Abstract
Males and females of Hermann's tortoise (Testudo hermanni) exhibit a conspicuous yellow patch on both their cheeks, whose origin and potential function are totally unknown. In this study, we measured the extent and the colour intensity of these patches in 29 male and 19 female tortoises in order to test for sexual difference in these features. In addition, we analysed the relationships between patch features and body condition to investigate the possible function of these ornaments as status signals. We detected symmetric yellow patches in all sampled females, while five males did not show at all the ornament, and two had a yellow patch only on the left cheek. Although head and scale size, as well as RGB values, did not differ between sexes, female patches were significantly larger than those of males. In addition, the extent of cheek patches was correlated to female body condition, suggesting that these ornaments may have evolved as honest signals of quality through sexual selection processes driven by female–female contests over rank or by male mate choice.
Introduction
The interest on animal conspicuous colourations (either more intense or larger coloured area) dates from Darwin (Citation1871), but has grown in recent decades due to their role as meaningful signals in assessment contexts. In fact, colour ornaments may evolve through a variety of sexual selection mechanisms (e.g. runaway selection, Fisher Citation1930; indicator processes, Zahavi Citation1975; Grafen Citation1990; status signal selection, Pryke et al. Citation2001; etc.), facilitating receiver assessment of signallers, thus improving the mating success of their bearers.
Vertebrate colour patches are complex traits that can be viewed as ornaments nested within ornaments and often appear to act as honest signals of condition (Hamilton & Zuk Citation1982; Figuerola et al. Citation1999; Faivre et al. Citation2003), since only the more healthy individuals can afford the costs of developing such colouration. In particular, red, orange and yellow colourations are produced mostly by carotenoids, which also play important immune and detoxification functions, and must be acquired in the diet. Carotenoid-dependent colouration has been shown to signal condition, playing a role in intraspecific communication (e.g. Hill et al. Citation1994; Hill Citation1996), including mate choice.
While the elaborate male traits resulting from sexual selection processes have been the primary focus of studies in evolutionary and behavioural ecology (see Andersson Citation1993 for a review), studies addressing the evolution of female ornaments by sexual selection are far less common, although they have been increasing in recent years (Rowland et al. Citation1991; Potti & Merino Citation1996; Amundsen et al. Citation1997, 2000; Jones & Hunter Citation1999; Amundsen & Forsgren Citation2001; Houde Citation2001). This is because female ornaments have been traditionally considered as a genetically correlated result of sexual selection on males, which are sexually selected to express ornaments (Darwin Citation1871; Lande Citation1980; Muma & Weatherhead Citation1989). However, it is now well recognised that female ornamentation may evolve via natural selection or sexual selection, acting directly on females (e.g. West-Eberhard Citation1983; Amundsen Citation2000).
Among reptiles, female ornaments have been documented in more than 30 lizard species (see Cooper & Greenberg Citation1992), although ornament function has been investigated experimentally in only a few cases. Most of these studies reported a relationship between female colourations and reproductive state (Ferguson Citation1976; Cooper et al. Citation1983; Watkins Citation1997; Cuadrado Citation2000; LeBas & Marshall Citation2000; Hager Citation2001; Weiss Citation2002; Baird Citation2004); for example, the throat colouration of female Sceloporus virgatus changes seasonally and the colour intensity peaks near the time of ovulation (Weiss Citation2002, Citation2006). In some cases, males have been shown to respond to female ornaments by increasing courtship towards ovulating females while reducing courtship to non-receptive females (Baird Citation2004).
In Chelonians, cryptic colourations prevail and conspicuous patches and stripes are limited to the head and legs, and are present only in few families, including Testudinidae (Ernst & Barbour Citation1989). In terrestrial tortoises, the courtship and copulatory behaviour is elaborate, and based on a multiple signalling system involving visual, olfactory, tactile and acoustic signals. For example, in some species, males bite the female's legs, and occasionally rams her shell, while smelling that particular region of the body; in addition, mounting males emit a regular series of loud whistles (Ernst & Barbour Citation1989), which appear to be honest, condition-dependent signals of male quality (Sacchi et al. Citation2003; Galeotti et al. Citation2005a,Citationb,Citationc).
In the promiscuous Hermann's tortoise (Testudo hermanni Gmelin, 1789), one of the three tortoise species endemic to Europe, sexual dimorphism is noticeable: males are smaller than females, and their plastron is concave thus facilitating the mount (Willemsen & Hailey Citation2003). However, both sexes exhibit a conspicuous yellow patch on both cheeks, extending backward from the eye to the tympanum, whose origin and potential function are totally unknown. As in other Testudinidae, during sexual interactions both males and females also display ‘head bobbing’ behaviour, in which the head is nodded regularly, the neck being usually fully extended (Carpenter & Ferguson Citation1977; Sacchi et al. Citation2003), and the yellow cheek patches are particularly evident. Considering the context in which the head is maximally exposed, it may be suggested that the expression of these ornaments may be correlated with adult condition and other qualities, thus enhancing the success of bearers in an assessment context. The fact that females also show such colour patches is intriguing, and rather than a ‘correlated response’ between the trait in males and females (Hill Citation1993; Cuervo et al. Citation1996; Tella et al. Citation1997), this ornament may be directly selected either through female–female competition or male choice (Darwin Citation1871; Jones & Hunter Citation1999; Amundsen Citation2000), signalling social dominance in contests over limited resources, or reproductive and genetic qualities preferred by choosy males. Thus, similar preferences for ornaments in both sexes and similar competition within both sexes can explain ornament monomorphism in this and other species with a promiscuous mating system without any parental care.
In this study, we measured the extent and the colour intensity of yellow patches in both male and female Hermann's tortoises in order to test for inter-sexual difference in these features, and analysed the relationship between patch size and body condition in order to investigate the possible function as status signals of these ornaments.
Materials and methods
We carried out this study during spring and summer 2003, at the ‘CARAPAX’ European Centre for Tortoise Conservation, located at Massa Marittima (Tuscany, Central Italy), where 8000 individuals (D. Ballasina, personal communication) of several tortoise species reproduce in enclosures, in semi-natural conditions and at high density. Data were collected from a sample of 29 male and 19 female Hermann's tortoises maintained in 11 different enclosures (150 to 300 m2 large), and belonging to the same Tuscan population. We weighed (g) each individual and measured (mm) carapace length, and width, length and height of the head. We used body mass × (carapace length)−3 as an index of individual body condition instead of the mass, because this latter is greatly influenced by carapace size (Peterson Citation1996). Since morphological measures of the head were highly intercorrelated (|r p| > 0.69, P < 0.0001, N = 48), we used a principal component analysis (PCA) to reduce the three head variables to a single ‘head size’ component. The PCA procedure extracted only one component (eigenvalue > 1), which accounted for 77.4% of the cumulative variance and was positively related to all head variables (the three variables entered PCA with component loadings > 0.81).
Patch description and measurements
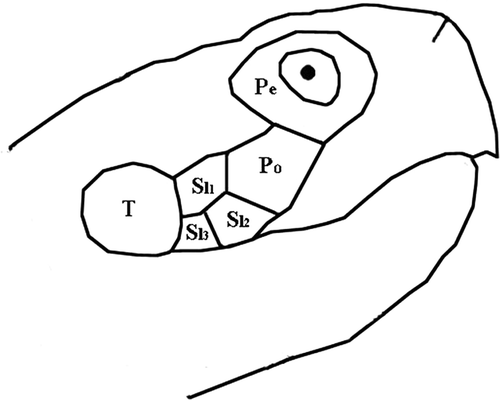
Right and left sides of the head of each tortoise were photographed in a darkroom against a reference centimetre scale and under highly standardised conditions (distance, light, exposure set constant for all pictures) using a digital camera (Nikon Coolpix 4300) with 1536 × 2048 pixels of resolution and 256 × 106 colours. Digital images were transferred to the computer and analysed with Adobe Photoshop CS2. The cheeks' yellow patch covers a variable area completely included within the four scales delimited by the eye, the ear opening and the upper jaw (one post-ocular and three super-labial scales, ). The area of the four scales as well as that of the yellow patch were measured (in pixels) using the ‘magic wand’ option (tolerance 32) of the Photoshop software. The reference centimetre scale was also measured (in pixels) with the ‘measure tool’ and the areas in pixels were appropriately transformed into mm2. The colour intensity of the yellow patches was measured using the ‘histogram tool’ of the software and expressed in the continuous scale of Red-Green-Blue (RGB, Villafuerte & Negro Citation1998). RGB values were then standardised following the procedure reported in Villafuerte & Negro (Citation1998).
Figure 1. Topography of the yellow patch on tortoise cheek. Pe: peri-ocular scale; Po: post-ocular scale; Sl1, Sl2, Sl3: supra-labial scales 1, 2, 3; T: tympanum. Coloured area covers Po, Sl1, Sl2, Sl3 scales.
The repeatability of the areas and RGB values (Lessels & Boag Citation1987), as measured on a repeated picture of 10 randomly selected tortoises (five males, five females), was very high (all r-values > 0.90, F 9,10 > 20.02, all P-values < 0.001), indicating that the measurement error was negligible.
Statistical analyses
Non-normal data were log-transformed. T-tests for paired samples were performed to check for differences between left and right cheek variables. We used univariate ANOVA to check for variation in morphological and mean patch variables between male and female tortoises. Discriminant analysis was performed in order to test if patch size could be used to distinguish statistically between the sexes. Since females are significantly larger than males (see ), a univariate GLM was carried out on yellow patch size using sex as a fixed factor, and body condition (log-transformed) and head size (PCA) as covariates, in order to disentangle the role of sex and body size in determining the difference between sexes. Interactions between sex and both covariates were also included in the initial model. The model was optimised by the stepwise elimination of non-significant terms to produce the minimal adequate models (Crawley Citation1993). Finally, a correlation test (Pearson procedure) was used to analyse bivariate relationships between patch variables and body condition of both sexes. All statistics were performed using the SPSS 15.0 package.
Table I. Sexual difference in body mass, body condition, head size, and cheek-patch features (values averaged on both cheeks) of adult Hermann's tortoises used in this study (one-way ANOVA)
Results
The symmetric yellow patches were present in all sampled females, while among males, five individuals did not show at all the ornament, and two had a yellow patch only on the left cheek. Thus, these males were discarded from the analysis on RGB values, but not from the analyses on morphology and scale or patch size.
In males, the area of the four scales significantly differed between the right and left cheeks (t = −3.61, d.f. = 28, P = 0.001), left scales being significantly larger than right scales (42 mm2 ± SD 11 vs. 41 mm2 ± 12); by contrast, neither the mean size of the coloured patch (t =1.11, d.f. = 21, P = 0.28) nor the mean RGB values differed between right and left cheeks (all P-values > 0.49, t-test for paired samples). In females, there were no differences in the mean four-scales area, patch size and RGB values between the two cheeks (all P-values > 0.35, same test).
Head size, scale size and standardised RGB values did not differ between the sexes, but the mean patch size of females was significantly larger than those of males (). In fact, males and females could be discriminated with good accuracy (81.3% of correctly classified cases) on the basis of the patch size alone.
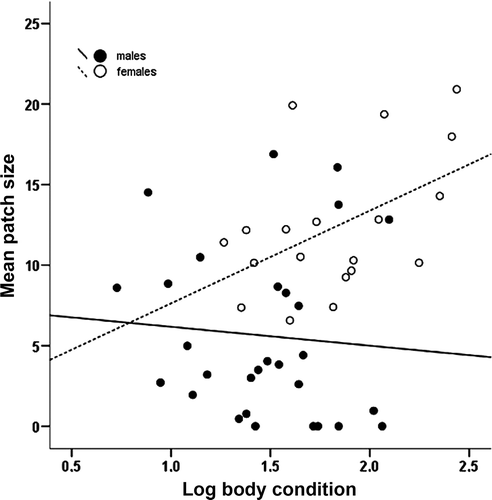
The larger extent of yellow patches in females did not depend on their larger size, however, because the univariate GLM showed that patch size was affected only by gender (F 1,46 = 21.64, P < 0.001) and not by body condition, head size or by sex × head size interaction (P-values at removal: 0.434, 0.786 and 0.983, respectively). The P-value at removal for the interaction sex × body condition was close to significance (0.093), suggesting that body condition affected patch size differently depending on sex (). Actually, the mean size of yellow patch was significantly and positively related to the index of body condition in females (r = 0.56, P = 0.012, N = 19, ), but not in males (r = −0.08, P = 0.681, N = 29). All results were qualitatively unchanged when the analyses (both GLM and correlation test) were performed modelling body condition by adding both body mass and carapace length to the statistical models (Darlington & Smulders Citation2001; Garcìa-Berthou Citation2001).
Discussion
The results of this study showed that the yellow cheek patches are larger in female than male Hermann's tortoises; thus, such ornamentation may represent a supplementary trait easy to assess for correctly sexing Hermann's tortoises. However, there are several reasons to expect that yellow cheek patches in females are unnecessary for sex recognition during courtship in this species. First, male Hermann's tortoises are markedly smaller in carapace size and shape (Sacchi et al. Citation2007), and their ability to detect and distinguish subtle visual cues is demonstrated by their differential responses to females of different size (P. Galeotti, R. Sacchi, D. Pellitteri-Rosa, unpublished data). Second, males can detect and distinguish the odour of female conspecifics from that of another species, as well as the sex and sexual maturity of females through only chemical cues (Galeotti et al. Citation2007).
The traditional explanation that female ornaments may constitute a genetically correlated result of sexual selection on males is also unlikely for Hermann's tortoises, since we found that yellow cheek patches were more conspicuous in females than in males, and a number of males did not show these ornaments at all. A higher expression of this trait in females than in males cannot be controlled and maintained through male–male competition or female mate choice. According to the recent models that consider female ornamentation from a functional perspective (Rowland et al. Citation1991; Potti & Merino Citation1996; Amundsen et al. Citation1997, 2000; Jones & Hunter Citation1999; Amundsen & Forsgren Citation2001), cheek patches in female Hermann's tortoises might have been selected to signal individual quality in assessment contexts such as inter-female fights or male mate choice. Actually, we found that the extent of these head ornaments provided a good indication of female body condition, suggesting that cheek patches were costly condition-dependent traits, able to indicate female quality to rivals and prospecting mates (Zahavi Citation1975, Citation1977). On the other hand, the lower expression of this trait in males and the lack of any relationships between trait and male condition suggested that the yellow cheek patches of males might be a selectively neutral response resulting from a genetic correlation between the trait in females and males in combination with the selection for the trait in females (e.g. Cuervo et al. Citation1996; Tella et al. Citation1997).
Despite the fact that female fighting may occur in Hermann's tortoises (D. Pellitteri-Rosa, personal observation), the hypothesis that yellow check patches may set up inter-female combats preventing escalated contests during assessment of social dominance appears unlikely. Female competition is expected to evolve when sexual (mates) or non-sexual (e.g. food or optimal basking sites) resources essential to female fitness are limited. However, in the Hermann's tortoise the sex ratio is close to 1:1 in mainland wild populations (Stubbs & Swingland Citation1985; Meek Citation1989; Paglione & Carbone Citation1990; Mazzotti et al. Citation2002) and females do not actively search for males, but the opposite is true (Willemsen & Hailey Citation2003). In addition, female tortoises are able to store viable sperm for up to four years within storage tubules located in the oviducts (Gist & Jones Citation1989; Kuchling Citation1999), thus avoiding having to mate at every breeding season in order to fertilise eggs. Taken together, these findings appear to rule out the possibility that female Hermann's tortoises may compete for mates. Also, Hermann's tortoises are herbivorous generalists, a condition that makes defending a territory pointless, since vegetable food resources are scattered and unpredictable, and when drought reduces food availability in summer, females may avoid competition by aestivation (Diaz-Paniagua et al. Citation1995). Moreover, contrary to freshwater turtles, terrestrial tortoises are not limited by the number of basking sites, which are plentiful in dry land. For all the above reasons, it would seem that yellow cheek patches in females did not evolve through female competition for mates or habitat resources.
On the other hand, courtship in Hermann's tortoises is energetically expensive for males, because they have to pursue females, while biting their legs and roaming their shells until females ‘accept’ to cooperate, which may occur after half an hour or more of such an endurance race (Hailey & Willemsen, Citation2000; P. Galeotti & R. Sacchi, personal observation). Thus, males are expected to avoid courting low-remunerative females in terms of clutch and egg size (i.e. small females). Actually, in most reptiles, female body size is a reliable predictor of female fecundity, and males clearly prefer to mate with larger females (Olsson Citation1993; Stuart-Smith et al. Citation2007). Also, in chelonians larger females lay larger clutches of large eggs (Hailey & Loumbourdis Citation1988, Citation1990), but males cannot reliably assess female condition by body size, since the carapace is a rigid structure which does not vary significantly according to variation in body mass or fat reserves. In other words, males may be unable to detect differences in body condition among females showing the same carapace size using only female shape as a cue. Since mating with a poor quality female is a high fitness cost for a male, any other trait than size which reliably reveals the female body condition before courtship starts should be strongly favoured by sexual selection. Since the yellow cheek patches of females correlate with body condition, males may rapidly assess female quality by paying attention to these condition-dependent signals, thus avoiding mating with poor-quality partners and preserving energy reserves (and sperm as well) for future, more remunerative matings. By choosing females in better condition, which may allocate more energy to eggs, males may gain more fitness in terms of progeny survival. Clearly, the effectiveness of the yellow patch size in signalling body condition would depend on how receivers perceive colour and size variation of cheek patches. Like other Chelonians (e.g. Ammermüller et al. Citation1995; Twig & Perlman Citation2004; Mathger et al. Citation2007), Hermann's tortoises exhibit good vision, based on a complex type of eye with rods and cones containing multicoloured oil droplets, which enable them to see and clearly distinguish images and to perceive and discriminate colours, particularly the yellow (Pellitteri-Rosa et al. Citation2010). Thus, females with larger cheek patches may actually be more attractive and stimulate males more efficiently, which could result in a larger amount of sperm for fertilising eggs.
In this scenario, the complex courtship of Hermann's tortoises should be therefore interpreted as the result of a mutual mate choice system, where both sexes assess the partner's quality to optimise their reproductive investments.
Acknowledgements
We are very grateful to Dr D. Ballasina, for his warm hospitality at the Carapax Centre for Tortoise Conservation (Massa Marittima, GR). Thanks are also due to Marco A. L. Zuffi and two anonymous referees for their useful comments on an early version of the manuscript. The study was financially supported by a MURST grant (COFIN 2000) to PG, and was carried out in conformity with the Italian current laws on tortoise detention.
References
- Ammermüller , J , Muller , JF and Kolb , H. 1995 . The organization of the turtle inner retina. II. Analysis of color-coded and directionally selective cells . The Journal of Comparative Neurology , 358 : 35 – 62 .
- Amundsen , T. 2000 . Why are female birds ornamented? . Trends in Ecology and Evolution , 15 : 149 – 155 .
- Amundsen , T and Forsgren , E. 2001 . Male mate choice selects for female coloration in a fish . Proceedings of the National Academy of Sciences USA , 98 : 13155 – 13160 .
- Amundsen , T , Forsgren , E and Hansen , LTT. 1997 . On the function of female ornaments: Male bluethroats prefer colourful females . Proceedings of the Royal Society of London B, Biological Sciences , 264 : 1579 – 1586 .
- Andersson , M. 1993 . Sexual selection , Princeton, NJ : Princeton University Press .
- Baird , TA. 2004 . Reproductive coloration in female collared lizards, Crotophytus collaris, stimulates courtship by males . Herpetologica , 60 : 337 – 348 .
- Carpenter , C and Ferguson , GW. 1977 . “ Variation and evolution of stereotyped behavior in Reptiles ” . In Biology of the Reptilia , Edited by: Gans , C and Tinkle , DW . Vol. 7 , 335 – 354 . New York, NY : Academic Press .
- Cooper , WE Jr , Adams , CS and Dobie , JL. 1983 . Female color change in the keeled earless lizard, Holbrookia propinqua: Relationship to the reproductive cycle . Southwestern Naturalist , 28 : 275 – 280 .
- Cooper , WE Jr and Greenberg , N. 1992 . “ Reptilian coloration and behavior ” . In Biology of the Reptilia , Edited by: Gans , C and Crews , D . Vol. 18 , 298 – 422 . Chicago, IL : University of Chicago Press . Hormones, brain, and behavior
- Crawley , MJ. 1993 . Glim for ecologists , Oxford : Blackwell Scientific .
- Cuadrado , M. 2000 . Body colors indicate the reproductive status of female common chameleons: Experimental evidence for the intersex communication function . Ethology , 106 : 79 – 91 .
- Cuervo , JJ , de Lope , F and Møller , AP. 1996 . The function of long tails in female barn swallows (Hirundo rustica): An experimental study . Behavioral Ecology , 7 : 132 – 136 .
- Darlington , RB and Smulders , TV. 2001 . Problems with residual analysis . Animal Behaviour , 62 : 599 – 602 .
- Darwin , C. 1871 . The descent of man and selection in relation to sex , London : John Murray .
- Diaz-Paniagua , C , Keller , C and Andreu , AC. 1995 . Annual variation of activity and daily distances moved in adult spur-thighed tortoises, Testudo graeca, in southwestern Spain . Herpetologica , 51 : 225 – 233 .
- Ernst , CH and Barbour , RW. 1989 . Turtles of the world , Washington, DC : Smithsonian Institution Press .
- Faivre , B , Grégoire , A , Prèault , M , Cezilly , F and Sorci , G. 2003 . Immune activation rapidly mirrored in a secondary sexual trait . Science , 300 : 103 – 103 .
- Ferguson , GW. 1976 . Color change and reproductive cycling in female collared lizards (Crotaphytus collaris) . Copeia , 1976 : 491 – 494 .
- Figuerola , J , Muñoz , E , Gutierrez , R and Ferrer , D. 1999 . Blood parasites, leucocytes and plumage brightness in the cirl bunting, Emberiza cirlus . Functional Ecology , 13 : 594 – 601 .
- Fisher , RA. 1930 . The genetical theory of natural selection , London : Oxford University Press .
- Galeotti , P , Sacchi , R , Fasola , M and Ballasina , D. 2005a . Do mounting vocalisations in tortoises have a communication function? A comparative analysis . Herpetological Journal , 15 : 61 – 71 .
- Galeotti , P , Sacchi , R , Fasola , M , Pellitteri-Rosa , D , Marchesi , M and Ballasina , D. 2005b . Courtships displays and mounting call are honest, condition-dependent signals that influence mounting success in Hermann's tortoises . Canadian Journal of Zoology , 83 : 1306 – 1313 .
- Galeotti , P , Sacchi , R , Pellitteri-Rosa , D and Fasola , M. 2005c . Female preference for fast-rate, high-pitched calls in Hermann's tortoises Testudo hermanni . Behavioral Ecology , 16 : 301 – 308 .
- Galeotti , P , Sacchi , R , Fasola , M , Pellitteri-Rosa , D and Fasola , M. 2007 . Olfactory discrimination of species, sex, and sexual maturity by the Hermann's Tortoise Testudo hermanni . Copeia , 2007 : 980 – 985 .
- Garcìa-Berthou , E. 2001 . On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance . Journal of Animal Ecology , 70 : 708 – 711 .
- Gist , DH and Jones , JM. 1989 . Sperm storage within the oviduct of turtles . Journal of Morphology , 199 : 379 – 384 .
- Grafen , A. 1990 . Biological signals as handicaps . Journal of Theoretical Biology , 144 : 517 – 546 .
- Hager , SB. 2001 . The role of nuptial coloration in female Holbrookia maculata: Evidence for a dual signalling system . Journal of Herpetology , 35 : 624 – 632 .
- Hailey , A and Loumbourdis , NS. 1988 . Egg size and shape, clutch dynamics, and reproductive effort in European tortoises . Canadian Journal of Zoology , 66 : 1527 – 1536 .
- Hailey , A and Loumbourdis , NS. 1990 . Population ecology and conservation of tortoises: Demographic aspects of reproduction in Testudo hermanni . Herpetological Journal , 1 : 425 – 434 .
- Hailey , A and Willemsen , RE. 2000 . Population density and adult sex ratio of the tortoise Testudo hermanni in Greece: Evidence for intrinsic population regulation . Journal of Zoology , 251 : 325 – 338 .
- Hamilton , WD and Zuk , M. 1982 . Heritable true fitness and bright birds: A role for parasites? . Science , 218 : 384 – 387 .
- Hill , GE. 1993 . Mate choice and the evolution of female plumage coloration in the house finch . Evolution , 47 : 1515 – 1525 .
- Hill , GE. 1996 . Redness as a measure of the production cost of ornamental coloration . Ethology Ecology and Evolution , 8 : 157 – 175 .
- Hill , GE , Montgomerie , R , Inouye , CY and Dale , J. 1994 . Influence of dietary carotenoids on plasma and plumage color in the house finch: Intra- and intersexual variation . Functional Ecology , 8 : 343 – 350 .
- Houde , AE. 2001 . Sex roles, ornaments, and evolutionary explanation . Proceedings of the National Academy of Sciences USA , 98 : 12857 – 12859 .
- Jones , IL and Hunter , FM. 1999 . Experimental evidence for mutual inter- and intrasexual selection favouring a crested auklet ornament . Animal Behaviour , 57 : 521 – 528 .
- Kuchling , G. 1999 . The reproductive biology of Chelonia , Berlin : Springer .
- Lande , R. 1980 . Sexual dimorphism, sexual selection, and adaptation in polygenic characters . Evolution , 34 : 292 – 305 .
- LeBas , NR and Marshall , NJ. 2000 . The role of colour in signalling and male choice in the agamid lizard Ctenophorus ornatus . Proceedings of the Royal Society of London B, Biological Sciences , 267 : 445 – 452 .
- Lessels , CM and Boag , PT. 1987 . Unrepeatable repeatabilities: A common mistake . Auk , 104 : 116 – 121 .
- Mathger , LM , Litherland , L and Fritsches , KA. 2007 . An anatomical study of the visual capabilities of the green turtle, Chelonia mydas . Copeia , 2007 : 169 – 179 .
- Mazzotti , S , Pisapia , A and Fasola , M. 2002 . Activity and home range of Testudo hermanni in Northern Italy . Amphibia–Reptilia , 23 : 305 – 312 .
- Meek , R. 1989 . The comparative population ecology of Hermann's tortoise, Testudo hermanni, in Croatia and Montenegro, Yugoslavia . Herpetological Journal , 1 : 404 – 414 .
- Muma , KE and Weatherhead , PJ. 1989 . Male traits expressed in females . Direct or indirect sexual selection? Behavioural Ecology and Sociobiology , 25 : 23 – 31 .
- Olsson , M. 1993 . Male-preference for large females and assortative mating for body size in the sand lizard (Lacerta agilis) . Behavioural Ecology and Sociobiology , 32 : 337 – 341 .
- Paglione , G and Carbone , M. 1990 . “ Biologia di popolazione di Testudo hermanni nel Parco della Maremma (GR) ” . In Atti del VI Convegno Nazionale dell'Associazione Alessandro Ghigi , 197 – 199 . Torino : Museo Regionale di Scienze Naturali .
- Pellitteri-Rosa , D , Sacchi , R , Galeotti , P , Marchesi , M and Fasola , M. 2010 . Do Hermann's tortoises (Testudo hermanni) discriminate colours? An experiment with natural and artificial stimuli . Italian Journal of Zoology , 77 : 481 – 491 .
- Peterson , CC. 1996 . Anhomeostasis: Seasonal water and solute relations in two population of the desert tortoise (Gopherus agassizii) during chronic drought . Physiological Zoology , 69 : 1324 – 1358 .
- Potti , J and Merino , S. 1996 . Decreased levels of blood trypanosome infection correlate with female expression of a male secondary sexual trait: Implications for sexual selection . Proceedings of the Royal Society of London B, Biological Sciences , 263 : 1199 – 1204 .
- Pryke , SR , Lawes , MJ and Andersson , S. 2001 . Agonistic carotenoid signalling in male red-collared widowbirds: Aggression related to the colour signal of both the territory owner and model intruder . Animal Behaviour , 62 : 695 – 704 .
- Rowland , WJ , Baube , CL and Horan , TT. 1991 . Signalling of sexual receptivity by pigmentation pattern in female sticklebacks . Animal Behaviour , 42 : 243 – 249 .
- Sacchi , R , Galeotti , P , Fasola , M and Ballasina , D. 2003 . Vocalizations and courtship intensity correlate with mounting success in marginated tortoises Testudo marginata . Behavioral Ecology and Sociobiology , 55 : 95 – 102 .
- Sacchi , R , Pupin , F , Pellitteri-Rosa , D and Fasola , M. 2007 . Bergmann's rule and the Italian Hermann's tortoises (Testudo hermanni): Latitudinal variations of size and shape . Amphibia–Reptilia , 28 : 43 – 50 .
- Stuart-Smith , J , Swain , R and Wapstra , E. 2007 . The role of body size in competition and mate choice in an agamid with female-biased size dimorphism . Behaviour , 144 : 1087 – 1102 .
- Stubbs , D and Swingland , IR. 1985 . The ecology of a Mediterranean tortoise (Testudo hermanni): A declining population . Canadian Journal of Zoology , 63 : 169 – 180 .
- Tella , JL , Forero , MG , Donazar , JA and Hiraldo , F. 1997 . Is the expression of male traits in female lesser kestrels related to sexual selection? . Ethology , 103 : 72 – 81 .
- Twig , G and Perlman , I. 2004 . Homogeneity and diversity of color-opponent horizontal cells in the turtle retina: Consequences for potential wavelength discrimination . Journal of Vision , 2004 : 403 – 414 .
- Villafuerte , R and Negro , JJ. 1998 . Digital imaging for colour measurement in ecological research . Ecology Letters , 1 : 151 – 154 .
- Watkins , GG. 1997 . Inter-sexual signalling and the functions of female coloration in the tropidurid lizard Microlophus occipitalis . Animal Behaviour , 53 : 843 – 852 .
- Weiss , SL. 2002 . Reproductive signals of female lizards: Pattern of trait expression and male response . Ethology , 108 : 793 – 813 .
- Weiss , SL. 2006 . Female-specific color is a signal of quality in the striped plateau lizard (Sceloporus virgatus) . Behavioral Ecology , 17 : 726 – 732 .
- West-Eberhard , MJ. 1983 . Sexual selection, social competition, and speciation . Quarterly Review of Biology , 58 : 155 – 183 .
- Willemsen , RE and Hailey , A. 2003 . Sexual dimorphism of body size and shell shape in European tortoises . Journal of Zoology , 260 : 353 – 365 .
- Zahavi , A. 1975 . Mate selection – A selection for a handicap . Journal of Theoretical Biology , 53 : 205 – 214 .
- Zahavi , A. 1977 . The cost of honesty (further remarks on the handicap principle) . Journal of Theoretical Biology , 67 : 603 – 605 .