Citation1986; Schratzberger & Warwick Citation1998; Liu et al. Citation2008). T. longicaudata seems to show affinity towards silty sediment type (Tietjen Citation1980) and this stands true in this part of the tropical Indian Ocean ().
Dominance of T. longicaudata from the intertidal regions of Eastern Australia and seagrass bed has been reported by Alongi (Citation1990) and Fisher and Sheaves (Citation2003), respectively. The dominance of T. longicaudata at most locations might be due to few factors, but the most evident is the silty sediment type.
The presence of T. longicaudata in most of the marine habitats indicates its adaptability to different type of sediments (Sergeeva Citation1991). Detailed phenotypic variation in T. longicaudata along with molecular evolutionary studies has already been initiated (Bhadury et al. Citation2005). Comparison of molecular data from various locations will probably provide direct evidence of genetic variability, if any, and be the pathway for determining worldwide distribution of this species. The present study confirms its presence from the coastal Indian Ocean and supports the notion of its ubiquity with species preference for silty sediments.
Acknowledgements
The authors thank the Director, NIO, Goa for the facilities. They would like to express their gratitude to all the cruise participants for their assistance in onboard sampling and Dr Prakash Babu for Organic Carbon Analysis. They acknowledge the help of Dr Melanie Austen, of Plymouth Marine Laboratory, Plymouth, UK, for confirming the nematode taxonomy through the Darwin nematode workshop and the three anonymous reviewers for providing helpful comments on the manuscript. This is the contribution no. 4678.NIO (CSIR) Goa.
References
- Alongi , DM . 1990 . Community dynamics of free-living nematodes in some tropical mangrove and sandflat habitats . Bulletin of Marine Science , 46 : 358 – 373 .
- Bhadury , P , Austen , MC , Bilton , DT , Lambshead , PJD , Rogers , AD and Smerdon , R . 2005 . Combined morphological and molecular analysis of individual nematodes through short-term preservation in formalin . Molecular Ecology Notes , 5 : 965 – 968 .
- Bongers , T , Alkemade , R and Yeates , GW . 1991 . Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index . Marine Ecology Progress Series , 67 : 135 – 142 .
- Chardy , P and Dauvin , JC . 1992 . Carbon flows in a subtidal fine sand community from the western English Channel: A simulation analysis . Marine Ecology Progress Series , 81 : 147 – 161 .
- Coull , BC and Chandler , GT . 1992 . Pollution and meiofauna: Field, laboratory and mesocosm studies . Oceanography and Marine Biology Annual Review , 30 : 191 – 271 .
- Danovaro , R . 1996 . Detritus–bacteria–meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean . Marine Biology , 127 : 1 – 13 .
- De Ley , P and Blaxter , M . 2001 . “ Systematic position and phylogeny ” . In Biology of nematodes , Edited by: Lee , DL . 1 – 30 . London : Harwood Academic Publishers .
- El Wakeel , SK and Riley , JP . 1957 . The determination of organic carbon in marine muds . Journal du Conseil Permanent International Pour l'Exploration de la mer , 22 : 180 – 183 .
- Fisher , R and Sheaves , MJ . 2003 . Community structure and spatial variability of marine nematodes in tropical Australian pioneer seagrass meadows . Hydrobiologia , 495 : 143 – 158 .
- Folk , RL . 1968 . Petrology of sedimentary rocks , Austin, TX : Hemphill Publishing Company .
- Heip , C , Vincx , M , Smol , N and Vranken , G . 1982 . The systematics and ecology of free-living marine nematodes . Helminthological Abstracts Series B , 51 : 1 – 31 .
- Heip , C , Vincx , M and Vranken , G . 1985 . The ecology of marine nematodes . Oceanography and Marine Biology Annual Review , 23 : 399 – 489 .
- Higgins , RR and Thiel , H . 1988 . Introduction to the study of Meiofauna , Washington, DC : Smithsonian Institution Press. 488 .
- Hodda , M and Nicholas , WL . 1985 . Meiofauna associated with mangroves in the Hunter River Estuary and Fullerton Cove, south-eastern Australia . Australian Journal of Marine and Freshwater Research , 36 : 41 – 50 .
- Holm-Hansen , O , Lorenzen , CJ , Holmes , RW and Strickland , JDH . 1965 . Fluorometric determination of chlorophyll . Journal du Conseil Permanent International Pour l'Exploration de la mer , 30 : 3 – 15 .
- Ingole , BS , Ansari , ZA and Parulekar , AH . 1998 . Spatial variations in meiofaunal abundance of some coralline beaches of Mauritius . Tropical Ecology , 39 : 103 – 108 .
- Ingole , BS , Ansari , ZA , Rathod , V and Rodrigues , N . 2001 . Response of deep-sea macrobenthos to a small-scale environmental disturbance . Deep-Sea Research-II , 14 : 3401 – 3410 .
- Ingole , BS , Goltekar , R , Gonsalves , S and Ansari , ZA . 2005 . Recovery of deep-sea meiofauna after artificial disturbance in the Central Indian Basin . Marine Georesources and Geotechnology , 23 : 253 – 266 .
- Ingole , BS and Koslow , JA . 2005 . Deep-sea ecosystems of the Indian Ocean . Indian Journal of Marine Sciences , 34 : 27 – 34 .
- Ingole , B , Sivadas , S , Goltekar , R , Clemente , S , Nanajkar , M , Sawant , R , D'Silva , C , Sarkar , A and Ansari , Z . 2006 . Ecotoxicological effect of grounded MV River Princess on the intertidal benthic organisms off Goa . Environment International , 32 : 284 – 291 .
- Kennedy , AD and Jacoby , CA . 1999 . Biological indicators of marine environmental health: Meiofauna – a neglected benthic component? . Environmental Monitoring Assessment , 54 : 47 – 68 .
- Lambshead , PJD . 1986 . Sub-catastrophic sewage and industrial waste contamination as revealed by marine nematode faunal analysis . Marine Ecology Progress Series , 29 : 247 – 260 .
- Lambshead , PJD and Boucher , G . 2003 . Marine nematode deep-sea biodiversity – Hyperdiverse or hype? . Journal of Biogeography , 30 : 475 – 485 .
- Liu , XS , Xu , WZ , Cheung , SG and Shin , PKS . 2008 . Subtropical meiobenthic nematode communities in Victoria Harbour, Hong Kong . Marine Pollution Bulletin , 56 : 1491 – 1497 .
- Meyer-Reil , LA and Faubel , A . 1980 . Uptake of organic matter by meiofauna organisms and interrelationships with bacteria . Marine Ecology Progress Series , 3 : 251 – 256 .
- Moens , T , Van Gansbeke , D and Vincx , M . 1999 . Linking estuarine nematodes to their suspected food: A case study from the Westerschelde estuary (south-west Netherlands) . Journal of the Marine Biological Association of the United Kingdom , 79 : 1017 – 1027 .
- Muthumbi , AW , Vanreusel , A , Soetaert , K , Duineveld , G and Vincx , M . 2004 . Meiofauna along the continental slope off the Kenyan coast on the Western Indian Ocean (WIO) with emphasis on nematode assemblages . International Review of Hydrobiologia , 89 : 188 – 205 .
- Naqvi , SWA , Jayakumar , DA , Narvekar , RV , Naik , H , Sarma , VVSS , D'souza , W , Joseph , S and George , MD . 2000 . Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf . Nature , 408 : 346 – 349 .
- Ndaro , SGM and Olafsson , E . 1999 . Soft-bottom meiofauna with emphasis on nematode assemblage structure in a tropical intertidal lagoon in eastern Africa: I . Spatial variability. Hydrobiologia , 405 : 133 – 148 .
- Novak , R . 1989 . Ecology of nematodes in the Mediterranean seagrass Posidonia oceanica (L.) Delile. 1. General part and faunistics of the nematode community . Marine Ecology , 10 : 335 – 363 .
- Platt , HM and Warwick , RM . 1983 . “ Free-living marine nematodes. Part I ” . In British Enoplids, Synopses of the British fauna (new series) 28 , Cambridge : Cambridge University Press .
- Platt , HM and Warwick , RM . 1988 . “ Freeliving marine nematodes. Part II: British chromadorids ” . In Pictorial key to world genera and notes for the identification of British species, Synopses of the British fauna (New Series) , Vol. 38 , Leiden : E.J. Brill .
- Pusceddu , A , Gambi , C , Zeppilli , D , Bianchelli , S and Danovaro , R . 2009 . Organic matter composition, metazoan meiofauna and nematode biodiversity in Mediterranean deepsea sediments . Deep-Sea Research II , 56 : 755 – 762 .
- Raes , M , De Troch , M , Ndaro , GM , Muthumbi , A , Guilini , K and Vanreusel , A . 2007 . The structuring role of microhabitat type in coral degradation zones: A case study with marine nematodes from Kenya and Zanzibar . Coral Reefs , 26 : 113 – 126 .
- Rao , BR and Veerayya , M . 2000 . Influence of marginal highs on the accumulation of organic carbon along the continental slope off western India . Deep-Sea Research II , 47 : 303 – 327 .
- Rzeznik-Orignac , J , Boucher , G , Fichet , D and Richard , P . 2008 . Stable isotope analysis of food source and trophic position of intertidal nematodes and copepods . Marine Ecology Progress Series , 359 : 145 – 150 .
- Schratzberger , M , Bolam , S , Whomersley , P and Warr , K . 2006 . Differential response of nematode colonist communities to the intertidal placement of dredged material . Journal of Experimental Marine Biology and Ecology , 334 : 244 – 255 .
- Schratzberger , M and Warwick , RM . 1998 . Effects of the intensity and frequency of organic enrichment on two estuarine nematode communities . Marine Ecology Progress Series , 164 : 84 – 95 .
- Schratzberger , M , Whomersley , P , Warr , K , Bolam , SG and Rees , HL . 2004 . Colonisation of various types of sediment by estuarine nematodes via lateral infaunal migration: A laboratory study . Marine Biology , 145 : 69 – 78 .
- Sergeeva , NG . 1991 . Unusual polyamphidity of natural population of Terschellingia longicaudata de Man, 1907 (Nematoda, Monhysterida, Linhomoeidae) in the Black Sea . Ecologiya Morya , 39 : 70 – 73 .
- Tietjen , JH . 1980 . “ Microbial–meiofaunal interrelationships: A review ” . In Microbiology 1980 , 135 – 138 . Washington, DC : American Society for Microbiology .
- Tita , G , Desrosiers , G , Vincx , M and Clement , M . 2002 . Intertidal meiofauna of the St Lawrence estuary (Quebec, Canada): Diversity, biomass and feeding structure of nematode assemblages . Journal of the Marine Biological Association of the United Kingdom , 82 : 779 – 791 .
- Travizi , A and Vidakovic , J . 1997 . Nematofauna in the Adriatic Sea: Review and check-list of free-living nematode species . Helgoländer Meerseunters , 51 : 503 – 519 .
- Vanaverbeke , J , Gheskiere , T and Vincx , M . 2000 . The meiobenthos of subtidal sandbanks on the Belgian Continental Shelf (Southern Bight of the North Sea) . Estuarine, Coastal and Shelf Science , 51 : 637 – 647 .
- Villano , N and Warwick , RM . 1995 . Meiobenthic communities associated with the seasonal cycle of growth and decay of Ulva rigida Arardh in the Palude Della Rosa, Lagoon of Venice . Estuarine, Coastal and Shelf Science , 4 : 181 – 194 .
- Warwick , RM , Platt , HM and Somerfield , PJ . 1998 . “ Freeliving marine nematodes ” . In Part III: British monhysterids. Pictorial key to world genera and notes for the identification of British species, Synopses of the British fauna (New Series) , Vol. 53 , Shrewsbury : Field Studies Council .
- Wieser , W . 1953 . Die Beziehung zwischen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden . Arkiv fur Zoolgie , 2/4 : 439 – 484 .