Abstract
Austropotamobius pallipes is a vulnerable crayfish, and therefore it is necessary to analyse reproduction in the extant populations. Details of the female annual reproductive cycle in a Mediterranean population are provided for two consecutive years. Maternal-size-related variables and date of collection have been considered in relation to the number of ovarian and pleopodal eggs. Reproductive data from 2587 females collected by hand and traps are reported. In total, 204 (14.69%) and 231 (15.93%) individuals in 2003 and 2004, respectively, were ovigerous. Demonstrable differences exist between ovarian and pleopodal egg counts. In particular, ovarian egg counts were considerably higher than expected pleopodal egg numbers. Mating begins in November and is followed by egg laying in early December–January. Berried females occur until May–June, when hatching occurs. The numbers of both ovarian and pleopodal eggs are correlated with the size of females, although fecundity slightly decreases in larger females. The histology of the reproductive organs of both sexes is also analysed.
Introduction
The infraorder Astacidea is represented by several species that have undergone an extensive adaptative radiation, today inhabiting several types of inland water habitats of all continents except Africa and Antartica (Hobbs Citation1988). Amongst the five European indigenous crayfish, the only species inhabiting the Italian inland waters is Austropotamobius pallipes (Lereboullet, 1858).
A. pallipes is extremely sensitive to slight changes in environmental conditions, living in fragmented habitats as a consequence of its restricted ecological habit (Demers & Reynolds Citation2002; Demers et al. Citation2003; Trouilhé et al. Citation2003), so that several authors classify it as a good bioindicator of water quality (Holdich & Reeve Citation1991; Reynolds et al. Citation2002; Scalici & Gibertini Citation2005; Renai et al. Citation2006; Brusconi et al. Citation2008; but see also Holdich et al. Citation2006; Trouilhé et al. Citation2007). However, as a consequence of several human activities, the white-clawed crayfish is becoming a more and more imperilled taxon, listed in the main international conventions for fauna and flora protection.
Since the threatened status, several aspects of its biology have been widely studied. A. pallipes is known as the largest and longest-lived freshwater invertebrate (Füreder et al. Citation2003; Scalici et al. Citation2008), playing a role in assuring the services offered by freshwater systems (Gherardi et al. Citation2004; Scalici & Gibertini Citation2007). Its occurrence is associated with the availability of boulders, boulder/cobble banks, and riffles (Naura & Robinson Citation1998; Brusconi et al. Citation2008). Among other characteristics of the habitat, fibrous and ramified roots provide shelter to crayfish and act as detritus traps (Bohl Citation1987; Smith et al. Citation1996; Broquet et al. Citation2002). Also, traditional and geometric morphometric studies were carried out on A. pallipes (Sint et al. Citation2007; Bertocchi et al. Citation2008; Scalici & Gibertini Citation2009; Scalici et al. Citation2009). However, all ecological and ‘morphological’ evidences proposed until now were achieved at regional level without providing useful comparison tools. Since some geographical differences emerged among the A. pallipes populations in its entire distribution area (see Albrecht Citation1982; Scalici et al. Citation2008), the main goal is to describe eventual geographical variations of some reproductive aspects of this species, in order to provide information for more detailed conservation activities. In fact, the Mediterranean climate and the geographic micro-conditions can influence mating, spawning, and development from site to site and from year to year.
For the most part, data on reproduction collected on wild populations are rare. Contrary to a great deal of studies in controlled rearing systems (see for example Woodlock & Reynolds Citation1988a; Celada et al. Citation1991; Reynolds et al. Citation1992; Carral et al. Citation1994, Citation2003; Pérez et al. Citation1998, Citation1999; Sáez-Royuela et al. Citation2005, 2006), data on the white-clawed crayfish reproductive biology collected in field seems to be very scarce and sparse, scattered throughout the European native range. Few studies have been concerned with wild populations (Rhodes & Holdich Citation1982; Brewis & Bowler Citation1985; Neveu Citation2007), also including the study of Brown and Bowler (Citation1977) carried out in a ‘semi-natural’ habitat. There are also no detailed studies on the gonad morphology and anatomy in Astacidae (Hobbs et al. Citation2007), but many on the gonad morphology in Cambaridae (for a review, see Erkan et al. Citation2009).
In particular, the purpose of this study was to collect data on some reproductive aspects of a native crayfish population in order to improve knowledge about fertility, fecundity, gonad development and structure of A. pallipes in a local basin in central Italy and to provide a baseline for comparison with other populations.
Materials and methods
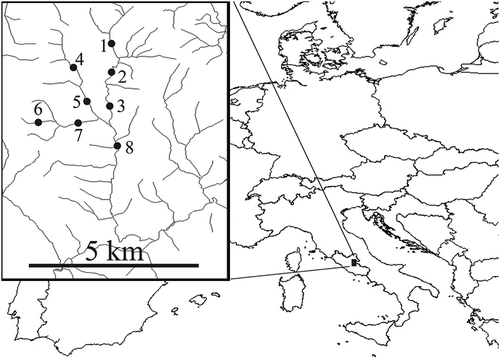
Data were collected monthly from January 2003 to December 2004 from 8 sampling sites (each being 40–180 cm deep and 170 cm wide on average) within the Licenza brook basin (central Italy, ).
Figure 1. Sampling sites within the study area, located between 450 and 600 m a.s.l., and from 42°05'48,8'N 12°54'36,6'E to 42°05'06,9'N 12°54'09,7'E. For the water physico-chemical and environmental features, see Scalici et al. (Citation2008).
Temperature was surveyed in each site using a digital thermometer with appropriate immersion probes (Multiline F/SET-3). Specimens were collected by hand-nets and traps, sexed and measured on the carapace from the ocular hollow to the terminal thorax portion (CL) using digital images taken on site and then analysed in the laboratory (see Scalici et al. Citation2008). Before their release, (1) the glair gland maturation (white colour indicates maturity: see Reynolds Citation2002), (2) mated (MF, with spermatophores) and berried (BF, with eggs) females, (3) the colour of the ovarian eggs (white for immature oocytes, yellow for maturing oocytes, and orange for mature eggs), (4) the number of pleopodal eggs (PE), and (5) the embryonic development stage (ES, only during 2004) were recorded. The colour of ovarian (internal) eggs was assessed by folding the abdomen under the thorax and observing the ovary through the thoraco-abdominal pleura. The CL value at which 50% of females were BF (CL50) was interpreted as the size at maturity. ESs (identified only for the external eggs in order to avoid excessive stress) were divided into (1) fertilized eggs with a black colour (ES1), (2) ‘half moon’ eggs (ES2, eggs with a half portion of a light colour and the other half dark, corresponding to the pulsating heart phase), (3) eggs containing eyed embryo (ES3), and (4) eggs with pre-hatching embryo (ES4) (according to a modification of Celada et al. Citation1991). The external egg diameter was also measured by analysis of images taken on sites using a digital camera and then analysed in the laboratory. Captured females were individually marked by waterproof paint on the carapace back in order to avoid duplication of measurements on the same crayfish, and then further stresses for animals and pseudo-replications during the statistical elaborations.
Moreover, a total of 55 crayfish (25 females + 30 males) ranging from 10 to 55 mm CL, collected 12 years ago within the same study area during an entire calendar year and preserved in absolute ethanol, were selected for histological analysis. These individuals were dissected to remove gonads, and the latter were fixed with Bouin's solution, dehydrated in a graded ethanol series, and embedded in paraffin. Then, 7–10 µm thick serial sections were stained with Carazzi's haematoxylin and eosin. Further, 20 sacrificed females were used to evaluate fertility (i.e. the ovarian egg number, OE).
All statistical analyses were performed using the STATISTICA Statsoft software version 7.0. First, the analysis of covariance (ANCOVA) was used to identify differences between the two temperature trends of 2003 and 2004 (with temperature as covariate). Then, χ2 tests (with the Yates correction) were used to verify the significance of the difference between the number of females collected in 2003 and 2004. Moreover, in order to test the size homogeneity of the subsamples collected in the two study years, data of body dimensions (CL) were first checked for normality using the Kolmogorov–Smirnoff test and then compared using Student's t-test and the F test for the mean and the variance, respectively. ANOVA, and eventually the Tukey's post-hoc, was used to identify differences among the egg diameters of the observed ESs. The three regressions (and the respective significance by t-test) ‘CL vs. PE’ (R1), ‘CL vs. OE’ (R2), and ‘CL vs. egg diameter mean value per female’ (R3) were computed.
Results
Field observations
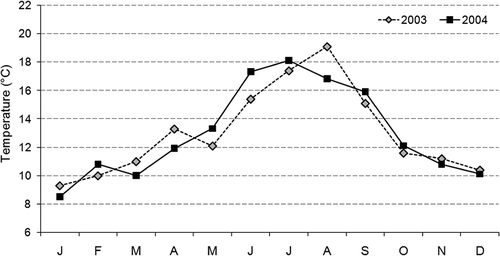
Through all the sampling sessions, mean and standard deviation values of the water temperature within the study area showed seasonal oscillations (). The temperature value trends of 2003 and 2004 did not show significant differences (P = 2.53 after ANCOVA).
Figure 2. Mean values of the water temperature of the study area surveyed from January 2003 to December 2004.
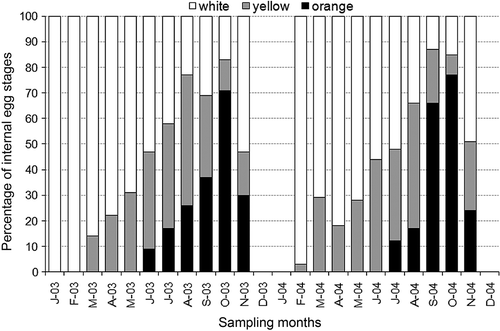
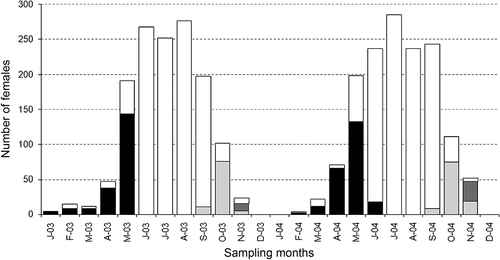
In total, 2587 females were collected during the study period. In particular, the analysed sample comprised 1286 females ranging between 11 and 40 mm CL in 2003, and 1301 from 13 and 40 mm CL in 2004 (). The difference in numbers of collected individuals between the two years was not significant (χ2 = 1.25, df = 1, ns). Also the mean (t = 4.24, df = 1, ns) and variance (F[1305,1411] = 0.84, ns) values of CL showed no differences between 2003 and 2004. In this case we used parametric tests to compare CL data of the two study years since the Kolmogorov–Smirnoff test confirmed the data set normality (KS = 0.354 and 0.411, both with p = 0.79 and 0.97, for 2003 and 2004, respectively; if the KS statistic is significant, then the hypothesis that the respective distribution is normal should be rejected). Females with matured glair glands (N tot. = 92 and 103 in 2003 and 2004, respectively) occurred from September (N = 11 and 9) to November (N = 5 and 19) in both years whereas MF individuals in November in both year, (N = 11 and 28) and BF from January to May (N tot. = 204, 15.86%) in 2003 and from February to June (N tot. = 231, 17.76%) in 2004.
Figure 3. Total number of collected females per sample. Within bars, the portion of females with matured glair gland (light grey), and both mated (dark grey) and berried (black) females is reported.
Each BF carried a different number of pleopodal eggs (PE). PE mean values per 2 mm CL are shown in .
Figure 4. Regression between the number of ovarian (from dissected females) and pleopodal (according to year) eggs, and the carapace length of Austropotamobius pallipes. For pleopodal eggs, mean values per 2 mm of carapace length are shown; the standard deviation values were omitted to facilitate the image comprehensiveness.
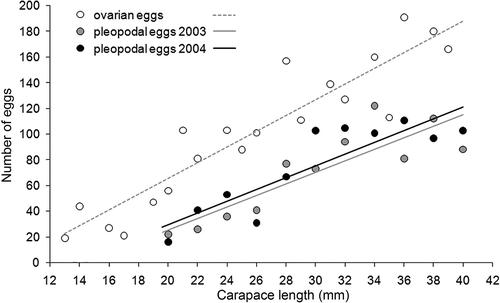
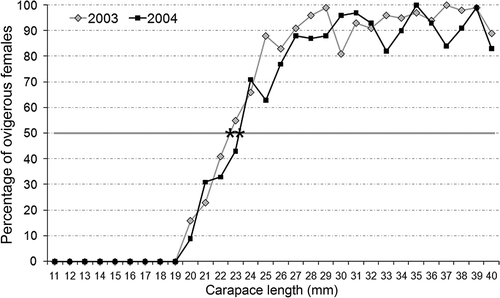
The regressions CL vs. PE of the two study years showed the same positive relation (). As OF concerns, CL50 was between 22 and 23 mm in 2003 and between 23 and 24 mm in 2004 ().
Table I. Regression data for both pleopodal (PE) and ovarian (OE) eggs versus carapace length (CL). N = number of individuals. T-test values were estimated on slopes: *p ≤ 0.05; **p ≤ 0.01
Figure 5. Percentage of ovigerous females per 1 mm of carapace length recorded in both study years. Stars indicate the length at which 50% of females carried pleopodal eggs.
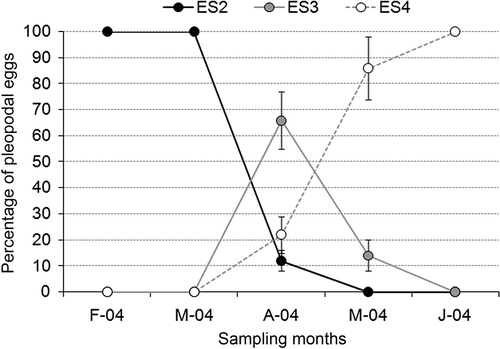
Three different ESs were observed during the 2004: ES2, ES3, and ES4. No recently spawned and fertilized eggs (ES1) were observed during the study period. The trend of the examined ESs is shown in
Figure 6. Mean percentage and standard deviation values for the number of pleopodal egg maturation stages observed during 2004. ES2: ‘half moon’ eggs; ES3: eggs containing eyed embryo; ES4: eggs with pre-hatching embryo.
The pleopodal egg diameter did not change during the embryo development (ANOVA: F[3,3227] = 3.53, P = 1.71) since the diameter of the three egg maturation stages showed similar values: 3.76±0.24 for ES2; 4.01±0.44 for ES3; 3.61±0.39 for ES4. Statistical significant differences were found for the regression between CL and the mean egg diameter per female (R 2 = 0.24, df = 224, P < 0.005; t = 3.34, P < 0.005).
Concerning OE, the colour of the egg maturation stages is shown in , while the regression between OE and CL is shown in (cf. ). Comparison between ovarian and pleopodal eggs is also shown in .
Histology
Both ovary and testis lie dorsally in the thorax between the floor of the pericardial sinus and the hindgut.
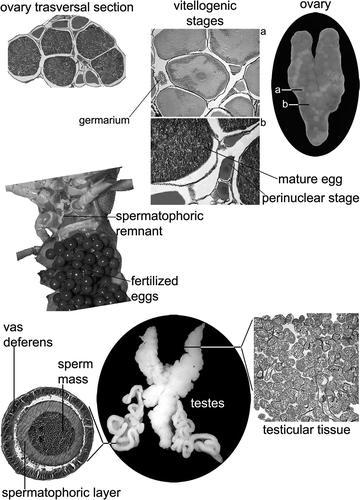
Female gonad ()
The female reproductive system is composed of the ovary (the only reproductive structure here described), the oviducts (which open into a large thick-walled reproductive orifice on the bases of the third pereiopods), and the external spermatophoric plate with the function of seminal receptacles. The ovary, tightly surrounded by a thin cellular membranous sheath, appeared to be a longitudinal Y-like sac containing oocytes in different stages of maturity. The ovary contains oocytes in various degrees of development. Mature oocytes have internal yolk granules, which indicate a stage of complete maturation. At the onset of vitellogenesis, the originally white, thin ovaries acquire a creamy colour and start to grow as a consequence of the deposition of yolk inside the oocytes. As this process continues, the ovary turns yellow and, finally, bright orange. Oocytes on the external surface of the ovary start to colour at about a diameter of 0.5 mm, and then they are considered vitellogenic. Mature eggs are laid, and next stored under the female abdomen. They are generally orange and have a diameter of about 1.5–2 mm. Each oocyte is enclosed in its own oogenetic pouch (arising from evagination and invagination of the inner part of the ovarian epithelium), the latter being the basic structure of the ovary. Each oogenetic pouch contains a single egg or a single vitellogenic or previtellogenic oocyte larger than about 250–350 µm in diameter. The size of the oogenetic pouch depends on the size of the egg or the oocyte it contains. Most of the oogenetic pouches are well developed and contain a fully developed vitellogenic oocyte or a mature egg (about 2 mm in diameter). Young oogenetic pouches contain a growing oocyte, 300–600 µm in diameter, early vitellogenic or previtellogenic. Oogonia and very early previtellogenic oocytes are localized in the germaria, the latter being a slightly swollen area of the ovarian epithelium containing cells, which are less than 50 µm in diameter. Germaria are located in the ovarian epithelium separately at the bases of the oogenetic pouches. Early previtellogenic oocytes (50–150 µm in diameter) do not stay in the germarium, and they are not yet enclosed by the oogenetic pouches. They migrate and begin to raise the ovarian epithelium to form their own oogenetic pouches.
Male gonad ()
The male reproductive system consists of testes, collecting tubes, a vasa deferentia (with regionally distinct functions), and an ejaculatory duct (the latter not discussed here). The testes are composed of a paired anterior testicular lobe and an elongated, unpaired posterior one, while the vas deferens comprises a highly convoluted duct that arises from each side of the testis and opens at the outside through small papillae. The entire testis was enveloped by an external layer. Histological observations revealed that the testicular epithelium is covered and supported by a connective tissue. The germinal epithelium is found on a ridge of the connective tissue and the gametes, mingled with the Sertoli cells, develop from the germinal layer of the testes, and give rise to spermatozoa that fill the whole lumen of the seminiferous tubules. The sexual cells appeared to be synchronic, and at least one or two developmental stages are found currently in a seminiferous tubule. At microscopic level, the seminiferous tubules fuse to form collecting ducts covered by a simple columnar epithelium that terminate in the two vasa deferentia, the latter begin as narrow, opaque, white, and swollen tubes running from the testes to form coiled masses on either side of the stomach. The section of a vas deferens shows two main regions: (1) the proximal region presents a thin single-layer epithelium; (2) the distal region is represented by a single-layer columnar epithelium, which secretes an eosinophilic amorphous matrix. Within the lumen of the seminiferous tubules, the gametes appear strongly grouped, and in the distal one they become more and more grouped due to the progressive filling of the lumen by the amorphous matrix that seems to be responsible for packaging the spermatozoa into spermatophores.
Determination of the size at maturity by histological analysis
Only individuals with CL > 18 and 22 mm for females and males, respectively, had gonads, while the remainder showed either no type of gonads or gonads with very reduced size. However, gonad with developing gametes occurred only in individuals with CL > 23 and 27 mm for females and males, respectively.
Discussion
In the present study the gonad structure was no different from the description reported in the literature in both sexes (see Reynolds Citation2002). The size at maturity in Austropotamobius pallipes by histological analysis was registered for 23 and 27 mm CL for females and males, respectively. With respect to females, the smallest individuals (12–20 mm CL) had few vitellogenic oocytes, but gonad preparing to mating appeared only in specimens with more than 23 mm CL. Probably in juvenile females some oogonia can begin oogenesis, but matured oocytes are reabsorbed after the breeding period since reproductive males have a preference to mate larger females (Brewis & Bowler Citation1985). For mate selection, pair formation, and mating behaviour and process in crayfish we refer to the minute description by Ingle and Thomas (Citation1974) and Villanelli and Gherardi (Citation1998). Histology also revealed that males showed a similar size at maturity to that of females, and behavioural studies indicates that ‘large/old males may face greater difficulties in gamete release than small/young ones, as shown by the lower number of females fertilized by large compared with small males, which may reflect the ongoing senescence of their reproductive performance’ (Rubolini et al. Citation2007; Galeotti et al. Citation2009). Data on the glair gland maturity and the percentage of ovigerous females confirmed the value of CL at maturity. In this case the registered value ranged between 22 and 24 mm CL, taking on the whole the two study years into account. The values of size at maturity did not seem to be different from other populations. In particular, various workers have used glair glands to distinguish mature females, recording a minimum maturity size of around 22 mm CL for A. pallipes (Thomas & Ingle Citation1971; Brown & Bowler Citation1977; Rhodes & Holdich Citation1979; Carral et al. Citation1993). Brewis and Bowler (Citation1985) found that in Northumberland, sexual maturity occurs when the females are between 22 and 27 mm CL. In the Vivier brook (France), CL at maturity was 25.5 mm (Neveu Citation2007). Gonzalez et al. (Citation1994) recorded a smaller size of 21.6 mm (CL) in Spain, and Mori et al. (Citation1998) reported females of 19 mm (CL) in Italy, probably in relation with water temperature. Irish A. pallipes may become functionally mature at 22–26 mm CL, but different minimum sizes at maturity are seen from year to year (see also Neveu Citation2007), apparently reflecting water trophic conditions and growth rates (Moriarty Citation1973; Brewis & Bowler Citation1982, 1985; Woodlock & Reynolds Citation1988b; Scalici et al. Citation2008). Maturity can also be deduced by changes in appearance of gonads, overall in females. Several ovarian egg stages may be distinguished: in pre-spawning crayfish the large, compact ovary has yellow–orange eggs, further darkening and enlarging in size just before reproduction.
At the study site latitude, the mating period occurs from November to December, when the water temperature mean value ranged from 10 to 12°C, and once commenced, mating normally concentrated into approximately 3 weeks (see also Reynolds et al. Citation1992). In fact, in the present study, mated females only occurred in November, and after the breeding probably all individuals undergo a physiological inactivity, as the reduced number of collected crayfish from November to April testifies. In other European populations, it is possible to observe different situations. In particular, the A. pallipes mating starts generally in October, extending into November, when water temperatures fall below about 10°C in Ireland (Woodlock & Reynolds Citation1988b), but are sometimes as high as 13.5°C in Spain (Carral et al. Citation1993, 1994). In England, in southern populations, mating and egg-laying occurred one month earlier (October) than in the Northumberland population (Thomas & Ingle Citation1971). Also the hatching period showed a certain type of shifting among sites. In the present study, females mainly release juveniles in May, whereas in England females release their young in July (S. Peay, personal communication). Hatching was also advanced in populations in Kent and the Midlands where it occurred in mid-June, and in Northumberland in mid-August (Brewis & Bowler Citation1985).
During the brooding period, eggs seemed to have a synchronous development. Fertilized eggs (ES1) were never observed probably due to the spawning in December, when crayfish are less active than in the other seasons. The remnant embryonic stages were observed from February to June in 2004. In particular, the half moon eggs (ES2) occurred until April, when the mean water temperature value was 11.9°C. Thereafter, the subsequent two stages (ES3 + ES4) followed one another in a rapid sequence conditioned by the water temperature. Unfortunately, in this case, similar data did not occur in literature, therefore comparisons are not possible.
The later mating and the earlier hatching in more southern populations is probably a result of warmer water temperatures in spring and early summer at Mediterranean latitude. The reason for the different mating dates in autumn is less obvious; water temperature differences may be involved, but a timing mechanism, by changes in photoperiod, cannot be excluded. Also site differences in terms of food availability, population structure and the actual nature of the environment may all be causative factors. In fact, there is some evidence suggesting that food availability and population density can affect crayfish reproductive potential (see Rhodes & Holdich Citation1982).
An investigation on the number of mature oocytes, just before reproduction, gives an indication on the potential fecundity of the species and the influence of body size. After laying, females retain some non-expelled oocytes, and comparisons between fertility and fecundity indicate that females show a deficit in eggs compared to the oocytes. Woodlock and Reynolds (Citation1988b) report a complete emptying of the ovaries and a pleopodal fecundity (in December) representing 61% of potential fecundity. Rhodes and Holdich (Citation1982) found a reduction of 33%, Mankampa (Citation1995) quoted a value of 78% for Limousin (France) crayfish, while Neveu (Citation2007) 85.7% in Normandy. In fact, many external factors control the real fecundity as summarized by Reynolds et al. (Citation1992). Also in the present study, fecundity (pleopodal egg counts) showed lower values than fertility (ovarian egg counts), and the number of spawned eggs did not show particular differences with studies on natural populations (Brown & Bowler Citation1977; Rhodes & Holdich Citation1982; Brewis & Bowler Citation1985; Neveu Citation2007).
With regard to pleopodal fecundity, many authors find that female size has an influence on the number of eggs fixed onto the abdomen (Brown & Bowler Citation1977; Rhodes & Holdich Citation1982; Woodlock & Reynolds Citation1988a; Gonzalez et al. Citation1994; Sàez-Royuela et al. 2006; Neveu Citation2007). As in previous studies, the number of pleopodal eggs was positively correlated with female size. Regression coefficients reported by different authors have shown a high variability. So, Rhodes and Holdich (Citation1982), Brewis and Bowler (Citation1985), Carral et al. (Citation1994), and Sáez-Royuela et al. (Citation2006) obtained in A. pallipes different relations. These differences are probably related to the different numbers of animals, different populations, reproductive seasons and the phase of embryonic development. Several authors correlated pleopodal egg numbers and body length measurements in a number of crayfish species, noting marked variability in the data obtained (see Rhodes & Holdich Citation1982). The relative number of eggs is linearly related to maternal size and hence dependent on the individual female. The present study suggests that such a maternal influence also exists in A. pallipes, although environmental factors (see above) may modify this relationship, and not all the ovarian potential is realized in pleopodal egg counts in the white-clawed crayfish. The discrepancy between ovarian and pleopodal egg counts may be due to egg attachment failure, failure to extrude at the time of laying or perhaps failure to become fertilized, and loss throughout the season due to the female activity and cannibalism (by mothers and/or juveniles). While many studies provide data on size and age at first maturity and on egg number per female, only a few studies take into consideration egg losses by individuals during embryonic development (Woodlock & Reynolds Citation1988a; Grandjean et al. Citation2000; Neveu Citation2007).
The ‘egg number/mother size’ relationship is highly variable according to different authors. A relatively low fecundity may be linked to cold habitats of low trophic quality (Neveu Citation2007). Significant relation was also found between egg size and mother size, differently by the study of Sàez-Royuela et al. (2006), but not between egg size and embryonic stages. However, Woodlock and Reynolds (Citation1988a) showed that larger females have bigger eggs and that is also the case in A. torrentium (Maguire et al. Citation2004).
Predictive lines indicate an increase in the numbers of pleopodal eggs with increasing size (and age) of the samples. The pleopodal egg number may be limited in different species by morphology, specifically to setal abundance. Thomas (Citation1991) showed that in Austopotamobius pallipes the eggs are attached by the glair to 53 groups of oosete, whose flexible setules become embedded in the soft eggshell. However, average egg production seemed to be 40% higher for a given size in a warm, mesotrophic Irish lake than in a cool stream (Reynolds Citation2002), and 31% higher in an English river than a deep reservoir (Rhodes & Holdich Citation1979). There were also marked year-to-year differences in fecundity in White Lake, where 35 mm CL crayfish produced an average of 110 eggs in 1979–1981 but only 80 in 1985 (Woodlock & Reynolds Citation1988a). Although fecundity is generally proportional to body size, variation in egg size may also influence egg number (Huner & Lundqvist Citation1991).
Mean pleopodal fecundity in captivity for A. pallipes from Spain was 64 (range 18–220) (Carral et al. Citation1994), while A. pallipes in England had a mean of 59 pleopodal eggs (Brown & Bowler Citation1977). The highest ovarian fecundity observed in Ireland was a female of 46.3 mm CL from White Lake with over 225 eggs (Reynolds Citation2002). In Britain, at least 75% of A. pallipes females in a pond were berried, but with 50–120 pleopodal eggs (Holdich & Domaniewski Citation1995). In general terms, larger females usually produce more eggs, but for long-lived crayfish species, such as A. pallipes, old females can show reduced fecundity (Momot Citation1984). According to this statement, we found a relatively low pleopodal egg production in big females.
Many reproductive aspects of A. pallipes seem to be affected by climatic and environmental features rather than genetic parameters. In particular, the white-clawed crayfish reproduction seems to be affected more by water temperature and trophic condition than genetic parameters. In fact, reproductive differences do not seem the result of genetic divergences amongst populations, since the geographic differences of the A. pallipes reproduction do not overlap on to the different haplogroups described by Grandjean et al. (Citation2002), Fratini et al. (Citation2005), and Trontelj et al. (Citation2005).
Some geographic variations appear evident, overall for the mating and brooding period, and the embryonic development. This implies a continuous monitoring of the crayfish populations in order to engage useful regionalized conservation activities. Moreover, this information should be taken into account during behavioural studies.
For a better comprehension of the crayfish reproductive biology, further issues of concern are to be solved, such as the influence of the population structure, sex ratio, and density on the reproduction.
Acknowledgements
We are indebted to two anonymous reviewers for their helpful comments on the manuscript improvement, and to Dr M. Kenyon for his revision of the language.
References
- Albrecht , H. 1982 . Das system der europaischen Flußkrebse (Decapoda, Astacidae): vorschlag und begrundung . Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut , 79 : 187 – 210 .
- Ando , H and Makioka , T. 1998 . Structure of the ovary and mode of oogenesis in a freshwater crayfish, Procambarus clarkii (Girard) . Zoological Science , 15 : 893 – 901 .
- Bertocchi , S , Brusconi , S , Gherardi , F , Buccianti , A and Scalici , M. 2008 . Morphometrical characterization of the Austropotamobius pallipes complex . Journal of Natural History , 42 : 2063 – 2077 .
- Bohl , E. 1987 . Comparative studies on crayfish brooks in Bavaria (Astacus astacus L., Austropotamobius torrentium Schr.) . Freshwater Crayfish , 7 : 287 – 294 .
- Brewis , JM and Bowler , K. 1982 . The growth of the freshwater crayfish Austropotamobius pallipes in Northumbria . Freshwater Biology , 12 : 187 – 200 .
- Brewis , JM and Bowler , K. 1985 . A study of reproductive females of the freshwater crayfish A. pallipes . Hydrobiologia , 121 : 145 – 149 .
- Broquet , T , Thibault , M and Neveu , A. 2002 . Distribution and habitat requirements of the white-clawed crayfish, Austropotamobius pallipes, in a stream from the pays de Loire region, France: An experimental and descriptive study . Bulletin Francais de la Pêche et de la Pisciculture , 367 : 717 – 728 .
- Brown , DJ and Bowler , K. 1977 . A population study of the British freshwater crayfish Austropotamobius pallipes (Lereboullet) . Freshwater Crayfish , 3 : 33 – 50 .
- Brusconi , S , Renai , B , Scalici , M , Souty-Grosset , C and Gherardi , F. 2008 . Stock assessment in the threatened crayfish Austropotamobius italicus in Tuscany (Italy): Implications for its conservation . Aquatic Conservation: Marine and Freshwater Ecosystems , 18 : 1227 – 1239 .
- Carral , JM , Celada , JD and Gonzalez , J. 1993 . Wild freshwater crayfish populations in Spain: Current status and perspectives . Freshwater Crayfish , 9 : 158 – 162 .
- Carral , JM , Celada , JD , Gonzalez , J , Sàez-Royuela , M and Gaudioso , VR. 1994 . Mating and spawning of freshwater crayfish (Austropotamobius pallipes Lereboullet) under laboratory conditions . Aquaculture and Fishery Management , 25 : 721 – 727 .
- Carral , JM , Sàez-Royuela , M , Celada , JD , érez , P JR , Melendre , PM and Aguilera , A. 2003 . Advantages of artificial reproduction techniques for white-clawed crayfish (Austropotamobius pallipes Lereboullet) . Bulletin Francais de la Pêche et de la Pisciculture , 370–371 : 181 – 184 .
- Celada , JC , Carral , JM and Gonzalez , J. 1991 . A study on the identification and chronology of the embryonic stages of freshwater crayfish Austropotamobius pallipes (Lereboullet, 1858) . Crustaceana , 61 : 225 – 232 .
- Demers , A and Reynolds , JD. 2002 . A survey of the white-clawed crayfish, Austropotamobius pallipes (Lereboullet), and of water quality in two catchments of Eastern Ireland . Bulletin Francais de la Pêche et de la Pisciculture , 367 : 729 – 740 .
- Demers , A , Reynolds , JD and Cioni , A. 2003 . Habitat preference of different size classes of Austropotamobius pallipes in an Irish river . Bulletin Francais de la Pêche et de la Pisciculture , 370–371 : 127 – 138 .
- Erkan , M , Tunali , Y and Sancar-Bas , S. 2009 . Male reproductive system morphology and spermatophore formation in Astacus leptodactylus (Eschscholtz, 1823) (Decapoda: Astacidae) . Journal of Crustacean Biology , 29 : 42 – 50 .
- Fratini , S , Zaccara , S , Barbaresi , S , Grandjean , F , Souty-Grosset , C , Crosa , G and Gherardi , F. 2005 . Phylogeography of the threatened crayfish (genus Austropotamobius) in Italy: Implications for its taxonomy and conservation . Heredity , 94 : 108 – 118 .
- Füreder , L , Oberkofler , B , Hanel , R , Leiter , J and Thaler , B. 2003 . The freshwater crayfish in South Tyrol: Heritage species and bioindicator . Bulletin Francais de la Pêche et de la Pisciculture , 370–371 : 79 – 95 .
- Galeotti , P , Rubolini , D , Pupin , F , Sacchi , R , Altobelli , E , Nardi , PA and Fasola , M. 2009 . Presence of rivals reduces mating probability but does not affect ejaculate size in the freshwater crayfish Austropotamobius italicus . Behaviour , 146 : 45 – 68 .
- Gherardi , F , Acquistapace , P and Santini , G. 2004 . Food selection in freshwater omnivores: A case study of crayfish Austropotamobius pallipes . Fundamental and Applied Limnology (Archiv fur Hydrobiolie) , 159 : 357 – 376 .
- Gonzalez , J , Carral , JM , Celada , JD , Saez-Royuela , M and Gaudioso , VR. 1994 . Mating and spawning of freshwater crayfish Austropotamobius pallipes, Lereboullet, under laboratory conditions . Aquaculture and Fishery Management , 25 : 721 – 727 .
- Grandjean , F , Cornuault , B , Archambault , S , Bramard , M and Otrebsky , G. 2000 . Life history and population biology of the white-clawed crayfish, Austropotamobius pallipes pallipes, in a brook from the Poitou-Charentes region (France) . Bulletin Francais de la Pêche et de la Pisciculture , 356 : 55 – 70 .
- Grandjean , F , Frelon-Raimond , M and Souty-Grosset , C. 2002 . Compilation of molecular data for the phylogeny of the genus Austropotamobius . One species or several? Bulletin Francais de la Pêche et de la Pisciculture , 367 : 671 – 680 .
- Hobbs , HH Jr. 1988 . “ Crayfish distribution, adaptative radiation and evolution ” . In Freshwater crayfish: Biology, management and exploitation , Edited by: Holdich , DM and Lowery , RS . 52 – 82 . London : Croom Helm .
- Hobbs , HH Jr , Harvey , MC and Hobbs , HH I II. 2007 . A comparative study of functional morphology of the male reproductive systems in the Astacidea with emphasis on the freshwater crayfishes (Crustacea: Decapoda) . Smithsonian Contributions to Zoology , 624 : 1 – 69 .
- Holdich , DM and Domaniewski , JCJ. 1995 . Studies on a mixed population of the crayfish Austropotamobius pallipes and Pacifastacus leniusculus in England . Freshwater Crayfish , 10 : 37 – 45 .
- Holdich , DM , Peay , S , Foster , J , Hiley , PD and Brickland , JH. 2006 . Studies on the white-clawed crayfish (Austropotamobius pallipes) associated with muddy habitats . Bulletin Francais de la Pêche et de la Pisciculture , 380–381 : 1055 – 1078 .
- Holdich , DM and Reeve , ID. 1991 . The distribution of freshwater crayfish in the British Isles with particular reference to crayfish plague, alien introductions and water quality . Aquatic Conservation: Marine and Freshwater Ecosystems , 1 : 139 – 158 .
- Huner , JV and Lundqvist , OV. 1991 . “ Special problems in freshwater crayfish egg production ” . In Crustacean egg production , Edited by: Wenner , A and Kuris , A . 235 – 246 . Rotterdam : AA Balkema .
- Ingle , RW and Thomas , WJ. 1974 . Mating and spawning of freshwater crayfish, Austropotamobius pallipes (Crustacea: Astacidae) . Journal of Zoology, London , 173 : 525 – 538 .
- Maguire , I , Klobucar , G , Lucic , A and Erben , R. 2004 . “ The relation between female size and eggs size in stone crayfish Austropotamobius torrentium ” . In Craynet 3rd Thematic Meeting, Innsbruck. Abstract
- Mankampa , M. 1995 . Approche de la diversité démographique, trophique et nutritionnelle é de cinq espèces d’écrevisses dans un secteur géographique limousin , Université de Limoges : Thèse Doctorat .
- Momot , WT. 1984 . Crayfish production: A reflection of community energetics . Journal of Crustacean Biology , 4 : 35 – 54 .
- Mori , M , Salvidio , S and Cresta , R. 1998 . Size, structure, relative growth and sanitary condition of a crayfish population (Austropotamobius pallipes) living in rocky pools . Spixiana , 21 : 135 – 144 .
- Moriarty , C. 1973 . A study of Austropotamobius pallipes in Ireland . Freshwater Crayfish , 1 : 57 – 67 .
- Naura , M and Robinson , M. 1998 . Principles of using River Habitat Survey to predict the distribution of aquatic species: An example applied to the native white-clawed crayfish Austropotamobius pallipes . Aquatic Conservation: Marine and Freshwater Ecosystems , 8 : 515 – 527 .
- Neveu , A. 2007 . Annual variability in reproduction of the white-clawed crayfish (Austropotamobius pallipes): Implications for survival . Acta Oecologica , 32 : 67 – 76 .
- érez , P JR , Carral , JM , Celada , JD , Munoz , C , Sáez-Royuela , M and Antolìn , JI. 1998 . Effects of stripping time on the success of the artificial incubation of white-clawed crayfish, Austropotamobius pallipes (Lereboullet), eggs . Aquaculture Research , 29 : 389 – 395 .
- Pérez , JR , Carral , JM , Celada , JD , Munoz , C , Sáez-Royuela , M and Antolìn , JI. 1999 . The possibilities for artificial incubation of white-clawed crayfish, Austropotamobius pallipes Lereboullet, eggs: Comparison between maternal and artificial incubation . Aquaculture , 170 : 29 – 35 .
- Renai , B , Bertocchi , S , Brusconi , S , Grandjean , F , Lebboroni , M , Parinet , B , Souty Grosset , C , Trouilhé , MC and Gherardi , F. 2006 . Ecological characterization of streams in Tuscany (Italy) for the management of the threatened crayfish Austropotamobius pallipes complex . Bulletin Francais de la Pêche et de la Pisciculture , 380–381 : 1095 – 1114 .
- Reynolds , JD. 2002 . “ Growth and reproduction ” . In Biology of freshwater crayfish , Edited by: Holdich , DM . 151 – 181 . London : Blackwell Science. p .
- Reynolds , JD , Celada , JC , Carral , JM and Matthews , MA. 1992 . Reproduction of the astacid crayfishes in captivity – Current developments and implications for culture, with special reference to Ireland and Spain . Invertebrate Reproduction and Development , 22 : 253 – 266 .
- Reynolds , JD , Gouin , N , Pain , S , Grandjean , F , Demers , A and Souty-Grosset , C. 2002 . Irish crayfish populations: Ecological survey and preliminary genetic findings . Freshwater Crayfish , 13 : 584 – 594 .
- Rhodes , CP and Holdich , DM. 1979 . On size and sexual dimorphism in Austropotamobius pallipes (Lereboullet) . Aquaculture , 17 : 345 – 358 .
- Rhodes , CP and Holdich , DM. 1982 . Observation on the fecundity of the freshwater crayfish, Austropotamobius pallipes (Lereboullet) in the British Isles . Hydrobiologia , 89 : 231 – 236 .
- Rubolini , D , Galeotti , P , Pupin , F , Sacchi , R , Nardi , PA and Fasola , M. 2007 . Repeated matings and sperm depletion in the freshwater crayfish Austropotamobius italicus . Freshwater Biology , 52 : 1898 – 1906 .
- Sáez-Royuela , M , Carral , JM , Celada , JD , Melendre , PM and Aguilera , A. 2005 . Comparison between individual and group mating of . Austropotamobius pallipes under controlled conditions. Bulletin Francais de la Pêche et de la Pisciculture , 376–377 : 699 – 704 .
- Sáez-Royuela , M , Carral , JM , Celada , JD , érez , P JR and González , A. 2006 . “ Pleopodal egg production of the white-clawed crayfish Austropotamobius pallipes Lereboullet under laboratory conditions: relationship between egg number, egg diameter and female size ” . In Bulletin Francais de la Pêche et de la Pisciculture Vol. 380–381 , 1207 – 1214 .
- Scalici , M , Belluscio , A and Gibertini , G. 2008 . Understanding the population structure and dynamics in threatened crayfish . Journal of Zoology, London , 275 : 160 – 171 .
- Scalici , M and Gibertini , G. 2005 . Can Austropotamobius italicus meridionalis be used as a monitoring instrument in Central Italy? Preliminary observations . Bulletin Francais de la Pêche et de la Pisciculture , 376–377 : 613 – 625 .
- Scalici , M and Gibertini , G. 2007 . Feeding habits of the crayfish Austropotamobius pallipes (Decapoda, Astacidae) in a brook of Latium (central Italy) . Italian Journal of Zoology , 74 : 157 – 168 .
- Scalici , M and Gibertini , G. 2009 . Sexual dimorphism and ontogenetic variation in the carapace of A. pallipes (Lereboullet, 1858) . Italian Journal of Zoology , 76 : 179 – 188 .
- Scalici , M , Macale , D and Gibertini , G. 2010 . Allometry in the ontogenesis of Austropotamobius pallipes species complex (Decapoda: Astacidae): The use of geometric morphometrics . Italian Journal of Zoology, 77:296–302. ,
- Sint , D , Dalla Via , J and Füreder , L. 2007 . Phenotypical characterization of indigenous freshwater crayfish populations . Journal of Zoology, London , 273 : 210 – 219 .
- Smith , GRT , Learner , MA , Slater , FM and Foster , J. 1996 . Habitat features important for the conservation of the native crayfish Austropotamobius pallipes in Britain . Biological Conservation , 75 : 239 – 246 .
- Thomas , WJ. 1991 . Aspects of egg attachment in Austropotamobius pallipes (Lereboullet, 1858) (Decapoda, Astacidae) . Crustaceana , 61 : 287 – 293 .
- Thomas , WJ and Ingle , RW. 1971 . The nomenclature, bionomics and distribution of the crayfish A . pallipes (Lereboullet) in the British Isles. Essex Naturalist , 32 : 349 – 360 .
- Trontelj , P , Machino , Y and Sket , B. 2005 . Phylogenetic and phylogeographic relationships in the crayfish genus Austropotamobius inferred from mithocondrial COI gene sequences . Molecular Phylogenetic and Evolution , 34 : 212 – 226 .
- Trouilhé , MC , Ricard , F , Parinet , B , Grandjean , F and Souty-Grosset , C. 2003 . Management of the white-clawed crayfish (Austropotamobius pallipes) in Western France: Abiotic and biotic factors study . Bulletin Francais de la Pêche et de la Pisciculture , 370–371 : 97 – 114 .
- Trouilhé , MC , Souty-Grosset , C , Grandjean , F and Parinet , B. 2007 . Physical and chemical water requirements of the white-clawed crayfish (Austropotamobius pallipes) in western France . Aquatic Conservation: Marine and Freshwater Ecosystems , 17 : 520 – 538 .
- Villanelli , F and Gherardi , F. 1998 . Breeding in the crayfish, Austropotamobius pallipes: Mating patterns, mate choice and intermale competition . Freshwater Biology , 40 : 305 – 315 .
- Woodlock , B and Reynolds , JD. 1988a . Laboratory breeding studies of freshwater crayfish Austropotamobius pallipes (Lereboullet) . Freshwater Crayfish , 19 : 71 – 78 .
- Woodlock , B and Reynolds , JD. 1988b . Reproduction in an Irish lake population of the crayfish Austropotamobius pallipes (Lereboullet) . Freshwater Crayfish , 19 : 79 – 86 .