Abstract
Pelagic larval duration (PLD) and trace elements in otoliths provide basic information to investigate the dispersal history of marine fishes. Due to the paucity of such information in the Mediterranean region, we assessed PLD, hatching and settlement duration and timing, and otolith microelemental composition of the White Sea bream (Diplodus sargus sargus) from multiple locations (hundreds of kilometers from each other) along the Italian coast (Mediterranean Sea). Otoliths were read to assess PLD and analyzed by a laser ablation inductively coupled plasma mass spectrometer in the core, larval and juvenile regions. PLD ranged from ∼14 to 17 days and significantly changed among locations, similarly to hatching (temporal windows of 10–24 days) and settlement (8–22 days) duration and timing, and microelemental fingerprints. These results show (1) a non-negligible large-scale variability in juvenile fish traits and otolith chemistry ever tested before for any Mediterranean fish; (2) the usefulness to properly estimate those traits and chemical features that may help shed light on spatial patterns of population connectivity and dispersal of marine fishes.
Introduction
Otoliths are acellular calcified structures located in the inner ear of teleosts (i.e. the stato-acoustic organ of fish; Campana & Neilson Citation1985; Tuset et al. Citation2008). They form in the period comprised between egg fecundation and hatching (Green et al. Citation2009) and then grow by apposition of daily increments (Campana & Neilson Citation1985).
Daily increments depart concentrically from the otolith primordium (the initial structure of the otolith forming prior to hatching) towards the external margin. In the otolith microstructure of some fish species, different marks can be detected, such as hatching and settlement marks, each representing a transition phase during the life cycle of fish. The hatching mark corresponds to the hatch and the release of the larva from the egg (Campana Citation1990), while the settlement mark corresponds to the settlement of the planktonic larva metamorphosing into the benthic juvenile (Campana & Neilson Citation1985; Wellington & Victor Citation1989; Vigliola & Meekan Citation2002). Three spatial regions can be identified in the otolith of juvenile fish: the core (prior the first increment), larval (between the first increment and the settlement mark) and juvenile regions (between settlement mark and outer otolith margin).
Otoliths have been used for a long time for estimating individual age of fish (Jackson Citation2007). Counting the daily growth increments from the otoliths of juvenile fish (Pannella Citation1971), in addition, may allow assessing pelagic larval duration (PLD, i.e. the number of days that larvae spend before they metamorphose into juveniles; Victor Citation1986; Raventos & MacPherson Citation2001) and age (in days) of post-settler fish, and back-calculating hatching and settlement timing from the estimated post-settlement age (Vigliola & Meekan Citation2002).
While growing, otoliths can incorporate in their calcium carbonate matrix both minor and trace elements (Green et al. Citation2009). Some elements (e.g. Sr, Ba) reflect their availability in the sea water where the fish lives (Campana Citation1999). Uptake of other elements (e.g. K, Na, Zn, Mn), instead, is likely to be mediated by physiological regulation (Limburg & Elfman Citation2010). Regardless of the mechanism regulating incorporation into otoliths, these elements can be used as permanent spatial signatures of the chemical marine environment experienced by the fish during the various phases of the life cycle (Campana & Neilson Citation1985; Campana et al. Citation1994; Gillanders & Kingsford Citation1996; Campana Citation1999; Green et al. Citation2009).
In this perspective, the chemical composition of different regions of otoliths (i.e. core, larval and juvenile regions) can be used to discriminate among groups of juveniles that use different locations, environments or habitats (e.g. lagoons, estuaries, open marine waters) during their ontogenetic development (Campana Citation1999; Miller & Shanks Citation2004).
Studies on PLD and otolith microchemistry may provide basic information to estimate connectivity among marine populations (Jones et al. Citation2009). Connectivity is defined as the ‘demographic linking of local populations through dispersal of individuals among them as larvae, juveniles or adults’ (Sale et al. Citation2005). This critical property of marine populations (Cowen et al. Citation2000) is strongly influenced by dispersal patterns of individuals, especially during the larval stage (Almany et al. Citation2009). From this general perspective, coupling information about PLD and oceanographic patterns (e.g. intensity and direction of currents) may inform about the maximum potential spatial scale of larval dispersal and, therefore, spatial connectivity.
A number of studies investigated the potential trajectories of larvae. Some studies treated larvae as passive objects (Cowen et al. Citation2000; Siegel et al. Citation2008; Cudney-Bueno et al. Citation2009), whereas others considered the possibility of active movements of larvae (Van Der Molen et al. Citation2007). Trace elements in fish otoliths have been used as natural tags to investigate the dispersal history of fish (Gillanders Citation2005) and to estimate larval connectivity (Ruttenberg et al. Citation2005). A successful detection of locations of larval origin has been achieved for nesting species (Planes et al. Citation2009). For species that spawn in open waters, instead, the identification of different groups of fishes has been used to infer about the spatial scales of larval dispersal and spatial heterogeneity of natal origin (Miller & Shanks Citation2004).
The White Sea bream Diplodus sargus sargus is an ecologically and economically relevant species in Mediterranean sublittoral rocky reefs (Sala et al. Citation1998; Guidetti Citation2006). This fish usually inhabits the littoral zone in shallow waters down to about 50 m (Tortonese Citation1965; Harmelin-Vivien et al. Citation1995). Adults are relatively sedentary and demersal, and produce eggs and larvae that develop in the pelagic waters before post-larvae metamorphose and settle in shallow coastal benthic habitats (Tortonese Citation1965; Harmelin-Vivien et al. Citation1995) at about 1.0 cm TL (Macpherson Citation1998). Juveniles recruit when they approximately reach 6–7 cm in size, ∼6 months after settlement (Garcia Rubies & Machperson Citation1995; Macpherson Citation1998). Before recruitment, juveniles mainly occupy shallow rocky habitats (within about 2 m depth; Macpherson Citation1998), whereas the subadults and adults thrive in deeper rocky bottoms and Posidonia oceanica beds (Tortonese Citation1965; Harmelin-Vivien et al. Citation1995; Macpherson Citation1998).
Basic data useful to estimate patterns of connectivity of this fish as well as of many other Mediterranean species are very scanty. Such data, however, are crucial to set up appropriate management (e.g. sustainable exploitation) and conservation measures (e.g. the creation of marine protected area networks).
Some papers reported PLD data of Mediterranean fishes (Raventos & Macpherson Citation2001; Macpherson & Raventos Citation2006), but little attention was paid to the potential spatial variability (Di Franco & Guidetti Citation2011). With regard to the otolith microchemistry, similarly, a few studies have been published in the Mediterranean Sea and mainly concerned pelagic species, such as Trachurus mediterraneus (Turan Citation2006) and Thunnus thynnus (Rooker et al. Citation2008), or fish targeted by trawling such as Merluccius merluccius and Helicolenus dactylopterus (Morales-Nin et al. Citation2005; Swan et al. Citation2006). Only Gillanders et al. (Citation2001) carried out chemical analyses on otoliths of littoral species in the Mediterranean, such as Diplodus vulgaris along the Spanish coast, but integrated assays across the entire lifetime of fish by performing elemental analyses on whole dissolved otoliths (i.e. without discriminating among the various otolith regions). Nowadays, no information is available for Diplodus sargus sargus. In addition, in spite of the potentially useful applications (e.g. identification of nursery areas or assessment of spatial dispersal of juvenile fish; see Gillanders Citation2005) for conservation and management purposes (Jones et al. Citation2009), no studies are available about any Mediterranean fish species that investigated PLD and/or otolith chemical composition at large (i.e. geographical) scale.
In this study, therefore, we used the White Sea bream as a model species to assess the variability among multiple Mediterranean locations in PLD, hatching and settlement timing and duration, and microelemental composition.
Material and methods
Study locations and fish collection
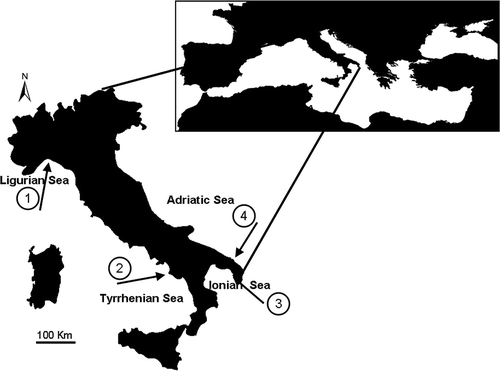
Juveniles of D. sargus sargus were collected at four different locations (Genoa, Ligurian Sea; Maratea, Central Tyrrhenian Sea; San Isidoro, Ionian Sea; Torre Guaceto, Southern Adriatic Sea) situated hundred of kilometers from each other along the coast of Italy (Mediterranean Sea, ). The selection of the four locations was done by a random extraction from a pool of 10 locations situated at large distance (hundreds of kilometers) from each other. Sampling was carried out around the end of June at Maratea, in the beginning of July at San Isidoro e Torre Guaceto, and at the end of July at Genoa 2008 (before the juvenile recruitment to the adult populations).
We collected a total of 36 newly settled individuals, nine at each of the four locations. The sample size from each location was decided considering the lowest number of individuals collected at Genoa in order to run analyses on equal numbers of samples from each location.
Specimens were collected by using a hand-made hand-net 3 m long, 1.5 m high, and with 2 mm mesh size. Care was taken to minimize suffering or distress of fish. After collection, juvenile specimens have been immediately immersed in cold water with ice (<5°C) and then stored in 95% ethanol (Ben-Tzvi et al. Citation2008; Ruttenberg et al. Citation2008). Results of previous work are equivocal about the effect of storage methods (i.e. by freezing or in ethanol) on the chemical composition of otoliths (Milton & Chenery Citation1998; Hedges et al. Citation2004). In our study, the storage method was consistent among locations to avoid any bias on results.
Since the specimens from the different locations were not collected simultaneously, they differed in size (and age) among locations (especially fish from Genoa that were clearly bigger in size than the others). This difference in size, however, did not affect the reliability of PLD evaluations (readings were always very clear) and microchemical comparisons that were conducted on the otolith portions of equivalent age estimated through increment back-calculation.
Otolith extraction, preparation and analysis
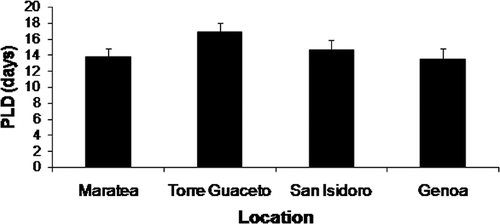
Estimating pelagic larval duration (PLD), and hatching and settlement timing and duration. Fish specimens were measured (standard length, ± 0.1 mm) before removing the otoliths. Fish size was comprised between 0.7 and 4.0 cm with mean values differing among locations ().
Table I. Hatching and settlement temporal windows, and standard length (SL, cm) of sampled fish specimens are reported for each study location
In D. sargus, the daily periodicity of increment formation on otoliths was validated both in captivity and in natural environment (Vigliola Citation1997).
PLD analyses were done using lapillar otoliths, as daily increments are clearer in lapillar than in sagittal otoliths (while the actual number of increments is identical; De Rinaldis Citation2008).
One lapillar otolith was removed from each specimen, cleansed of soft tissue using dissecting pins and then mounted onto a glass slide. We used two different grained sandpapers (3 μm and 1 μm Imperial lapping film, 3M) to obtain thin sections, to expose all the growth layers and improve optical resolution. Otoliths were read using a high-powered microscope and polarized transmitted light (Nikon Z100) coupled with Nikon DS-5MC camera.
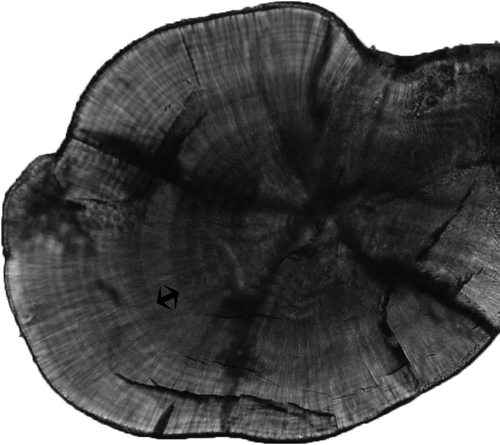
The settlement mark in D. sargus sargus appears as a series of three shaded daily increments (type Ib according to Wilson & McCormick Citation1999, ) quite clearly identifiable by experienced reader (Raventos, personal communications, but see Vigliola et al. Citation2000). The pre-settlement increments were counted from the primordium (the initial structure of the otolith identifying egg fecundation) to the settlement mark along one axis (Bay et al. Citation2006). Pre- and post-settlement increments were counted directly under the microscope (×400). Daily growth increments were counted by two different readers and both PLD and total age in days were calculated starting from the primordium. First increment was assumed to form at hatching (see Vigliola Citation1997 for further details on D. sargus).
Figure 2. Otolith of juvenile Diplodus sargus sargus. Arrows indicate the settlement mark (image set to highlight settlement mark).
Hatching and settlement timing and duration (indicating ‘when’ hatching and settlement events took place and ‘for how many days’ they lasted, respectively, at each study location) were back-calculated from the post-settlement age.
Otolith microchemistry
Sagittal otoliths are routinely used for elemental analyses as they are bigger in size and have wider increments than lapillar otoliths. This allows ablation with the laser of a bigger surface, collecting a greater amount of material and thus increasing the analytical precision of elemental analyses. Before laser ablation, sagittae were prepared following the same procedure previously reported for lapillar otoliths and aged in order to analyze equivalent age among fishes through increment back-calculation. After polishing with lapping films, otoliths were rinsed and sonicated for 10 min in ultra-pure water. All otoliths were analyzed using a Thermo Elemental X7 inductively coupled plasma mass spectrometer (ICP-MS) and NewWave Research UP213 with aperture imaging laser ablation system.
We analyzed otoliths for 13 elements selected from both a bibliographic analysis and a preliminary analysis of dissolved otoliths.
The LA-ICPMS system was calibrated using 612 glass. Calcium was used as internal standard (Vasconcelos et al. Citation2008) to take into account variation in ablation and aerosol efficiency. All the elements were expressed as ratios relative to Ca.
Within-otolith variability was assessed by ablating three spots from each region of each otolith, i.e. from the core, larval and juvenile regions (for a total of 324 replicate spots). The three replicates from the core were represented by three sequential pits (Miller & Shanks Citation2004; Patterson & Swearer Citation2007) vertically collected after having visually detected the core (Ruttenberg et al. Citation2005, Citation2008). Each run generally consisted of 40 s acquisition: 10 s blank to correct for background, 10 s ablation resulting in a pit about 10 μm deep and 20 s for washout.
An average of 10 s of blank counts was used for subtraction from each sample before each ablation. To maintain instrumental precision, we analyzed solid glass standard material from the National Institute of Standards and Technology (NIST 612) every six samples and a linear interpolation between the two consecutive sets of standards carried out. Detection limits were calculated from the concentration of analyte yielding a signal equivalent to 3× the standard deviation of the blank signal, and for each of the elements were: 1.833 μg g−1 (Li), 3.901 μg g−1 (Na), 1.146 μg g−1 (Mg), 80.055 μg g–1 (K), 2.876 μg g–1 (Mn), 0.131 μg g–1 (Co), 4.578 μg g–1 (Ni), 3.571 μg g−1 (Cu), 1.763 μg g−1 (Zn), 0.02 μg g−1 (Sr), 0.227 μg g−1 (Ba), 0.104 μg g−1 (Pb), 13.31 μg g−1 (Ca).
The otoliths were placed in the ablation chamber and viewed remotely on a computer screen where the area for ablation has been selected. The laser was focused on the sample surface and fired through the microscope objective lens (Gillanders Citation2002) using a spot size of 30 μm (identified previously as the approximate size of the cores in a conservative way). Prior to analysis, samples were pre-ablated to remove any surface contamination (laser at 50% power). He gas was flushed into the ablation cell to reduce the deposition of ablated aerosols and to improve signal intensities. The ablated aerosol was then mixed with argon before entering the ICP torch (Gillanders Citation2002). The recorded values of Li, Ni and Cu were consistently below the detection limits and therefore excluded from the analyses. Mean estimates of precision (%RSD, relative standard deviation) based on replicate measurements of NIST 612 standard were: 5.62% (Na), 7.32% (Mg), 22.91% (K), 4.59% (Mn), 4.59% (Co), 14.46% (Zn), 8.10% (Sr), 7.40% (Ba), 9.55% (Pb), 8.52% (Ca).
Data analysis
To test for potential spatial differences in PLD among locations, a one-way analysis of variance (ANOVA) was performed. The only factor included in the analysis was ‘Location’ (Lo) (random factor with four levels). In order to prevent potential effects of fish size (different from site to site) on spatial comparisons of PLDs, fish standard length (SL) was set as covariate. Prior to analysis, homogeneity of variance was tested by Cochran's test (Underwood Citation1997).
To test for potential differences among locations and among otoliths (within location) in terms of multi-element fingerprints, three different two-way PERMANOVAs were run, one for each of the three otolith regions. ‘Location’ (Lo) was treated as a random factor (four levels), ‘Otolith’ (Ot) as a random factor nested in (Lo) (nine levels). Three ablations were conducted in each region of each otolith: the assessment of intra-otolith variability is, in fact, instrumental to then assess the ‘among-otoliths variability’.
Data were ln(x+1) transformed prior to analyses. Transforming data reduced heterogeneity of the within-group variance–covariance matrices in multivariate analyses (Quinn & Keough Citation2002).
Both multivariate components of variation and the ratio ‘estimated magnitude of variance for each factor/estimated residual variance’ (ø) were calculated for the two random factors considered in the PERMANOVA analyses (Terlizzi et al. Citation2007; Gray et al. Citation2009).
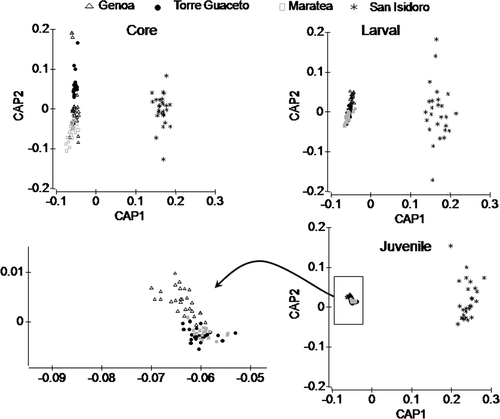
Canonical analyses of principal coordinates (CAP), as discriminant analysis (see Anderson & Willis Citation2003 for details), and cross-validation (or leave-one-out) procedure were performed to assess how accurately samples were assigned to locations. The analyses, based on a Euclidean distance resemblance matrix, were performed separately for each otolith region (core, larval and juvenile).
The GMAV5 package (University of Sydney) and the PRIMER 6 (Clarke & Gorley Citation2006) and Permanova+B20 package (Plymouth Marine Laboratory) were used to perform the statistical analyses.
Results
Pelagic larval duration (PLD), and hatching and settlement timing and duration
PLD was significantly different among locations (p<0.001) and no effect of SL was recorded (p>0.05) in determining the observed pattern. Mean PLD values did not show dramatic change among the four locations, ranging between 13.7 and 16.8 days (). Within-location variability was very low (RSD values ranging from 6.2% at Torre Guaceto to 9.1% at Genoa).
Back-calculated hatching events took places in general from 23 April to 8 June 2008. Hatching differed among locations both in terms of timing and duration (). The longest duration was identified at Genoa (24 days) and the shortest at San Isidoro (10 days).
Settlement events, in general, occurred between 8 May and 21 June 2008, with differences among locations in terms of timing and duration (from 8 days at San Isidoro to 22 days at Genoa, see ).
Both hatching and settlement events at the northernmost location, i.e. Genoa, were anticipated in time and lasted longer compared to the other locations ().
Otolith microchemistry
Microelemental composition significantly differed among locations and among otoliths within location (), with differences that were coherent for the three otolith regions.
Table II. PERMANOVA (multivariate analysis) on log(x+1) transformed data of chemical composition in each otolith region. ***, significant at p<0.001. See text for factor labels
Components of variation show that the most of variability is associated with the factor ‘Location’, while far lower was the variability associated with the factor ‘otolith’ and the residuals ().
Table III. Components of variation derived from PERMANOVA
The core was the region showing the highest average level of correct classification for location (85.20%). Lower levels of average correct classification were recorded both for larval (75.70%) and for juvenile region (78.78%). Otolith from San Isidoro had the 100% of correct classification for all the three otolith regions considered (). This result, particularly for juvenile region, highlights the high dissimilarity of the samples from San Isidoro compared to other samples, as shown by CAP analysis ().
Table IV. Results from a cross-validation procedure. Results are given as percentage of the total number classified to each location, with correct percentages reported in bold
Discussion
The use of new scientific approaches and technologies to investigate spatial connectivity of fish populations, including those applied to fish otoliths, has dramatically increased in the last years (Botsford et al. Citation2009; Jones et al. Citation2009). This is reflected in an increasing number of published papers that mostly deal with tropical regions and, to a lesser extent, with temperate regions (i.e. Forrester & Swearer Citation2002; Vasconcelos et al. Citation2007, Citation2008; Standish et al. Citation2008).
This increasing effort in investigating connectivity is due to the importance of this process for management purposes, like the creation of marine reserve networks and the recovery of exploited fish stocks via larval replenishment (Standish et al. Citation2008; Jones et al. Citation2009; Planes et al. Citation2009). Connectivity, in fact, is a process that for many fishes primarily occurs through dispersal of planktonic larvae (Almany et al. Citation2009), especially for coastal fishes that have fairly sedentary adult stages. The extent to which fish populations are connected via larval dispersal, however, still remains one of the fundamental unresolved issues in marine ecology (Standish et al. Citation2008; Jones et al. Citation2009).
The lack of information on fish population connectivity in the Mediterranean basin is particularly evident and sounds contradictory despite the effort done in this region to establish marine reserves (Abdulla et al. Citation2008) or the call for a better management of fisheries stocks (Sherman et al. Citation2009). The available literature reports very few examples of connectivity studies in the Mediterranean (Galarza et al. Citation2009).
A reliable assessment of patterns of connectivity stems also from the availability of correct estimates of PLD and/or otolith chemical composition. In other regions of the world, the variability in PLD values (both in space and in time) was found to range, depending on the species considered, from 2 to >15 days when evaluated on specimens taken from areas separated by 10 to 100 km (Bay et al. Citation2006; Colleye et al. Citation2008; Di Franco & Guidetti Citation2011), and up to 15 days in specimens collected in different years (Juncker et al. Citation2006; Sponaugle et al. Citation2006). These differences may reflect environmentally mediated variations (i.e. temperature, currents) in growth rates during the pre-competent phase, and potential behaviorally controlled delays in settlement (Sponaugle & Cowen Citation1997) that control larval transportation and permanence in the water (Sabates et al. Citation2007). Conversely, only one investigation was done in the Mediterranean that assessed spatial variability, e.g. of PDL (Di Franco & Guidetti Citation2011), with the risk of failing the estimates of connectivity because PLDs just estimated in one place and/or in one year are used to provide generalizations about connectivity patterns investigated elsewhere or in different years. Estimates of PLD are largely used for modeling larval dispersal (Cowen et al. Citation2000; Cudney-Bueno et al. Citation2009) and are available for a number of Mediterranean fishes (Macpherson & Raventos Citation2006). However, the information mostly comes from specimens collected in relatively restricted areas (e.g. NW Mediterranean), probably from single local populations and in specific years. This makes it difficult to generalize patterns of PLD for a given species at greater spatial or temporal scales.
PLD values observed for D. sargus sargus in this study differed significantly among the locations sampled along the Italian coast. PLD values reported here (from ∼14 to 17 days) are quite similar to those reported by Di Franco and Guidetti (Citation2011), but far lower than those reported by Vigliola et al. (Citation2000) more than 10 years ago from Marseille (French Mediterranean; i.e. ∼28 days). Due to the paucity of data available, it is not possible to ascertain whether this difference is due to spatial or temporal variability in PLD. Another potential source of difference among PLD values reported in the present study and in Vigliola et al. (Citation2000) could be related to the difference in the size of the specimens examined. In fact, Vigliola et al. (Citation2000) examined larger (i.e. average SL ∼5 cm) specimens that potentially experienced selective mortality, a mechanism that a priori we cannot exclude in having driven differences in both hatching and settlement timing and duration of samples from Genoa compared to the other locations examined in the present study.
In any case, an oceanographic dispersal model based on PLD data will provide very different connectivity scenarios considering 14–17 or 28 days. An important caveat, therefore, is that PLD estimates should be used cautiously to estimate dispersal (e.g. using oceanographic models), unless they have been properly evaluated in space and time.
A prerequisite for the use of otolith chemistry to assess patterns of dispersal (e.g. movement among different habitats) is the detection of different elemental composition from source to sink locations, environments or habitats, e.g. for fish living in estuaries as juvenile and in open marine waters as adult (Vasconcelos et al. Citation2008). Detection of this ontogenetic movement is possible provided that chemical composition is assessed for the different otolith regions (corresponding to different phases of the fish life history; Campana Citation1999).
One important novelty of this study on otolith chemistry is the adoption of a formal sampling design, where potential within-otolith variability was taken into account (Underwood Citation1997). Multiple ablations, in fact, were collected from each region (i.e. core, larval and juvenile) of each otolith. Except for a preliminary extra-Mediterranean study showing that variation among otoliths from different specimens was greater than within-otolith variability (Gillanders Citation2002), this aspect has been mostly overlooked. The present study thus stressed the importance of proper replication within each region of each otolith of D. sargus sargus to appropriately assess variability among-otoliths and potential differences at various spatial scales.
The results obtained in the present study allow the following specific (i.e. on the species studied) and general conclusions to be drawn.
| 1. | Timing and duration of hatching and settlement, and PLD of D. sargus sargus, showed significant spatial differences in addition to the previously observed differences in time, i.e. among years (Vigliola et al. Citation1998). Such differences, which may reflect differences in the timing of settlement peaks, can be related both to variations in the timing of reproduction and duration of the pelagic life (Miller & Shanks Citation2004) due to variability in space and time of sea-surface temperatures, available food sources and other environmental features. | ||||
| 2. | Our assessment of a non-negligible variability in space of biological features (e.g. duration of larval life, hatching and settlement timing and duration) and otolith chemistry stresses the need to pay major attention when data available in the literature (e.g. of PLD) coming from single or few sites are used to provide general models of larval dispersal. | ||||
In conclusion, connectivity is increasingly recognized as a key process for population persistence, recovery from disturbance and conservation (Almany et al. Citation2009; Jones et al. Citation2009). We thus need more and more accurate estimates of spatial connectivity that, for instance, may provide invaluable help effectively design both single marine protected areas and marine protected area networks (e.g. reserve location, size and spacing: Almany et al. Citation2009; Planes et al. Citation2009), but to do so we should properly incorporate spatial and temporal variability into dispersal models.
Acknowledgements
The authors wish to thank Prof. K. Robert Clarke (Plymouth Marine Laboratory, Plymouth, UK) for his invaluable suggestions about statistics. The authors thank two anonymous referees for critically reviewing an early draft of the manuscript.
The study was funded by Total Foundation and by Italian Ministry of Instruction, University and Research (MIUR-PRIN 2008).
References
- Abdulla , A , Gomei , M , Maison , E and Piante , C. 2008 . Status of Marine Protected Areas in the Mediterranean Sea , Vol. 152 , France : IUCN, Malaga and WWF .
- Almany , GR , Connolly , SR , Heath , DD , Hogan , JD , Jones , GP , McCook , LJ , Mills , M , Pressey , RL and Williamson , DH. 2009 . Connectivity, biodiversity conservation, and the design of marine reserve networks for coral reefs . Coral Reefs , 28 : 339 – 351 .
- Anderson , MJ and Willis , TJ. 2003 . Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology . Ecology , 84 : 511 – 525 .
- Bay , LK , Buechler , K , Gagliano , M and Caley , M. 2006 . Intraspecific variation in the pelagic larval duration of tropical reef fishes . Journal of Fish Biology , 68 : 1206 – 1214 .
- Ben-Tzvi , O , Kiflawi , M , Gaines , SD , Al-Zibdah , M , Sheehy , MS , Paradis , GL and Abelson , A. 2008 . Tracking recruitment pathways of Chromis viridis in the Gulf of Aqaba using otolith chemistry . Marine Ecology Progress Series , 35 : 229 – 238 .
- Botsford , LW , White , JW , Coffroth , MA , Paris , CB , Planes , S , Shearer , TL , Thorrold , SR and Jones , GP. 2009 . Connectivity and resilience of coral reef metapopulations in marine protected areas: Matching empirical efforts to predictive needs . Coral Reefs , 28 : 327 – 337 .
- Campana , SE. 1990 . How reliable are growth back-calculations based on otoliths? . Canadian Journal of Fisheries and Aquatic Sciences , 47 : 2219 – 2227 .
- Campana , SE. 1999 . Chemistry and composition of fish otoliths: Pathways, mechanisms and applications . Marine Ecology Progress Series , 188 : 263 – 297 .
- Campana , SE , Fowler , AJ and Jones , CM. 1994 . Otolith elemental fingerprinting for stock identification of Atlantic cod (Gadus morhua) using laser ablation ICPMS . Canadian Journal of Fisheries and Aquatic Sciences , 51 : 1942 – 1950 .
- Campana , SE and Neilson , JD. 1985 . Microstructure of fish otoliths . Canadian Journal of Fisheries and Aquatic Sciences , 42 : 1014 – 1032 .
- Clarke , KR and Gorley , RN. 2006 . Primer v6: User Manual/Tutorial , Plymouth : PRIMER-E .
- Colleye , O , Brie , C , Malpot , E , Vandewalle , P and Parmentier , E. 2008 . Temporal variability of settlement in Carapidae larvae at Rangiroa atoll . Environmental Biology of Fishes , 81 : 77 – 285 .
- Cowen , RK , Lwiza , KMM , Sponaugle , S , Paris , CB and Olson , DB. 2000 . Connectivity of marine populations . Open or closed? Science , 287 : 857 – 859 .
- Cudney-Bueno , R , Lavín , MF , Marinone , SG , Raimondi , PT and Shaw , WW. 2009 . Rapid effects of marine reserves via larval dispersal . PLoS ONE , 4 : e4140
- De Rinaldis , G. 2008 . Durata larvale pelagica del sarago maggiore Diplodus sargus sargus (Linnaeus, 1758) , Lecce, , Italy : University of Salento . [Bachelor thesis]
- Di Franco A, Guidetti P. 2011. Patterns of variability in early-life traits of fishes depend on spatial scale of analysis. Biology Letters, doi:10.1098/rsbl.2010.1149. Available from http://rsbl.royalsocietypublishing.org/content/early/2011/01/18/rsbl.2010.1149.full (http://rsbl.royalsocietypublishing.org/content/early/2011/01/18/rsbl.2010.1149.full) (Accessed: 31 January 2011 ).
- Forrester , GE and Swearer , SE. 2002 . Trace elements in otoliths indicate the use of open-coast versus bay nursery habitats by juvenile California halibut . Marine Ecology Progress Series , 241 : 201 – 213 .
- Galarza , JA , Carreras-Carbonnel , J , Macpherson , E , Pascual , M , Roques , S , Turner , GF and Rico , C. 2009 . The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species . Proceedings of the National Academy of Sciences , 106 : 1473 – 1478 .
- Garcia-Rubies , A and Macpherson , E. 1995 . Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral . Marine Biology , 124 : 35 – 42 .
- Gillanders , BM. 2002 . Temporal and spatial variability in elemental composition of otoliths: Implications for determining stock identity and connectivity of populations . Canadian Journal of Fisheries and Aquatic Sciences , 59 : 669 – 679 .
- Gillanders , BM. 2005 . Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats . Estuarine Coastal and Shelf Science , 64 : 47 – 57 .
- Gillanders , BM and Kingsford , MJ. 1996 . Elements in otoliths may elucidate the contribution of estuarine recruitment to sustaining coastal reef populations of a temperate reef fish . Marine Ecology Progress Series , 141 : 13 – 20 .
- Gillanders , BM , Sanchez-Jerez , P , Bayle-Sempere , J and Ramos-Espla , A. 2001 . Trace elements in otoliths of the two-banded bream from a coastal region in the southwest Mediterranean . are there differences among locations? Journal of Fish Biology , 59 : 350 – 363 .
- Gray , CA , Rotherham , D , Chapman , MG , Underwood , AJ and Johnson , DD. 2009 . Spatial scales of variation of assemblages of fish in coastal lakes sampled with multi-mesh gillnets: Implications for designing research surveys . Fisheries Research , 96 : 58 – 63 .
- Green , BS , Mapstone , B , Carlos , G and Begg , GA. 2009 . Tropical fish otoliths: Information for assessment, management and ecology , New York, NY : Springer .
- Guidetti , P. 2006 . Marine reserves re-establish lost predatory interactions and cause community-wide changes in rocky reefs . Ecological Application , 16 : 963 – 976 .
- Harmelin-Vivien , ML , Harmelin , JG and Leboulleux , V. 1995 . Microhabitat requirements for settlement of juvenile sparid fishes on Mediterranean rocky shores . Hydrobiologia , 300–301 : 309 – 320 .
- Hedges , KJ , Ludsin , SA and Fryer , BJ. 2004 . Effects of ethanol preservation on otolith microchemistry . Journal of Fish Biology , 64 : 923 – 937 .
- Jackson , JR. 2007 . Earliest references to age determination of fishes and their early application to the study of fisheries . Fisheries , 32 : 321 – 328 .
- Jones , GP , Almany , GR , Russ , GR , Sale , PF , Steneck , RS , Van Oppen , MJH and Willis , BL. 2009 . Larval retention and connectivity among populations of corals and reef fishes: History, advances and challenges . Coral Reefs , 28 : 307 – 325 .
- Juncker , M , Wantiez , L and Ponton , D. 2006 . Flexibility in size and age at settlement of coral reef fish: Spatial and temporal variations in Wallis Islands (South Central Pacific) . Aquatic Living Resources , 19 : 339 – 348 .
- Limburg , K and Elfman , M. 2010 . An index showing breakdown of seriation, related to disturbance, in a coral-reef assemblage . Canadian Journal of Fisheries and Aquatic Sciences , 67 : 597 – 604 .
- Macpherson , E. 1998 . Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes . Journal of Experimental Marine Biology and Ecology , 220 : 127 – 150 .
- Macpherson , E and Raventos , N. 2006 . Relationship between pelagic larval duration and geographic distribution of Mediterranean littoral fishes . Marine Ecology Progress Series , 327 : 257 – 265 .
- Miller , JA and Shanks , AL. 2004 . Evidence for limited larval dispersal in black rockfish (Sebastes melanops): Implications for population structure and marine reserve design . Canadian Journal of Fisheries and Aquatic Sciences , 61 : 1723 – 1735 .
- Milton , DA and Chenery , SR. 1998 . The effect of otolith storage methods on the concentrations of elements detected by laser-ablation ICPMS . Journal of Fish Biology , 53 : 785 – 794 .
- Morales-Nin , B , Swan , SC , Gordon , JDM , Palmer , M , Geffen , AJ , Shimmield , T and Sawyer , T. 2005 . Age-related trends in otolith chemistry of Merluccius merluccius from the north-eastern Atlantic Ocean and the western Mediterranean Sea . Marine and Freshwater Research , 56 : 599 – 607 .
- Pannella , G. 1971 . Fish otoliths: Daily growth layers and periodical patterns . Science , 173 : 1124 – 1127 .
- Patterson , HM and Swearer , SE. 2007 . Long distance dispersal and local retention as mechanisms of recruitment in an island population of a coral reef fish . Austral Ecology , 32 : 122 – 130 .
- Planes , S , Thorrold , SR and Jones , GP. 2009 . Larval dispersal connects fish populations in a network of marine protected areas . Proceedings of the National Academy of Sciences , 106 : 5693 – 5697 .
- Quinn , GP and Keough , MJ. 2002 . Experimental design and data analysis for biologists , Cambridge : Cambridge University Press .
- Raventos , N and Macpherson , E. 2001 . Planktonic larval duration and settlement marks on the otoliths of Mediterranean littoral fishes . Marine Biology , 138 : 1115 – 1120 .
- Rooker , JR , Secor , DH , DeMetrio , G , Kaufman , AJ , Belmonte Ríos , A and Ticina , V. 2008 . Evidence of trans-Atlantic movement and natal homing of bluefin tuna from stable isotopes in otoliths . Marine Ecology Progress Series , 368 : 231 – 239 .
- Ruttenberg , BI , Hamilton , SL , Hickford , MJH , Paradis , GL , Sheehy , MS , Standish , JD , Ben-Tzvi , O and Warner , RR. 2005 . Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags . Marine Ecology Progress Series , 297 : 273 – 281 .
- Ruttenberg , BI , Hamilton , SL and Warner , RR. 2008 . Spatial and temporal variation in the natal otolith chemistry of a Hawaiian reef fish: Prospects for measuring population connectivity . Canadian Journal of Fisheries and Aquatic Sciences , 65 : 1181 – 1192 .
- Sabates , A , Olivar , MP , Salat , J , Palomera , I and Alemany , F. 2007 . Physical and biological processes controlling the distribution of fish larvae in the NW Mediterranean . Progress in Oceanography , 74 : 355 – 376 .
- Sala , E , Boudouresque , CF and Harmelin-Vivien , M. 1998 . Fishing, trophic cascades, and the structure of algal assemblages: Evaluation of an old but untested paradigm . Oikos , 82 : 425 – 439 .
- Sale , PF , Cowen , RK , Danilowicz , BS , Jones , GP , Kritzer , JP , Lindeman , KC , Planes , S , Polunin , NVC , Russ , GR Sadovy , YJ . 2005 . Critical science gaps impede use of no-take fishery reserves . Trends in Ecology and Evolution , 20 : 74 – 80 .
- Sherman , K , Belkin , IM , Friedland , KD , O'Reilly , J and Hyde , K. 2009 . Accelerated warming and emergent trends in fisheries biomass yields of the world's large marine ecosystems . Ambio , 38 : 215 – 224 .
- Siegel , DA , Mitarai , S , Costello , CJ , Gaines , SD , Kendall , BE , Warner , RR and Winters , KB. 2008 . The stochastic nature of larval connectivity among nearshore marine populations . Proceedings of the National Academy of Sciences , 105 : 8974 – 8979 .
- Sponaugle , S and Cowen , RK. 1997 . Early life history traits and recruitment patterns of Caribbean wrasses (Labridae) . Ecological Monograph , 67 : 177 – 202 .
- Sponaugle , S , Grorud-Colvert , K and Pinkard , D. 2006 . Temperature mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida Keys . Marine Ecology Progress Series , 308 : 1 – 15 .
- Standish , JD , Sheehy , M and Warner , RR. 2008 . Use of otolith natal elemental signatures as natural tags to evaluate connectivity among open-coast fish populations . Marine Ecology Progress Series , 356 : 259 – 268 .
- Swan , SC , Geffen , AJ , Morales-Nin , B , Gordon , JDM , Shimmield , T , Sawyer , T and Massutı , E. 2006 . Otolith chemistry: An aid to stock separation of Helicolenus dactylopterus (bluemouth) and Merluccius merluccius (European hake) in the Northeast Atlantic and Mediterranean . ICES Journal of Marine Science , 63 : 504 – 513 .
- Terlizzi , A , Anderson , MJ , Fraschetti , S and Benedetti-Cecchi , L. 2007 . Scales of spatial variation in Mediterranean subtidal sessile assemblages at different depths . Marine Ecology Progress Series , 332 : 25 – 39 .
- Tortonese , E. 1965 . Biologie comparée de trois espèces méditerranéennes de Diplodus (Pisces, Sparidae) . Rapport Commission International pour l'Exploration Scientifique de la Mer Méditérranée , 18 : 189 – 192 .
- Turan , C. 2006 . The use of otolith shape and chemistry to determine stock structure of Mediterranean horse mackerel Trachurus mediterraneus (Steindachner) . Journal of Fish Biology , 69 ( Supplement C ) : 165 – 180 .
- Tuset , VM , Lombarte , A and Assis , CA. 2008 . Otolith atlas for the western Mediterranean, north and central eastern Atlantic . Scientia Marina , 72S1 : 7 – 198 .
- Underwood , AJ. 1997 . Experiment in ecology , Cambridge : Cambridge University Press .
- Van Der Molen , J , Rogers , SI , Ellis , JR , Fox , CJ and McCloghrie , P. 2007 . Dispersal patterns of the eggs and larvae of spring-spawning fish in the Irish Sea, UK . Journal of Sea Research , 58 : 313 – 330 .
- Vasconcelos , RP , Reis-Santos , P , Tanner , S , Fonseca , V , Latkoczy , C , Güntherb , D , Costa , MJ and Cabral , H. 2007 . Discriminating estuarine nurseries for five fish species through otolith elemental fingerprints . Marine Ecology Progress Series , 350 : 117 – 126 .
- Vasconcelos , RP , Reis-Santos , P , Tanner , S , Maia , A , Latkoczy , C , Güntherb , D , Costa , MJ and Cabral , H. 2008 . Evidence of estuarine nursery origin of five coastal fish species along the Portuguese coast through otolith elemental fingerprints . Estuarine Coastal and Shelf Science , 79 : 317 – 327 .
- Victor , BC. 1986 . Duration of the planktonic larval stage of one hundred species of Pacific and Atlantic wrasses (family Labridae) . Marine Biology , 90 : 317 – 326 .
- Vigliola , L. 1997 . Validation of daily increment formation in otoliths for three Diplodus species in the Mediterranean Sea . Journal of Fish Biology , 51 : 349 – 360 .
- Vigliola , L , Harmelin-Vivien , ML , Biagi , F , Galzin , R , García-Rubies , A , Harmelin , JG , Jouvenel , JY , LeDireach-Boursier , L , Macpherson , E and Tunesi , L. 1998 . Spatial and temporal patterns of settlement among sparid fishes of the genus Diplodus in the northwestern Mediterranean . Marine Ecology Progress Series , 168 : 45 – 56 .
- Vigliola , L , Harmelin-Vivien , M and Meekan , MG. 2000 . Comparison of techniques of back-calculation of growth and settlement marks from the otoliths of three species of Diplodus from the Mediterranean Sea . Canadian Journal of Fisheries and Aquatic Sciences , 57 : 1292 – 1299 .
- Vigliola , L and Meekan , MG. 2002 . Size at hatching and planktonic growth determine post-settlement survivorship of a coral reef fish . Oecologia , 131 : 89 – 93 .
- Wellington , GM and Victor , BC. 1989 . Planktonic larval duration of one hundred species of Pacific and Atlantic damselfishes (Pomacentridae) . Marine Biology , 101 : 557 – 568 .
- Wilson , DT and McCormick , MI. 1999 . Microstructure of settlement-marks in the otoliths of tropical reef fishes . Marine Biology , 134 : 29 – 41 .