Abstract
The present paper deals with spionid species (Spionidae) collected from various depths (4–183 m) and biotopes at 100 stations along the eastern part of the Aegean Sea in June–September 2000. A total of 35 species and 16 genera were identified, of which two species (Laonice norgensis and Spiophanes algidus) are new to the Mediterranean fauna, two species (Laonice bahusiensis and Polydora agassizi) are new to the eastern Mediterranean fauna, three species (Prionospio (Prionospio) saccifera, P. (P.) depauperata and Spiophanes afer) are new to the fauna of the Aegean Sea and four species are new to the fauna of Turkey. Prionospio (Prionospio) fallax and P. (P.) steenstrupi were represented by the highest dominance values, and Laonice cirrata and P. (M.) cf. multibranchiata by the highest frequency index values. The maximum number of species (28) were found on muddy bottom at 5–25 m. Nine species [Laonice norgensis, Paraprionospio coora, Polydora cornuta, Prionospio (Prionospio) depauperata, P. (P.) saccifera, P. (Minuspio) pulchra, Pseudopolydora paucibranchiata, Spiophanes algidus and Streblospio gynobranchiata] can be classified as alien.
Introduction
The family Spionidae, occurring in almost all benthic habitats from coastal areas to the deep sea, has a wide distribution pattern from Arctic to Antarctica. Several species of this family are considered cosmopolitan. Many species are transported worldwide by ballast water of ships and constitute dense populations in harbors and bays (Carlton Citation1985; Çinar et al. Citation2005a,Citationb, Citation2006).
Spionids represent a wide range of feeding habits, from detritivores to carnivores, and also constitute an excellent food source for many marine benthic organisms. They show different life history traits and reproduction modes (Blake & Arnofsky Citation1999). They have pelagic larvae of long life (Blake Citation1996). Spinoids mostly inhabit soft bottoms such as mud and sand in coastal waters.They also occur in various substrates such as algae, rock and seagrass meadows. Some species are closely associated with sponges, decapods, molluscs and colonies of bryzoans. A total of 35 species are known to be parasitic with other species (Martin & Britayev Citation1998).
Spionidae are represented worldwide by almost 470 species and 74 species in the Mediterrranean (author's database). The faunistic data of this family in the Mediterranean Sea mainly come from the publications by Claparède (Citation1869, Citation1870), Fauvel (Citation1928), Laubier (Citation1966, Citation1970), Laubier & Ramos (Citation1974), Maciolek (Citation1985), Guérin (Citation1990), Martin (Citation1996) and Dagli & Çinar (Citation2009).
Prior to this study, a total of 16 genera and 43 species were reported from the coasts of Turkey. The first spionid record in the region was given by Ostroumoff (Citation1896), who found Scolelepis fuliginosa in the Sea of Marmara. Kiseleva (Citation1981) was the first to encounter spionid species (Laonice cirrata, Prionospio cirrifera and Scolelepis cantabra) in the eastern part of the Aegean Sea. Afterwards, many species were reported from the Turkish Aegean Sea coasts by Ergen (Citation1976, Citation1992), Ergen et al. (Citation2002, Citation2006), Çinar & Ergen (Citation1998, Citation1999a), Çinar et al. (Citation2001, Citation2004, Citation2005a, Citation2006), Dogan et al. (Citation2005), Dagli & Çinar (Citation2008), Dagli et al. (Citation2008), Hisashi et al. (Citation2010) and Dagli & Çinar (Citation2010).
The present study focuses on ecological characteristics of Spionidae in the Aegean Sea, with special emphasis on the species newly reported from the Mediterranean and eastern Mediterranean regions.
Materials and methods
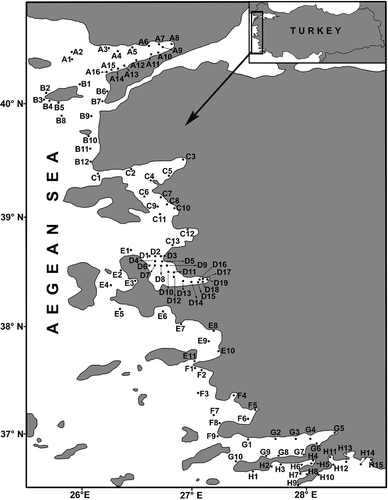
Benthic material was collected by the R/V Hippocampus during a cruise to the eastern coast of the Aegean Sea in July–September 2000. Benthic samples were collected at 100 stations (). Samples were taken by a Van Veen Grab, a dredge and a beam trawl between 4 and 183 m depth. On board the R/V Hippocampus, benthic samples were sieved with a 0.5-mm mesh and the retained material was placed in jars containing 10% seawater–formalin solution. In the laboratory, samples were rinsed in fresh water, sorted according to taxonomic groups using a stereomicroscope, and preserved in 70% ethanol. The spionids were identified and counted, using stereo- and compound microscopes. The length (excluding palps) and width (at chaetiger 10, excluding chaetae) of worms were measured using an ocular micrometer. Drawings were made with the aid of a camera lucida.
Jaccard's Similarity Index was used to distinguish species assemblages in the area. Soyer's Frequency Index (F) was used for classifying species according to their occurrences in samples. According to this index, species with F≥50% are considered ‘constant’, those with F between 25 and 49% are ‘common’, while F values <25% are considered as ‘rare’.
The material examined was deposited at the Museum of the Faculty of Fisheries (ESFM), Ege University, Turkey.
Results
Faunistic analysis of benthic samples collected from different depths and biotopes along the Aegean coasts of Turkey revealed a total of 35 species and 3056 individuals belonging to 16 genera (). Among these species, two species (Laonice norgensis and Spiophanes algidus) are new records for the Mediterranean Sea, two species (Laonice bahusiensis and Polydora agassizi) for the eastern Mediterranean Sea, three species for the Aegean Sea, and four species for the Turkish coasts.
Table I. Spionid species found on the Aegean coast of Turkey and their total abundance (DR: depth range; M: mud; S: sand; SM: sandy mud; Po: Posidonia oceanica; Al: algae; H: hard substratum)
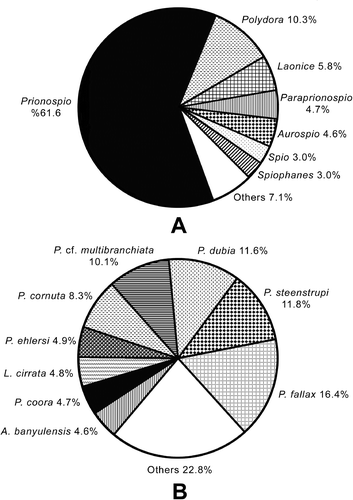
The genus Prionospio had the majority of specimens (1848 individuals, 61.6% of the total specimens), followed by Polydora (316 individuals, 10.3%) and Laonice (176 individuals, 5.8%), respectively (). Nine genera (Streblospio, Scolelepis, Pseudopolydora, Aonides, Malacoceros, Microspio, Laubieriellus, Dipolydora and Apoprionospio) were represented by only a few individuals.
Prionospio (P.) fallax was the numerical dominant species in the area, comprising 16.4% of the total number of specimens (502 individuals) (). The other dominant species were Prionospio (P.) steenstrupii (361 individuals, 11.8%), Prionospio (P.) dubia (356 individuals, 11.6%), Prionospio (M.) cf. multibranchiata (309 individuals, 10.1%) and Polydora cornuta (253 individuals, 8.3%).
According to Soyer's frequency index values, 2 species (Prionospio (M.) cf. multibranchiata and Laonice cirrata) could be classified as constant, 6 species as common and 27 species as rare. Four species (Apoprionospio caspersi, Dipolydora armata, Scolelepis cantabra and Spiophanes algidus) were found only at one station ().
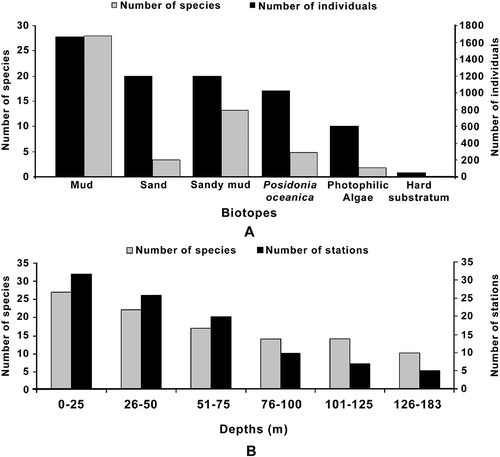
The highest number of species were found on muddy (28 species) and sandy (20 species) bottoms (). Aurospio banyulensis, Laonice cirrata, Prionospio (P.) fallax, Prionospio (P.) steenstrupi, P. (M.) cf. cirrifera, P. (M.) cf. multibranchiata and Spio decoratus occurred in all habitat types (except hard substratum). Malacoceros fuliginosus, Apoprionospio caspersi, P. (M.) pulchra, P. (P.) depauperata, Polydora agassizi, P. cornuta, Pseudopolydora paucibranchiata, Spiophanes algidus and Streblospio gynobranchiata were found only on muddy bottoms; Dipolydora armata only within shell fragments as a boring species; Scolelepis cantabra only on sandy mud bottoms; and Laubieriellus salzi only on Posidonia oceanica meadows. The highest number of individuals were found on muddy bottoms (1673 individuals), followed by sandy mud bottoms (788 individuals) and P. oceanica meadows (287 individuals), respectively (). The majority of species and individuals were found at the depth interval of 0–25 m ().
Figure 3. The distribution of number of species and individuals to biotopes (A) and the depth intervals (B).
Nine alien species [Laonice norgensis, Paraprionospio coora, Polydora cornuta, Prionospio (P.) depauperata, P. (P.) saccifera, P. (M.) pulchra, Pseudopolydora paucibranchiata, Spiophanes algidus and Streblospio gynobranchiata] were found in the area. Apart from P. coora and P. saccifera, other species were encountered in the polluted inner part of Izmir Bay. Polydora cornuta (970 ind. m−2), S. gynobranchiata (310 ind. m−2), P. (M.) pulchra (210 ind. m−2) and P. paucibranchiata (190 ind. m−2) were the dominant species in the polluted zone of Izmir Bay. Laonice norgensis and S. algidus were rare species in the area and only found at depths ranging from 109 to 217 m.
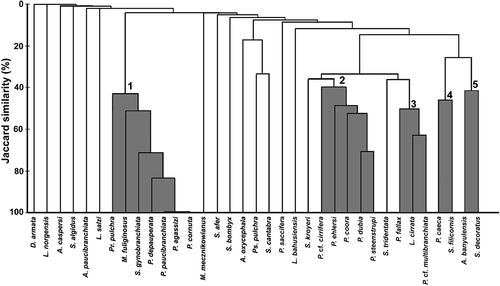
Based on Jaccard's Similarity Index, five species associations were encountered along the Turkish Aegean coast (). The first group (group 1) included the pollution-tolerant species such as Polydora cornuta, P. agassizi, Pseudopolydora paucibranchiata, Prionospio (P.) depauperata, Streblospio gynobranchiata, Malacoceros fuliginosus and Prionospio (M.) pulchra, which were found only in the inner part of Izmir Bay. The second group (group 2) comprised five species, namely Prionospio (M.) cf. cirrifera, P. (P.) ehlersi, P. (P.) steenstrupi, P. (P.) dubia and Paraprionospio coora. These species preferred depths of about 100 m and deeper. The third group (group 3) comprised the most frequent species (Prionospio (P.) fallax, Laonice cirrata, and Prionospio (M.) cf. multibranciahata). The species Polydora coeca and Spio filicornis, which were found on P. oceanica and muddy sand bottoms, comprised group 4. The last association (group 5) included the species Aurospio banyulensis and Spio decoratus, which occurred in all shallow water habitats (4–86 m).
Figure 4. Dendrogram showing similarity among spionids species found in different biotopes and depths along the Turkish Aegean coast.
Morphological and distributional features of the species that are new to the whole Mediterranean and eastern Mediterranean Seas are discussed below.
Laonice bahusiensis Söderström, 1920
Laonice bahusiensis Söderström Citation1920: 223, fig. 78–82; Sikorski Citation2002: 413–417, ; Sikorski Citation2003; 320–325, , a,b, fig. 6f
Material examined
ESFM–POL/00–531, 4 specimens, 29 July 2000, C2, 39°31′28″N, 26°29′08″E, 24 m, mud; ESFM–POL/00–532, 3 specimens, 28 July 2000, C6, 39°09′30″N, 26°40′20″E, 30 m, Posidonia oceanica; ESFM–POL/00–533, 3 specimens, 15 September 2000, F3, 37°23′55″N, 27°06′52″E, 71 m, mud; ESFM–POL/00–534, 1 specimen, 17 September 2000, F5, 37°16′00″N, 27°35′30″E, 13 m, mud; ESFM–POL/00–534, 3 specimens, 18 September 2000, G1, 36°59′00″N, 27°32′35″E, 47 m, algae; ESFM–POL/00–535, 2 specimens, 20 September 2000, G9, 36°47′58″N, 27°41′25″E, 51 m, algae; ESFM–POL/00–536, 3 specimens, 21 September 2000, H3, 36°45′08″N, 27°47′00″E, 47 m, Posidonia oceanica; ESFM–POL/00–537, 2 specimens, 21 September 2000, H4, 36°47′58″N, 28°07′00″E, 10 m, mud; ESFM–POL/00–538, 3 specimens, 21 September 2000, H8, 36°38′30″N, 28°05′15″E, 13 m, Posidonia oceanica.
Description
Largest specimen incomplete, 1.02 mm wide, 13.2 mm long, with 46 chaetigers. Body widest anteriorly, elliptical in cross-section, becoming cylindrical posteriorly. Color in alcohol pale yellowish brown.
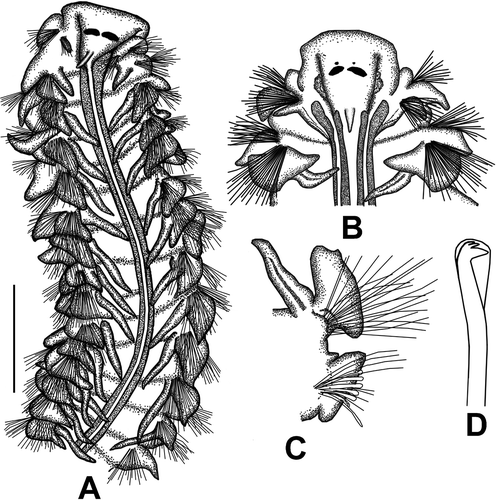
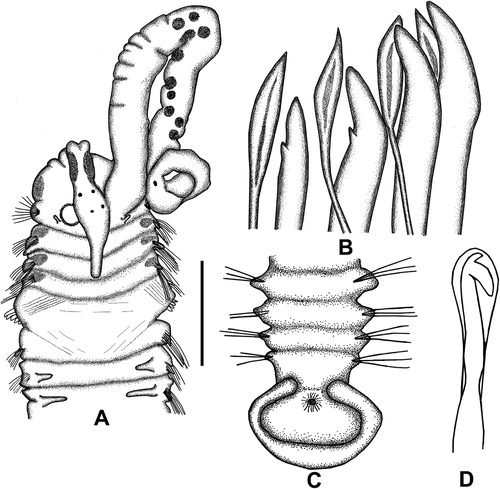
Prostomium triangular, anteriorly wide, truncated; fused with peristomium at anterior angles, clearly visible in dorsal view (). Caruncle together with nuchal organ extending to chatigers 30–31. Two pairs of eyes; anterior pair as single eyespots, posterior pair larger than anterior ones; elliptical in shape (). Large specimens with only posteriors pairs (). Occipital tentacle large.
Branchiae present from chaetiger 2, numbering 28 pairs; first pairs shorter than subsequent pairs; as long as notopodial postchaetal lamellae of chaetiger 2 (). Branchiae twice as long as notopodial lamellae in middle chaetigers.
Notopodial postchaetal lamellae of chaetiger 1 small, triangular. Notopodial postchaetal lamellae of branchial region ear-like, longer than wide, distal part wider than basal wide; becoming small in postbranchial region and gradually diminishing in size; leaf-shaped in last chaetigers. Dorsolateral margin of notopodial postchaetal lamellae in posterior branchial region forming a distinct small peak (). Notopodial lamellae united across dorsum on posterior chaetigers, forming low dorsal crests. Neuropodial postchaetal lamellae of branchial segments triangular with pointed tips (). Neuropodial postchaetal lamellae in postbranchial segments gradually diminishing in size, becoming rounded. Interparapodial lateral pouches first appearing from chaetiger 12–14 to 20.
Notopodial chaetae capillary; anterior chaetae broad, narrowly sheathed, lightly granulated, arranged in two rows. Neuropodial hooded hooks from chaetiger 26–32, up to six per fascicle; hooks tridentate, with two small teeth above main fang (). Sabre chaeta from chaetiger 12–14; one or two per fascicle; narrow, broadly sheathed.
Pygidium missing.
Remarks
Laonice bahusiensis was originally described from the western Sweden (Atlantic Ocean) by Söderström (Citation1920) and subsequently from the Mediterranean and north Atlantic by Sikorski (Citation2003). We observed three slight differences between our and the Sikorski's specimens. The neuropodial lamellae of our specimens are smaller than those of the Sikorski's specimens. The number of sabre chaetae is up to two per fascicle in our specimens, whereas it is up to three in the Sikorski's specimens. The largest diameter of mature oocytes is around 0.27 mm in Sikorski's specimens, whereas it is around 0.24 mm in our specimens.
Reproduction
Some specimens of Laonice bahusiensis collected in September 2000 had eggs in the coelomic cavity between chaetigers 26 and 39. Egg diameters ranged from 80 to 240 μm (mean: 184.5 μm ± 5.08 SE).
Distribution
This species was previously reported on silty sand and silt bottoms at 10–200 m depth from the northeastern Atlantic Ocean and western Mediterranea Sea (Sikorski Citation2003). It is new to the eastern Mediterranean fauna.
Laonice norgensis Sikorski,Citation2003
Laonice norgensis Sikorski Citation2003: 333–338, ,f, 9, 10.
Material examined
ESFM–POL/00–591, 3 specimens, 14 September 2000, E5, 39°09′30″N, 26°17′40″E, 113 m, sand; ESFM–POL/00–592, 3 specimens, 18 September 2000, G3, 36°58′30″N, 27°57′10″E, 109 m, sandy mud.
Description
All specimens incomplete. Largest 1.04 mm wide, 6.1 mm long, with 36 chaetigers. Body slender, enlarged anteriorly, gradually tapering to posterior end. Color in alcohol yellowish pale brown.
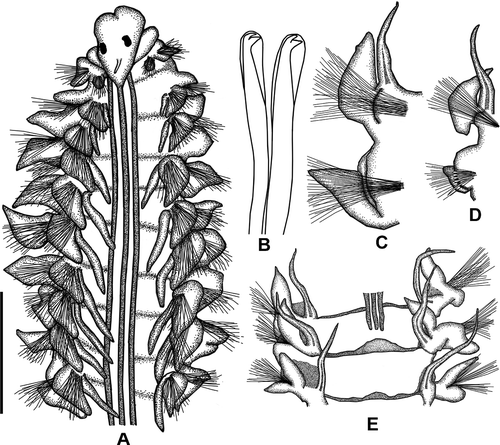
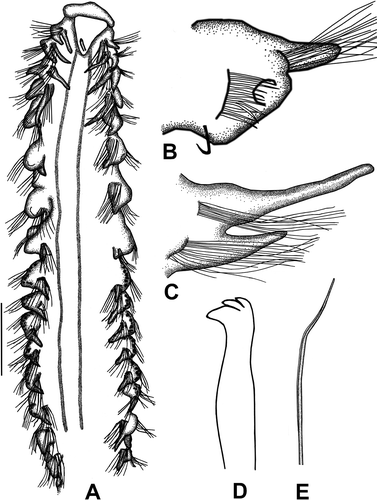
Prostomium triangular; anterior margin broadly rounded; with small apical incision (). Prostomium not fused with peristomium (). Caruncle together with nuchal organ extending to chaetiger 20. One pair brownish eyes; crescent-shaped (). Occipital tentacle finger-like.
Branchiae present from chaetiger 2, numbering 28 pairs; first pair shorter than notopodial lamellae; second pair as long as notopodial postchaetal lamellae; then increasing in size on subsequent chaetigers ().
Notopodial postchaetal lamellae of chaetiger 1 small, triangular. Notopodial postchaetal lamellae of chaetigers 2 and 3 smaller than subsequent notopodial lamellae, subrectangular in shape; notopodial postchaetal lamellae in branchial region ear-like, longer than wide, becoming rounded in postbranchial region; gradually diminishing in size in last chaetigers (). Postchaetal notopodial lamellae on chaetigers 10–13 with peaks near tips (). Notopodial lamellae united across dorsum in middle and posterior chaetigers, forming low dorsal crests. Neuropodial postchaetal lamellae in branchial segments subtriangular with pointed tips (). Neuropodial postchaetal lamellae in postbranchial segments gradually diminishing in size (). Interparapodial lateral pouches first appearing from chaetiger 10.
Notopodial chaetae capillary, narrowly sheathed, lightly granulated, arranged in three rows on anterior chaetigers. Neuropodial hooded hooks first emerging from chaetiger 19; up to eight per fascicle; hooks bidentate (). Sabre chaetae emerging from chaetiger 11–12, one or two per fascicle.
Pygidium missing.
Remarks
Laonice norgensis was first described from the North Sea and Norwegian shelf (Atlantic Ocean) by Sikorski (Citation2003). Although the main morphological features of the Mediterranean specimens of L. norgensis are similar to those of the original descriptions, some slight differences were observed. These differences are: (1) peaks are present on tips of the notopodial postchaetal lamellae of chaetigers 10–13 in the Mediterranean specimens, vs. chaetigers 8–12 in the Atlantic specimens; (2) notopodial lamellae in the branchial region of our specimens are smaller than those of Sikorski's specimens; (3) sabre chaetae numbered 2 per fascicle in the Mediterranean specimens vs. up to four per fascicle in the Atlantic specimens.
Reproduction
Specimens of Laonice norgensis collected in September 2000 had eggs in the coelomic cavity between chaetigers 24 and 32. Egg diameters ranged from 30 to 50 μm (mean: 37.8 μm ± 2.8 SE).
Distribution
This species was previously reported on sandy bottom at 106–298 m depth in the northeastern Atlantic Ocean (Sikorski Citation2003). It is new to the Mediterranean fauna. This species could have been introduced to the Mediterranean via ships.
Polydora agassizi Claparède, 1869
Polydora agassizi Claparède Citation1869: 54–58, pl. 22, ; Marion & Bobretzky Citation1875: 83–84.?Polydora cf. agassizi; Radashevsky & Hsieh Citation2000: 204–205, .
Material examined
ESFM–POL/00–598, 1 specimen, 14 July 2000, D16, 38°25′20″N–27°02′48″E, 13 m, mud; ESFM–POL/00–599, 28 specimens, 15 July 2000, D17, 38°26′05″N, 27°04′32″E, 18 m, mud; ESFM–POL/00–600, 9 specimens, 15 July 2000, D18, 38°26′25″N, 27°06′20″E, 12 m, mud; ESFM–POL/00–601, 3 specimens, 15 July 2000, D19, 38°26′00″N, 27°06′20″E, 15 m, mud; ESFM–POL/00–602, 12 specimens, 14 July 2000, D20, 38°27′01″N, 27°09′20″E, 9 m, mud.
Description
Largest specimen complete, 9.65 mm long, 0.82 mm wide, with 58 chaetigers. Body pale yellowish, slightly flattened dorsoventrally; enlarged in anterior part; gradually decreasing in width towards posterior. Black pigmentation present on dorsolateral sides of prostomium; two black bars on dorsal sides of peristomium and on different parts of palps (); black bars present on dorso-lateral sides of chaetigers 2–4. Prostomium anteriorly incised, enlarged at level of eyes; caruncle extending to posterior margin of chaetiger 3. Two pairs of black eyes in square arrangement; eyes small and rounded. Nuchal tentacle absent. Palps long, extending almost to chaetigers 15–18; with irregular black bars especially in middle region.
Chaetiger 1 with a small, finger-like notopodial lobe, without chaetae (). Neuropodial lamellae well developed, with capillaries. Chaetigers 2–4 and 6 with only capillaries in both noto- and neuropodia. From chaetiger 7 to posterior end of body, neuropodia with only hooded hooks, notopodia with only capillary chaetae. Capillaries in anterior segments numbering 9–12; those in posterior segments numbering 4–5; capillaries on posterior parapodia thinner and shorter than those on anterior parapodia. Neurochaetae on chaetigers 2–7 with about 6–9 capillaries per fascicle. Neuropodial hooded hooks first present on chaetiger 7, reaching up to six per fascicle in posterior parapodia. Hooks bidentate, with small distal tooth; a well-defined constriction present on shaft; not accompanied by capillary chaetae ().
Chaetiger 5 modified, larger than others. Chaetiger 5 with five or six large spines, accompanied by pennoned chaetae (); ventral tuft with 3–5 short-winged capillaries. Major spines falcate, with small lateral accessory tooth.
Branchiae present from chaetiger 7 to 27–30. Length of anterior branchiae almost same in size, gradually decreasing in size towards posterior end.
Pygidium cup-shaped, with a distinct dorsal gap ().
Remarks
Polydora agassizi was previously reported from the western Mediterranean by Claparède (Citation1869) and Marion & Bobretzky (Citation1875). This species was subsequently synonymized with Polydora ciliata by Carazzi (Citation1893), which was originally described from the coast of England by Johnston (Citation1838). However, there are many differences between these two species (according to the original and subsequent descriptions). For example, the caruncle extends to chaetiger 3 in P. agassizi (vs. to the end of chaetiger 2 in P. ciliata); palps of P. agassizi have black spots (vs. without black spots in P. ciliata); the accessory structure of major spines has a small tooth in P. agassizi (vs. a large tooth in P. ciliata). Therefore, P. agassizi could be accepted as a distinct species as proposed by Radashevsky & Hsieh (Citation2000), based on the material collected from the coast of China (Kinmen Island, northeastern Pacific Oceans). The Mediterranean and Chinese species show some differences: (1) the width of the Chinese specimens (0.4 mm) is less than that of specimens from the eastern (0.82 mm, this study) and western [1 mm, Carazzi (Citation1893)] Mediterranean; (2) the anterior-lateral sides of the prostomium have black bands in the Mediterranean specimens, whereas such pigmentation is absent in the Chinese specimens (black pigmentation present on the dorso-lateral sides of the peristomium); (3) palps of the Chinese specimens possessed up to 12 pairs of black spots (each pair located on either side of ciliated food groove), whereas those of the Mediterranean specimens have 8 or 10 black spots that are irregularly scattered (); and (4) our specimens occurred on muddy bottoms vs. those of Radasehvsky and Hsieh inhabited a horseshoe crab.
Reproduction
Specimens of Polydora agassizi collected in July 2000 had eggs in the coelomic cavity between chaetigers 14 and 26. Egg diameters ranged from 70 to 110 μm (mean: 88.7 μm ± 9.34 SE).
Distribution
This species was originally described from the Gulf of Naples (Italy, western Mediterranean) by Claparède (Citation1869) and subsequently from the coast of Marseille (France, western Mediterranean) by Marion & Bobretzky (Citation1875). It is a new species to the eastern Mediterranean fauna. This species was also questionably reported from the Pacific Ocean (coast of China) in mud tubes in association with the ventral anterior part of the carapace and walking legs of the horseshoe crab Tachypleus tridentatus (Radashevsky & Hsieh Citation2000).
Material examined
ESFM–POL/00–116, 2 specimens, 13 August 2000, B2, 40°10′40″N, 25°40′50″E, 104 m, mud.
Description
Largest specimen incomplete, 8.05 mm long, 0.79 mm wide, with 26 chaetigers. Body slender, subcylindrical; enlarged in anterior part; gradually decreasing towards posterior end. Color in alcohol yellowish, opaque white; very faint brownish pigments laterally on parapodia 9–15.
Prostomium broad anteriorly, bell-shaped, tapering posteriorly (). Occipital antenna present. Eyes absent. Nuchal organs, as two broad mediolateral lines, extending to chaetiger 15. Peristomium moderately developed. Notopodial postchaetal lamellae on chaetigers 1–4 long, cirriform; longest on chaetigers 1–2 (). Neuropodial postchaetal lamellae subulate to subtriangular in chaetigers 1–2, with slender tip. Chaetigers 5–8 each with rounded notopodial and reduced neuropodial postchaetal lamellae (). From chaetigers 9–14, notopodial lamella cirriform to subulate (except for chaetigers 15–18). Neuropodial lamella reduced in posterior parapodia. Glandular opening, with semicircular chaetal slit, well developed in chaetigers 5–7 (,B); absent in chaetiger 8; glandular organ of chaetigers 9–14 opening as lateral vertical slit. Ventrolateral intersegmental genital pouches present between chaetigers 15 and 16. Dorsal ciliated crests apparent from chaetiger 17.
Chaetiger 1 with 1–2 stout, crook-like chaetae in neuropodium, other chaetae capillaries; neurochaetae arranged in two rows. Chaetigers 2–4 with simple, unilimbate capillaries (); latter becoming more numerous and sheaths becoming more distinctive from chaetigers 2 to 4. Chaetigers 5–13 with stout, bilimbate neurochaetae, arranged in 1–2 rows; notochaetae each with broad distal sheath, arranged in three rows. Neuropodial hooded hooks first present from chaetiger 15, up to six per fascicle; hooks quadridentate without hoods (tridentate in lateral view) (). Bacillary chaetae missing on specimens examined. Ventral sabre chaetae from chaetiger 4; granulated near tip.
Pygidium missing.
Remarks
The Mediterranean specimens of Spiophanes algidus agree well with the original description of the species from the coast of France (Crozet Island) by Meißner (Citation2005). However, some slight differences were observed. The anterior margin of the prostomium in the Atlantic specimens had two short antero-lateral projections, whereas it is almost rounded in the Mediterranean specimens. Chaetiger 1 of our specimens possessed one, but often two, stout crook-like chaetae, whereas that of the Meißner's specimens had only one stout crook-like chaeta.
Reproduction
Specimens of Spiophanes algidus collected in August 2000 had eggs in the coelomic cavity between chaetigers 16 and 23. Egg diameters ranged from 40 to 110 μm (mean: 70.9 μm ± 9.51 SE).
Distribution
This species was originally described from the French Crozet Island (near South Africa, Indian Ocean) at depths between 210 and 217 m (Meißner Citation2005). It is new to the Mediterranean fauna. This species could have been introduced to the Mediterranean by ships.
Discussion
The present study raised the known number of spionid species in the Mediterranean from 73 to 75; that in the eastern Mediterranean from 57 to 61; and that in the Turkish Seas from 43 to 51. Prior to this study, a total of 16 genera and 43 spionid species were reported from the coasts of Turkey; 21 species from the Mediterranean coast of Turkey (Dagli & Çinar Citation2009, Citation2010); 33 species from the Turkish Aegean Sea coast (Ergen Citation1976; Çinar & Ergen Citation1999a; Çinar et al. Citation2001, Citation2004, Citation2005a, Citation2006, Citation2008; Ergen et al. Citation2002, Citation2006; Dağlı & Çinar Citation2008, Citation2010; Dağlı et al. Citation2008; Hisashi et al. Citation2010); 16 species from the Sea of Marmara (Ostroumoff Citation1896; Demir Citation1952; Çağlar 1954; Rullier Citation1963; Caspers Citation1968); and 11 species from the Turkish Black Sea coast (Dumitresco Citation1960, Citation1962; Topaloğlu & Kihara Citation1993; Gillet & Ünsal Citation2000; Çinar & Gönlügür-Demirci Citation2005). The species that are newly reported from the Mediterranean Sea and eastern Mediterranean Sea are Spiophanes algidus, Laonice norgensis, L. bahusiensis, and Polydora agassizi.
Spionids generally reproduce in spring and summer months (Blake Citation1969). However, individuals of the same species have different generation time in different regions. For instance, Hartmann-Schröder (Citation1996) postulated that specimens of Polydora coeca reproduced from February to April in Öresund (Baltic Sea) and from June to August in the North Sea. Meißner (Citation2005) mentioned that Spiophanes kroyeri spawned in winter (November to February) in north longitutides. Blake (Citation1996) also reported that this species spawned from November to February on the Swedish coasts and had an egg diameter of 160 μm. In the present study, this species possessed mature eggs in July and October, and had a maximum egg diameter of 120 μm.
Malacoceros fuliginosus, Polydora ciliata, P. cornuta, Spio decoratus, S. filicornis, Streblospio shrubsolii, P. (M.) cf. multibranchiata and P. (P.) fallax are known to form dense populations in organically polluted bottoms. In addition, the ship-transferred species such as Streblospio gynobranchiata and Pseudopolydora paucibranchiata were reported to dominate polluted soft bottoms (Çinar et al. Citation2005a; Dagli & Çinar Citation2008). Ergen et al. (Citation2002; Citation2006) and Doğan et al. (Citation2005) reported S. decoratus (6720 ind. m−2), P. ciliata (50,820 ind. m−2), P. cornuta (1940 ind. m−2), S. shrubsolii (14,256 ind. m−2), S. filicornis (12,580 ind. m−2), M. fuliginosus (3990 ind. m−2), P. (P.) fallax (1080 ind. m−2) and P. (M.) cf. multibranchiata (2090 ind. m−2) in polluted and semi-polluted waters of Izmir Bay. In this study, S. decoratus (150 ind. m−2), P. cornuta (970 ind. m−2), M. fuliginosus (70 ind. m−2), P. (P.) fallax (120 ind. m−2) and P. (M.) cf. multibranchiata (210 ind. m−2) were found at stations located in and near the polluted inner part of Izmir Bay. However, the presence of P. ciliata on the coast of Turkey (Mediterranean in general) seems to be dubious, probably confused with the other Polydora species such as P. cornuta (see Çinar et al. Citation2005a) and P. agassizi (this study). Therefore, the reports of P. ciliata in the Mediterranean Sea should be re-examined.
Almost 95 alien polychaete species are known from the Mediterranean Sea (Zenetos et al. Citation2008; Çinar Citation2009; Dağlı & Çinar Citation2009). Ten alien spionid species were reported to have been established in the Mediterrenean Sea. All these species were also known from the coast of Turkey. The first alien spionid species (Prionospio (P.) saccifera) on the coasts of Turkey was reported from the Manavgat River Delta (Çinar & Ergen Citation1999b). Afterwards, Streblospio gynobranchiata (max. 60,480 ind. m−2), Polydora cornuta (3170 ind. m−2) and Pseudopolydora paucibranchiata (6180 ind. m−2) were found in Alsancak Harbour (Aegean Sea) (Çinar et al. Citation2005a; Dağlı & Çinar Citation2008). Dağlı & Çinar (Citation2009) encountered four alien spionid species (P. (P.) saccifera, P. (A.) sexoculata, P. (P.) depauperata and P. (A.) krusadensis) on the southern coast of Turkey. Finally, P. (P.) paucipinnulata and Parapionospio coora were reported from the Turkish coasts (Dağlı & Çinar Citation2010). Among these species, two [P. (P.) saccifera, P. (A.) sexoculata] were considered to be Lessepsian migrants (i.e. species that migrated from the Red Sea to the Mediterranean through the Suez Canal) and the others were considered to have been introduced to the region by ballast water from ships. The species S. gynobranchiata, P. cornuta, P. paucibranchiata, and P. (M.) pulchra seem to be invasive. In this study, we found seven alien spionid species [P. coora, P. cornuta, P. (P.) depauperata, P. (P.) saccifera, P. (Minuspio) pulchra, S. gynobranchiata, and P. paucibranchiata]. The majority of species (five) were encountered in the polluted zone of Izmir Bay. They formed dense populations there, acting as pollution indicator species. After the establishment of these species, populations of the other opportunistic species such as Capitella sp. and Scolelepis fuliginosus were reported to have decreased (Çinar et al. Citation2005a), possibly reflecting a competition between the species. The newly reported species, Spiophanes algidus and Laonice norgensis, were only known from the South Africa and North Sea, respectively. Their presence in the eastern Mediterranean, a distinct place from their type localities, could be explained by the introduction via ships. However, these species were recently described and we do not have enough data regarding their natural distributional limits. Therefore, more data are required for classifying these species as alien or native.
The present study sheds more light on the spionid fauna and its taxonomic and ecological features in the benthic environments of the Aegean Sea, where no detailed study regarding this family has been undertaken. The numbers of species given here in fact do not reflect the real spionid diversity of the Aegean Sea, since the deep-water (more than 200 m) fauna still remains largely unexplored. Future such studies to be undertaken in the area would enable us to understand the true spionid diversity of the Aegean Sea and their functional roles in biotopes.
Acknowledgements
We wish to thank Dr. Karin Meißner (Institut fuer Angewandte Oekologie, Rostock, Germany) and Dr. Andrey V. Sikorski (Zoological Museum of the Moscow State University, Moscow, Russia) for confirmation of the specimens of Spiophanes algidus, Laonice bahusiensis, and Laonice norgensis. We also thank our colleagues at the Department of Hydrobiology, Ege University for collecting and sorting benthic material.
References
- Blake , JA. 1969 . Reproduction and larval development of Polydora from northern New England (Polychaeta: Spionidae) . Ophelia , 7 : 1 – 63 .
- Blake , JA. 1996 . “ Family Spionidae Grube, 1850 ” . In Taxonomic atlas of the benthic fauna of the Santa Maria Basin and Western Santa Barbara Channel, volume 6. The Annelida Part 3. Polychaeta: Orbiniidae to Cossuridae , Edited by: Blake , JA , Hilbig , B and Scott , PH . 81 – 221 . Santa Barbara, CA : Santa Barbara Museum of Natural History .
- Blake , JA and Arnofsky , PL. 1999 . Reproduction and larval development of spioniform Polychaeta with application to systematics and phylogeny . Hydrobiologia , 402 : 57 – 106 .
- Carazzi , D. 1893 . Revisione del genere Polydora Bosc e cenni su due specie che vivono sulle striche . Mittheilungen aus der Zoologischen Station zu Neapel , 11 : 4 – 45 .
- Carlton , JT. 1985 . Transoceanic and interoceanic dispersal of coastal marine organisms: The biology of ballast water . Oceanography and Marine Biology. An Annual Review , 23 : 313 – 371 .
- Claparède , E . 1869 . Les Annélides Chétopodes du Golfe de Naples . Second Partie. Annélides sédenteries. Memoires de la Sociedad Physiques Historia Natural Genève , 20 : 1 – 225 .
- Claparède , E . 1870 . Les Annélides Chétopodes du Golfe de Naples. Troisième partie . Mémoires de la Société de Physique et d'Histoire naturelle de Genève , 20 ( 2 ) : 365 – 542 .
- Caspers , H. 1968 . La Macrofaune benthique du Bosphore et les problèmes de l'infiltration des èlèments Mèditerranèes dans la mer Noire . Rapport de la Commission İnternational Exploration de la Mer. Mediterranée , 19 : 107 – 115 .
- Çaglar , M. 1954 . Mytilus galloprovincialis kabuklarında yaşayan oyucu bir Polydora türü . Istanbul University Hydrobiology Institute Publications series , A ( II ) : 67 – 73 . in Turkish
- Çinar , ME. 2009 . Alien polychaete species (Annelida: Polychaeta) on the southern coast of Turkey (Levantine Sea, eastern Mediterranean), with 13 new records for the Mediterranean Sea . Journal of Natural History , 43 : 2283 – 2328 .
- Çinar , ME , Bilecenoglu , M , Öztürk , B , Katagan , T and Aysel , V. 2005b . Alien species on the coasts of Turkey . Mediterranean Marine Science , 6 : 119 – 146 .
- Çinar , ME and Ergen , Z. 1998 . Polychaetes associated with the sponge Sarcotragus muscarum Schmidt, 1864 from the Turkish Aegean coast . Ophelia , 48 : 167 – 183 .
- Çinar , ME and Ergen , Z. 1999a . A preliminary study on Polychaeta fauna of the Marmaris Bay (Southern Aegean Sea) . Istanbul University Journal of Fisheries, special issue , : 47 – 59 .
- Çinar , ME and Ergen , Z. 1999b . Occurrence of Prionospio saccifera (Spionidae: Polychaeta) in the Mediterranean Sea . Cahiers de Biologie Marine , 40 : 105 – 112 .
- Çinar , ME , Ergen , Z and Dagli , E. 2004 . New records of polychaetes from the Turkish Aegean coast . Rapport de la Commission İnternational Exploration de la Mer Mediterranée , 37 : 508
- Çinar , ME , Ergen , Z , Dagli , E and Petersen , ME. 2005a . Alien species of spionid polychaetes (Streblospio gynobranchiata and Polydora cornuta) in Izmir Bay, eastern Mediterranean . Journal of the Marine Biological Association of the United Kingdom , 85 : 821 – 827 .
- Çinar , ME , Ergen , Z , Kocataş , A and Katağan , T. 2001 . Zoobenthos of the probable dumping area in İzmir Bay (Aegean Sea) . Rapport de la Commission International Exploration de la Mer Mediterranée , 36 : 374
- Çinar , ME and Gönlügür-Demirci , G. 2005 . Polychaete assemblages on shallow-water benthic habitats along the Sinop Peninsula (Black Sea, Turkey) . Cahiers de Biologie Marine , 46 : 253 – 263 .
- Çinar , ME , Katagan , T , Koçak , F , Öztürk , B , Ergen , Z , Kocatas , A , Önen , M , Kirkim , F , Bakir , K Kurt , G . 2008 . Faunal assemblages of the mussel Mytilus galloprovincialis in and around Alsancak Harbour (Izmir Bay, eastern Mediterranean) . Journal of Marine Systems , 71 : 1 – 17 .
- Çinar , ME , Katagan , T , Öztürk , B , Egemen , Ö , Ergen , Z , Kocatas , A , Önen , M , Kirkim , F , Bakir , K Kurt , G . 2006 . Temporal changes of soft bottom zoobenthic communities in and around Alsancak Harbor (Izmir Bay, Aegean Sea), with special attention to the autoecology of exotic species . Marine, Ecology , 27 : 229 – 246 .
- Dagli , E and Çinar , ME. 2008 . Invasion of polluted soft substrate of Izmir Bay (Aegean Sea, eastern Mediterranean) by the spionid polychaete worm . Pseudopolydora paucibranchiata (Polychaeta: Spionidae). Cahiers de Biologie Marine , 49 : 87 – 96 .
- Dagli , E and Çinar , ME. 2009 . Species of the subgenera Aquilaspio and Prionospio (Polychaeta: Spionidae: Prionospio) from the southern coast of Turkey (Levantine Sea, eastern Mediterranean), with description of a new species and two new reports for the Mediterranean fauna . Zootaxa , 2275 : 1 – 20 .
- Dagli , E and Çinar , ME . 2010 . Presence of the Australian spionid species, Prionospio paucipinnulata (Polychaeta: Spionidae), in the Mediterranean Sea . Cahiers de Biologie Marine , 51 : 311 – 317 .
- Dagli , E , Ergen , Z and Çinar , ME . 2008 . The taxonomic and ecological characteristics of Longosomatidae and Spionidae (Annelida: Polychaeta) distributed in Saros Bay, Turkey . Journal of Fisheries Sciences , 2 : 198 – 209 .
- Demir , M. 1952 . Boğazlar ve Adalar sahillerinin omurgasız dip hayvanları . Istanbul University Hydrobiology Institute Publications , 2 : 1 – 654 . in Turkish
- Dogan , A , Çinar , ME , Önen , M , Ergen , Z and Katagan , T. 2005 . Seasonal dynamics of soft-bottom zoobenthic communities in polluted and unpolluted areas of Izmir Bay (Aegean Sea) . Senckenbergiana Maritima , 35 ( 1 ) : 133 – 145 .
- Dumitresco , H . 1960 . Contribution a la connaissance des polychètes de la Mer Noire, specialement des eaux prébosphoriques . Travaux de Muséum d'Histoire Naturelle ‘Gregor Antipa’ , II : 69 – 85 .
- Dumitresco , H. 1962 . Nouvelle contribution a l’étude des polychètes de la Mer Noire . Trauaux de Muséum d'Histoire Naturelle ‘Gregor Antipa’ , 3 : 61 – 68 .
- Ergen , Z. 1976 . İzmir Körfezi ve Civarı Poliketlerinin Ekolojik ve Taksonomik Özellikleri . Ege Üniversitesi Fen Fakültesi İlmi Raporlar Serisi , 209 : 73
- Ergen , Z. 1992 . The latest status of Polychaeta in the soft substrate of İzmir Bay . Rapport de la Commission İnternational Exploration de la Mer Mediterranée , 33 : 36
- Ergen , Z , Çinar , ME , Dagli , E and Kurt , G. 2006 . Seasonal dynamics of soft-bottom polychaetes in Izmir Bay (Aegean Sea, eastern Mediterranean) . Scientia Marina , 70S3 : 197 – 207 .
- Ergen , Z , Dora , Ç and Çinar , ME. 2002 . Seasonal analysis of Polychaeta from the Gediz River Delta (İzmir Bay, Aegean Sea) . Acta Adriatica , 43 ( 2 ) : 29 – 42 .
- Fauvel , P. 1928 . Annélides polychètes nouvelles de l'Inde. Pt. 2 . Bulletin du Musee d'Histoire Naturelle de Paris , 34 : 159 – 165 .
- Gillet , P and Unsal , M. 2000 . Résultats de la campagne océanographique du ‘Bilim’: Annélides polychètes de la Mer de Marmara, du Bosphore et des régions prébosphoriques de la Mer Noire (Turquie) . Mésogée , 58 : 85 – 91 .
- Guérin , JP. 1990 . Description d'une nouvelle espece de spionide (annelides, polychetes) Boccardia semibranchiata . Annales de l'Institut Oceanographique , 66 : 37 – 45 .
- Hartmann-Schröder , G. 1996 . “ Annelida, Borstenwürmer, Polychaeta. Die Tierwelt Deutschlands, 58 ” . In , 2nd , 648 Jena : Gustav Fischer .
- Hisashi , Y , Dagli , E and Çinar , ME. 2010 . First record of Paraprionospio coora Wilson, 1990 (Polychaeta: Spionidae) from the Mediterranean Sea . Mediterranean Marine Science , 11 : 133 – 141 .
- Johnston , G. 1838 . Miscellanea zoologica . Aricidea. Magazine of Botany, Edinburgh , 2 : 62 – 73 .
- Kiseleva , MI. 1981 . Bentos râhlâh gruntov Cernogo moria , 168 Kiev : Izd-vo Naukova dumka .
- Laubier , L. 1966 . Le coralligène des Albères . Monographie biocénotique. Annales Institut océanographique , 43 : 137 – 316 .
- Laubier , L. 1970 . Prionospio salzi sp. nov., un spionidien (Annelide Polychète) des côtes Méditerranéennes d'Israël . Israel Journal of Zoology , 19 : 183 – 190 .
- Laubier , L and Ramos , J. 1974 . Polydora guillei sp. nov. nouvelle espèce de polychète spionidien en Méditerranée Occidentale . Vie et Milieu , XXIV ( 3A ) : 479 – 486 .
- Maciolek , NJ. 1985 . A revision of the genus Prionospio Malmgren, with special emphasis to the genera Apoprionospio Foster, and Paraprionospio Caullery (Polychaeta: Spionidae) . Zoological Journal of the Linnean Society , 84 : 325 – 383 .
- Marion , AF and Bobretzky , N. 1875 . Étude des Annélides du Golfe de Marseilles . Annales des Sciences Naturelles , 6 ( 2 ) : 1 – 106 .
- Martin , D. 1996 . A new species of Polydora (Polychaeta, Spionidae) associated with the excavating sponge Cliona viridis (Porifera, Hadromerida) in the northwestern Mediterranean Sea . Ophelia , 45 ( 3 ) : 159 – 174 .
- Martin , D and Britayev , TA. 1998 . Symbiotic polychaetes: Review of known species . Oceanography and Marine Biology An Annual Review , 36 : 217 – 340 .
- Meißner , K. 2005 . Revision of the genus Spiophanes (Polychaeta, Spionidae); With new synonymies, new records and descriptions of new species . Mitteilungen aus dem Museum für Naturkunde in Berlin/Zoologische Reihe , 81 : 3 – 66 .
- Ostroumoff , A. 1896 . Compates rendus des dragages et du plancton de l'expedition de ‘Selianik’ . Bulletion de L'Académia Impériale des Sciences Saint Petersburg , 5 : 33 – 92 .
- Radashevsky , VI and Hsieh , HL. 2000 . Polydora (Polychaeta: Spionidae) species from Taiwan . Zoological Studies , 39 : 203 – 217 .
- Rullier , F. 1963 . Les Annélides Polychètes du Bosphore, de la Mer de Marmara et de la Mer Noire, en Relation Avec Celles de La Méditerranée. Rapport de la Commission International Exploration de la Mer . Mediterranée , XVII : 161 – 260 .
- Sikorski , AV. 2002 . On distinguishing of Laonice cirrata (Sars, 1851) and Laonice bahusiensis Söderström, 1920 (Polychaeta: Spionidae) the two morphologically related species . Zoologicheskiy Zhurnal , 81 : 406 – 419 .
- Sikorski , AV. 2003 . Laonice (Polychaeta, Spionidae) in the Arctic and the North Atlantic . Sarsia , 88 : 316 – 345 .
- Söderström , A. 1920 . Studien über die Polychaeten familie Spionidae . PhD thesis, Uppsala Universitet. , : 288
- Topaloglu , B and Kihara , K. 1993 . Community of Mediterranean Mussel Mytilus galloprovincialis Lamarck, 1819 in the Bosphorus Strait . Journal of Tokyo University of Fisheries , 80 : 113 – 120 .
- Zenetos , A , Meriç , E , Verlaque , M , Galli , P , Boudouresque , CF , Giangrande , A , Çinar , ME and Bilecenoglu , M. 2008 . Additions to the annotated list of marine alien biota in the Mediterranean with special emphasis on Foraminifera and Parasites . Mediterranean Marine Science , 9 : 119 – 165 .