Abstract
A solitary hydroid of the genus Ectopleura was described associated with several Mediterranean anthozoans: the black coral Antipathella subpinnata and three gorgonian species, Eunicella cavolinii, Paramuricea clavata and Paramuricea macrospina. This find represents the first record of a hydroid species epibiont of an antipatharian coral and also the first record of such association with Mediterranean gorgonians. Hydroids arise from the branches of the hosting corals and are enveloped by a thin sheet of their skeleton and by a layer of living coenenchyme up to the neck of the hydranths. The relationship causes no apparent damage to the host, while the epibiotic habitus allows the hydroid to avoid siltation and to gain in defence and support. It is hypothesised that actinulae of Ectopleura sp. are able to settle on the corals (both skeleton or tissue) and, during their growth, to be enveloped by the skeletons of their host.
Introduction
Hydroids are able to establish epibiotic associations with the majority of marine phyla due to their fast growth rate and adaptability to a wide range of primary and secondary substrates (Riedl Citation1966; Boero Citation1984). In some cases, this epibiotic aptitude may shift to a mutualistic or parasitic relationship (Boero & Bouillon Citation2005; Puce et al. Citation2008b). Symbiotic associations involving hydroids and anthozoans were recently reviewed by Puce et al. (Citation2008a) (). Associations with living octocorals are well described, with 12 species belonging to six families (Asyncorynidae, Cladocorynidae, Corynidae, Ptilocodiidae, Tubulariidae, and Zancleidae) found associated with these corals. Particularly, the genus Ralpharia (Tubulariidae) is almost exclusively known to be associated with octocorals, with two species living on soft corals and four on gorgonians (Puce et al. Citation2008a). In contrast to associations with octocorals, the relationships between hydroids and hexacorals are almost unknown, with the exception of Zanclea margaritae Pantos and Bythell, Citation2010, which was recently described associated with Acropora muricata (Linnaeus, 1758) (Pantos & Bythell Citation2010). Some observations were also made for other scleractinian species, for example Zanclea sp. and Zanclea gilii Boero, Bouillon and Gravili, Citation2000 living in association with unidentified hard corals (Millard & Bouillon Citation1973, Millard Citation1975; Boero et al. Citation2000). Moreover, recently, a tubulariid hydroid, tentatively classified as Hybocodon cfr. prolifer (Agassiz, 1862), was photographed in association with living branches of Madrepora oculata (Linnaeus, 1758) in the white coral banks located at 500 m depth of Santa Maria di Leuca (Ionian Sea) (Mastrototaro et al. 2009; Vertino et al. Citation2009).
Table I. Known associations between hydroids and anthozoans
For benthic passive filter feeders, epibiosis on vertically branched anthozoans is particularly attractive (Bayer Citation1961) in fact, epibionts may increase their filtration efficiency (Linskens Citation1963; Oswald & Seed Citation1986; Zea Citation1993) and exploit the organic matter and bacteria entrapped in the coral mucus (Goh et al. Citation1999).
For these reasons, octocorals host a highly diversified epibiotic community. Bayer (Citation1961), for example, reported numerous species of commensal invertebrates such as hydroids, polychaetes, crustaceans and mollusks associated with gorgonians. Goh et al. (Citation1999) recorded that half of the 31 gorgonian species known from Singapore are associated with sponges, hydroids, polychaetes, crustaceans, bryozoans or echinoderms. Also, antipatharian corals may host a rich associated fauna, whether or not commonly through species-specific relations, which generally occur on the living portions of the colonies (Tazioli et al. Citation2007). The majority of the sessile organisms, including hydroids, usually live on the dead portions of these corals, and can reach extremely high abundances (Love et al. Citation2007; Di Camillo et al. Citation2008; Bo et al. in press).
This study aims to describe the peculiar association of a tubulariid hydroid with colonies of the black coral Antipathella subpinnata (Ellis and Solander, 1786) and some gorgonian species of the genera Eunicella and Paramuricea recorded in different mesophotic coral assemblages along the southern coasts of Italy (Mediterranean Sea).
Materials and methods
Hydroids epibiont of anthozoans were found on two samples of Antipathella subpinnata (family Myriopathidae) (Opresko Citation2001; Bo et al. Citation2008) and on one colony each of Paramuricea clavata (Risso, 1826) and Paramuricea macrospina (Koch, 1882) (family Plexauridae) (Carpine & Grasshoff Citation1975), collected in August 2009 on board of the R/V Astrea of ISPRA on a rocky shoal at 110–120 m depth in front of Vibo Marina (Calabria, South Tyrrhenian Sea, St. Eufemia Gulf; 38.8460°N–16.1430°E). The studied shoal is part of a system of rocky structures, about 50 m high, lying on the flat soft bottom of the Gulf, exposed to moderate currents and characterised by peculiar mesophotic coral assemblages, which have already been described (Bo et al. in press). Hydroids were found also on two colonies of Eunicella cavolinii Koch, 1887 collected in July 2010 between 60 and 90 m depth along the rocky coast of the Gulf of Salerno (Secca dei Galli, 40.5900°N–14.4876°E) and Ischia Island (Punta S. Angelo, 40.6912°N–13.8939°E), respectively ().
Figure 1. Map of the sampling regions. Locations of the explored areas along the western Tyrrhenian coast: Ischia Island, shoal Secca dei Galli and S. Eufemia Gulf (black dots).
Samples were collected with a jaw grabber mounted on a Remotely Operated Vehicle survey (ROV ‘Pollux’).
The collected samples were fixed in 4% formaldehyde for morphological observations. Drawings were made from fixed material and the colours mentioned in the descriptions have been determined from the videos and photographs of living colonies. For the scanning electron microscopy (SEM) analysis, some portions of coral colonies, associated with the hydroids, were rinsed and gradually dehydrated in an ethanol gradient. Then all samples were dried in a critical point dryer, coated with gold–palladium in a Balzer Union evaporator and examined with a Philips XL20 SEM.
The histological examinations were conducted after resin inclusion. The samples were dehydrated in a graded ethanol series (one-day steps) then included in a cold-curing resin (Technovit 8100), and finally mounted on plastic supports. The sections (9 μm thick) obtained by a microtome were coloured with Toluidine blue, then analysed on a compound microscope.
Results
Description of the hydroid
Specimens recorded on the studied anthozoans were very similar in morphology, size, cnidome and number of tentacles (,B, 3). The lumen of the stem of all specimens appeared open in transverse sections, without parenchymatic and longitudinal endodermal canals (,D). Hydranths were vasiform, about 1 mm high, without an evident collar on the neck region. This region was white–yellow coloured, while the portion including aboral and oral tentacles was milky white (, inset, 2B, inset). They bore about 25 pseudofiliform aboral tentacles and about 15 filiform oral tentacles, both types arranged in one whorl. The hypostome was cone-shaped. Hydranths arose from short, erect hydrocauli, 1–4 cm high, with a diameter of about 0.3 mm, the perisarc surrounding stem and stolons were beige coloured.
Figure 2. Description of Ectopleura sp. A, solitary polyp of Ectopleura sp. emerging from a branchlet of Antipathella subpinnata. Note the antipatharian polyps irregularly arranged on the hydroid stem. Inset: SEM close-up of the hydranth. B, solitary polyp of Ectopleura sp. emerging from a branchlet of Eunicella cavolinii. Inset: Close-up of the hydranth. C, transverse section of a hydrocaulus (hy) surrounded by the black coral (bc). Spines are visible on the golden skeleton of the coral (sk), while no divisions are found in the lumen of the hydroid. D, transverse section of a hydrocaulus (hy) surrounded by the gorgonian tissues (go). The gorgonian skeleton (sk), surrounded by the coenosarc bearing sclerites, covers the perisarc (p). Immature gonophores of Ectopleura sp. found in A. subpinnata (E) and E. cavolinii (white arrows) (F). Scale bars: B, 2 mm. A, B inset, 1 mm. A inset, 0.5 mm. C,D,F, 0.2 mm. E, 50 μm.
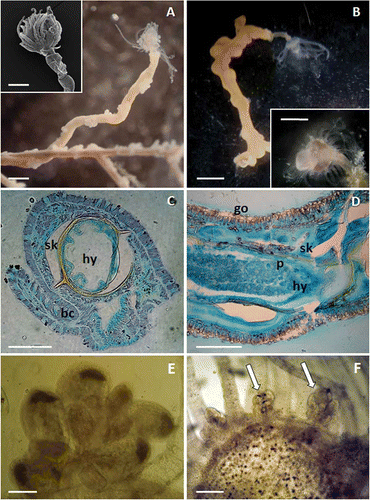
Only a few fertile polyps were observed. Fertile polyps found on the black coral showed immature gonophores just over the aboral tentacles characterised by a white, refringent distal extremity (). The fertile polyp found on E. cavolinii showed slightly larger gonophores, suggesting they were in an advanced stage of development ().
Three cnidocyst types were observed: (a) stenoteles of two sizes (8.5–9 × 7.5–8 μm and 7 ×6.7–7 μm), (b) desmonemes (6 × 5–5.5 μm) and (c) basitrichous isorhizas (8–11 × 5.5 μm). The cnidocysts were scattered in the polyp body and coenosarc, while they were concentrated in the tentacles.
Remarks
All the described tubulariids showed a stem with an open lumen, characteristic shared by both genera Ectopleura and Hybocodon (for recent descriptions see Bouillon et al. Citation2004; Schuchert Citation2010). However, in our specimens, unlike Hybocodon, the hydranths bore one whorl of oral filiform tentacles and lacked an evident collar on the neck region as described for Ectopleura. In the Mediterranean Sea, five species of Ectopleura and one of Hybocodon have been described: E. crocea (Agassiz, 1862), E. dumortierii (Van Beneden, 1844), E. larynx (Ellis and Solander, 1786), E. wrighti Petersen, 1979, E. sacculifera Kramp, 1957 (Bouillon et al. Citation2004), and Hybocodon prolifer L. Agassiz, 1862. Their distribution has been reported by Gravili et al. (Citation2008).
Hybocodon prolifer was typically found associated with sponges and, with some doubts, with Madrepora oculata in Mediterranean deep waters (Mastrototaro et al. 2009; Vertino et al. Citation2009). This species strongly differs from our specimens for the presence of about 50 short oral filiform tentacles in arranged two closely set whorls and about 30 longer aboral filiform tentacles arranged in one whorl (Bouillon et al. Citation2004). For these reasons we identify our hydroids as belonging to the genus Ectopleura. In the Mediterranean Sea, five species of Ectopleura are known: E. larynx and E. crocea are colonial hydroids, E. dumortierii is solitary, and E. wrighti is solitary or forms small colonies of a few polyps. The latter species differs from the other Mediterranean species by the presence of capitate or moniliform oral tentacles. The polyp stage of E. sacculifera is still unknown. The features of the hydroid described here agree with those of the solitary tubularid E. dumortierii, but a definitive specific attribution is impossible due to the lacking of mature reproductive structures.
Relationship with the hosts
The colonies of Antipathella subpinnata hosting Ectopleura sp. were bush-like, up to 30 cm high, with an almost planar arrangement of pseudo-pinnules (). They were collected from a mixed coral assemblage settled on a shoal of the St. Eufemia Gulf, including several gorgonian (Callogorgia verticillata (Pallas, 1766), Eunicella cavolinii, Paramuricea macrospina) and antipatharian (A. subpinnata, Antipathes dichotoma Pallas, 1766, and Parantipathes larix (Esper, 1790)) species. Sertulariid hydroids were occasionally observed living on the skeleton of dead branches, while the thinner branchlets and pinnules were spotted by numerous polyclad egg cocoons. Ectopleura sp. was settled on the living branches of the black corals (,C). The hydroid stems arose perpendicularly from the black coral branchlets () or showed a short basal hydrorhiza running along them (, ) that, however, never ramified within the coral's tissues. Some hydrocauli were fused with one or more coral branchlets. The hydroids were camouflaged when the hydranths were contracted, and were distinguishable from the antipatharian branches only by their slightly contorted shape (). As many as 15 Ectopleura sp. polyps were recorded on a single 30 cm high colony of A. subpinnata, indicating relatively high density.
Figure 4. Relationship of Ectopleura sp. with the hosts. A, colony of Antipathella subpinnata characterised by a lax, non-arborescent ramification system. Several foreign elements (both sediment and epibionts) are visible dispersed on the branches (while arrows). B, hydroid polyp emerging perpendicularly from a black coral branchlet. C, hydrocaulus running along the black coral branchlet. D, naked hydrocaulus of Ectopleura sp. settled on Paramuricea clavata. E,F, Ectopleura sp. polyps settled on Paramuricea macrospina. G, hydroid polyp emerging perpendicularly from a branchlet of Eunicella cavolinii. H, peeled portion of E. cavolinii showing the place of settlement of the hydrocaulus. J, portion of hydrocaulus (hy) surrounded by the gorgonin layer (sk). K, perisarc of Ectopleura sp. covered by the spiny skeleton of A. subpinnata. Along the hydrocaulus diameter decreases are visible as skeleton constrictions, indicating different growing stages of the hydroid. L, SEM enlargement of the transversal section adjacent to the area of contact between Ectopleura sp. and A. subpinnata: on the outer side the black coral coenenchyme and spiny skeleton (bc), on the inner side the perisarc and the coenosarc of the hydroid (hy). M, black coral branchlet (bc) hosting Ectopleura sp. (hy). Cylindrical, pointed, irregularly arranged spines are visible near the attachment area of the hydrocaulus. Scale bars: A, 5 cm. D, 2 mm. B,C,E–H,K,M, 1 mm. J, 0.5 mm. L, 10 μm.
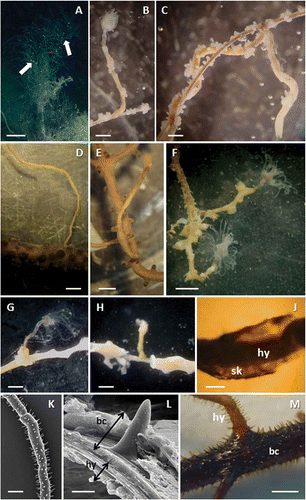
The colonies of E. cavolinii hosting Ectopleura sp. were collected on the top of two rocky cliffs (Secca dei Galli 88 m depth, Gulf of Salerno; Punta St. Angelo 48 m depth, Gulf of Naples) where the coral assemblages were mainly characterised by a mixed community of P. clavata. The density of Ectopleura sp. was lower than what was found on A. subpinnata, with four and one hydranths, respectively, for two colonies 30 cm tall. Moreover, two specimens, one of P. clavata and one of P. macrospina, hosting Ectopleura sp. were also recorded at 100–120 m depth in the Gulf of S. Eufemia (,E). In contrast to all other specimens, the hydrocaulus emerging from P. clavata was naked, with only the basal part surrounded by the gorgonian coenosarc (). In the case of both specimens of P. macrospina, as for A. subpinnata and E. cavolinii, the host's tissue instead covered most of the hydroid stem (,F). In no case was the hydrorhiza ramified under the coenosarc of the host.
In all the examined specimens, with the exception of P. clavata, the anthozoan reacted to the hydroid settling by depositing a thin skeletal layer (about 5 μm) along the entire length of the hydroid's perisarc leaving exposed only a small region below the hydranth (). The anthozoan skeleton was surrounded by the living coenenchyme bearing normal-sized polyps (,B, 4C,E,F). In the case of octocorals, the gorgonin skeleton almost completely surrounded the hydrocaulus (–J), which was reinforced by the overlying presence of sclerites. In the case of A. subpinnata, the coral skeleton was characterised by cylindrical, pointed spines (–M) covering the hydroid's perisarc, as happens for normal ramifications, and, occasionally, small coral branchlets. Spines were on average 0.11 mm high and 0.02 mm wide, as reported in the description of the species (Bo et al. Citation2008). This portion of the skeleton displayed a higher density of spines – on average 12 spines mm–1 – as well as several irregular aggregations of spines in the region near the attachment site of the hydroid ().
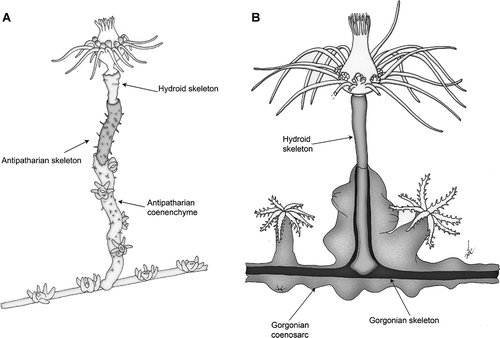
Figure 3. Scheme of the two associations. A, Ectopleura sp. on Antipathella subpinnata. B, Ectopleura sp. on Eunicella cavolinii. The distal portions of the hydrocauli have been represented as the coral tissue was removed to show the skeletons beneath.
The transverse section of the black coral branchlet near the basal region of the hydrocaulus showed the tubular perisarc of the hydroid surrounded by concentric layers of the antipatharian skeleton due to successive skeletal deposits. The basal extremity of the hydroid is settled on the coral skeleton in the proximity of the central canal, which is surrounded by layers of the oldest skeletal material.
Discussion
The previously described cases of hydroids associated with living portions of anthozoans involved a number of species (Puce et al. Citation2008a) (), of which six were tubulariids belonging to the genus Ralpharia. To these records must be added Hybocodon cfr. prolifer found on Madrepora oculata in Mediterranean deep waters (Mastrototaro et al. 2009; Vertino et al. Citation2009). The present study documents for the first time a species of Ectopleura establishing epibiotic associations with anthozoan corals. This increases the species of tubulariids involved in such relationships to eight. Within this family, there are several species reported in association with other organisms (Puce et al. Citation2005): Hybocodon includes species mainly associated with sponges and one associated with a scleractinian, Zyzzyzus includes only species associated with sponges and a further species of Ectopleura, E. exxona, lives associated with an unidentified sponge (Watson Citation1978). All of these evidences strongly suggest that the tubulariid evolution may have had a role in the possibility to establish symbiotic relationships with different benthic groups (Puce et al. Citation2008a).
In the Mediterranean Sea, the species of Ectopleura are reported to live predominantly on artificial substrates (Morri & Boero Citation1986; Genzano Citation2001; Zintzen et al. Citation2008a), especially in areas exposed to currents (Mullineaux & Garland Citation1993; Lemire & Bourget Citation1996). Since wrecks and other structures of anthropic origin, as well as deep coral assemblages, are sparse, the free-swimming stage of these organisms is hypothesised to have a high dispersal ability (Bacchiocchi & Airoldi Citation2003).
Hydroid dispersal may occur in different ways, including the release of jellyfish, planulae and actinulae or autotomy of floating portions (e.g. Wasserthal & Wasserthal Citation1973; Cornelius Citation1992; Pagliara et al. Citation2000). Tubulariids produce a particular post-embryonic stage known as actinula, exhibiting an ability for microhabitat selection (Nellis & Bourget Citation1996). We hypothesise that actinulae are able to colonise distant coral populations as a result of their notable dispersion ability (up to 10 km) (Zintzen et al. Citation2008b). The polyps of Ectopleura sp. were neither observed on other benthic organisms collected from the same study area nor on surrounding hard or soft substrates. Considering that the two studied localities, and in particular the S. Eufemia Gulf, are characterised by high sedimentation levels (Bo et al. in press), we suggest that the hydroid actinulae preferentially settle on large, erect anthozoans as a strategy to avoid siltation and enhance their survival chances. This also implies that actinulae should be immune to antifouling substances produced by the coral host. The result could be a long-lasting relationship, especially in the case of Antipathella subpinnata. Deep antipatharians, in fact, generally show slow growth rates (Roark et al. Citation2006); therefore, the fact that the hydroid was deeply settled within the black coral concentric skeletal layers suggests a long time of residence within the host.
It is likely that coral hosts do not suffer any particular damage from the presence of Ectopleura sp. polyps, while the hydroids gain the advantage of thickening and strengthening their perisarc. The thickening provided by the coral skeleton may also allow Ectopleura sp. polyps to extend the length of their stolons. Moreover, the coverage of skeleton and living tissue by the host may protect the hydroid from possible nudibranch attacks. In fact, these gastropods are known to perforate the perisarc in several species for feeding (Di Camillo et al. Citation2010). A similar situation was observed for Ralpharia neira (Petersen, Citation1990) found on the gorgonian Ellisella sp. and for Pteroclava krempfi (Billard, Citation1919) on Astrogorgia sp., both characterised by a hydrorhiza completely embedded in the coenosarc of their host (Puce et al. Citation2008a). In the case of R. neira, the juvenile settles on the gorgonian living tissue, which reacts by producing a kind of gall of coenosarc that envelops the base of the young polyp. Afterwards the coenenchyme grows along the hydrocaulus covering it up to the hydranth neck region (Puce et al. Citation2008a). In all observations, this area of the hydroid stem is always naked, suggesting that the growth of the hydroid is faster than that of the host.
Some species belonging to the genus Ectopleura form colonies made of several polyps and forming large tufts arising from a common hydrorhiza. Species associated with anthozoans, however, have always been reported as solitary, and probably this is also the case of Ectopleura sp., as put in evidence by the absence of ramified hydrorhiza under the host's tissues. The latter, growing around the hydroid perisarc, unable further growth of the stolons and development of a colony.
We observed that the coral tissues did not always cover the epibiotic hydrocauli, as in the case of Ectopleura sp. growing on P. clavata (). A possible mechanical constraint to the coverage of the hydrocaulus could be the size of the polyps of the coral host, as pointed out, for example, for parasitic zoanthids (Di Camillo et al. Citation2009). Thin ramifications, in fact, are unable to withstand large heavy polyps, without breaking. In our case, P. clavata, the gorgonian species showing the thickest branches and tallest polyps, is the only one not able to cover the hydrocaulus. This may represent a limit in the spreading of the hydroid on different anthozoans species.
The observation of Ectopleura sp. epibiotic on A. subpinnata is the first record of a tubulariid hydroid living associated with an antipatharian coral. The arborescent colonies of black corals offer substrate and refuge to a high number of organisms, and can therefore, be considered as centres for symbiotic relationships, both in tropical coral reefs (Molodtsova & Budaeva Citation2007; Tazioli et al. Citation2007) and in deep environments. Despite the fact that the majority of the sessile epibionts live on the dead portions of these corals, in some cases they are also able to interact with the living tissue of black corals inducing morphological modifications and rearrangements of parts of the colonies (Molodtsova & Budaeva Citation2007). In the associations described here, no traces of modifications were observed for the black coral skeleton covering the hydroid, except for a higher density and a more irregular arrangement of the spines along the hydrocaulus. In the case of black corals, the coverage of the perisarc could be enhanced by the chitinous component, which is similar in both skeletons. The presence of a higher number of Ectopleura sp. polyps on A. subpinnata than on gorgonians could be due to the health status of the coral colonies. Antipatharians generally do not host stable epibionts within their tissues, which are often covered by a dense mucous. In the Gulf of S. Eufemia the colonies, probably because of the heavy sedimentation, are not tall and arborescent as those observed in habitat of strong currents, such as near the Strait of Messina (Bo et al. Citation2009). The tissues are thin and damaged thus possibly favouring the settlement of actinulae on the underlying denuded skeleton.
Acknowledgements
The Calabrian exploration has been conducted by ISPRA (ex ICRAM), within the project no. 327 MoBioMarCal, and financed by the Calabrian Regional Council for Environment. The work undertaken through MoBioMarCal is affiliated to the European Census of Marine Life. The investigations conducted in Campania are part of the Red Coral project, financed by MATTM. Authors would like to thank the crew of R/V Astrea for their help during the field work.
References
- Bacchiocchi , F and Airoldi , L. 2003 . Distribution and dynamics of epibiota on hard structures for coastal protection . Estuarine and Coast Shelf Science , 56 : 1157 – 1166 .
- Bayer , F. 1961 . The shallow water Octocorallia of the West Indian region , Vol. 373 , Netherlands : Martinus Nijhoff .
- Billard , A. 1919 . Note sur une espèce nouvelle d'hydroide gymnoblastique (Clava krempfi), parasite d'un Alcyonaire . Bulletin du muséum national d'Histoire naturelle, Paris , 25 : 187 – 188 .
- Bo , M , Bavestrello , G , Canese , S , Giusti , M , Angiolillo , M , Cerrano , C , Salvati , E and Greco , S. Coral assemblage off the Calabrian Coast (South Italy) with new observations on living colonies of . Antipathes dichotoma. Italian Journal of Zoology , doi: doi: 10.1080/11250001003652619
- Bo , M , Bavestrello , G , Canese , S , Giusti , M , Salvati , E , Angiolillo , M and Greco , S. 2009 . Characteristics of a black coral meadow in the twilight zone of the central Mediterranean Sea . Marine Ecology Progress Series , 397 : 53 – 61 .
- Bo , M , Tazioli , S , Spanò , N and Bavestrello , G. 2008 . Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas . Italian Journal of Zoology , 75 : 185 – 195 .
- Boero , F. 1984 . The ecology of marine hydroids and effects of environmental factors . A review. Marine Ecology , 5 : 95 – 118 .
- Boero , F and Bouillon , J. 2005 . Cnidaria and Ctenophora (cnidarians and comb jellies). In: Rhode K, editor. Marine parasitology. Wallingford . CABI Publishing, CSIRO Publishing. , : 177 – 182 .
- Boero , F , Bouillon , J and Gravier-Bonnet , N. 1995 . The life cycle of Pteroclava krempfi (Cnidaria, Hydrozoa, Cladocorynidae), with notes on Asyncoryne philippina (Asyncorynidae) . Scientia Marina , 59 : 65 – 76 .
- Boero , F , Bouillon , J and Gravili , C. 2000 . A survey of Zanclea, Halocoryne and Zanclella (Cnidaria, Hydrozoa, Anthomedusae, Zancleidae) with a description of new species . Italian Journal of Zoology , 67 : 93 – 124 .
- Bouillon , J. 1967 . Révision de la famille des Ptilocodiidae avec la description d'un nouveau genre et d'une nouvelle espèce . Bulletin Académie royale de Belgique , 53 : 1106 – 1131 .
- Bouillon , J , Medel , MD , Pagès , F , Gili , JM , Boero , F and Gravili , C. 2004 . Fauna of the Mediterranean Hydrozoa . Scientia Marina , 68 : 1 – 449 .
- Carpine , C and Grasshoff , M. 1975 . Les gorgonaires de la Méditerranée . Bulletin de l'Institut océanographique, Monaco , 71 : 1 – 140 .
- Cornelius , PFS. 1992 . Medusae loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated lifecycles among their remote island faunae: An interim view . Scientia Marina , 56 : 245 – 261 .
- Coward , WE. 1909 . On Ptilocodium repens a new gymnoblastic hydroid epizoic on a Pennatulid . Verslagen der Zittingen van de Wis-en Natuurkundige Afdeeling der Koninklijke Akademie van Wetenschappen, Amsterdam, Section of Sciences , 11 : 635 – 641 .
- Di Camillo , CG , Bavestrello , G , Valisano , L and Puce , S. 2008 . Spatial and temporal distribution in a tropical hydroid assemblage . Journal of the Marine Biological Association of the United Kingdom , 88 : 1589 – 1599 .
- Di Camillo , CG , Bo , M , Puce , S and Bavestrello , G. 2009 . Association between Dentitheca habereri (Cnidaria: Hydrozoa) and two zoanthids . Italian Journal of Zoology , 77 : 81 – 91 .
- Di Camillo CG, Giordano G, Bo M, Betti F, Mori M, Puce S, Bavestrello G. 2010. Life history of Ectopleura crocea (Cnidaria: Hydrozoa) on a wreck in the North Adriatic Sea. 7th Workshop of the Hydrozoan Society, Symposium Life cycles and reproduction, September 2010, University of Salento. p. 27.
- Genzano , GN. 2001 . Associated fauna and sediment trapped by colonies of Tubularia crocea (Cnidaria, Hydrozoa) from the rocky intertidal of Mar del Plata, Argentina . Biociências , 9 : 105 – 119 .
- Gili , JM , Lopez-González , PJ and Bouillon , J. 2006 . A new Antarctic association: The case of the hydroid Sarsia medelae (new sp.) associated with gorgonians . Polar Biology , 29 : 624 – 631 .
- Goh , NKC , Ng , PKL and Chou , LM. 1999 . Notes on the shallow water gorgonian-associated fauna on coral reefs in Singapore . Bulletin of Marine Science , 65 : 259 – 282 .
- Gravili , C , Boero , F and Licandro , P. 2008 . Hydrozoa. in Checklist della flora e della fauna dei mari italiani (G. Relini) . Biologia Marina Mediterranea , 15 : 71 – 91 .
- Hirohito Emperor Showa . 1988 . “ Athecata ” . In The hydroids of Sagami Bay , 1 – 179 . Tokyo : Biological Laboratory Imperial Household .
- Korotneff , A. and de . 1887 . Zwei neue Coelenteraten . Zeitschrift fur Wissenschaftliche Zoologie , 45 : 468 – 490 .
- Lemire , M and Bourget , E. 1996 . Substratum heterogeneity and complexity Influences micro-habitat selection of Balanus sp. and Tubularia crocea larvae . Marine Ecology Progress Series , 135 : 77 – 87 .
- Linskens , HF. 1963 . Oberflächenspannung an marinen Algen . Verhandelingen der Koninklijke Nederlandse Akademie van Vetenschappen , 66 : 205 – 217 .
- Love , MS , Yoklavich , MM , Black , BA and Andrews , AH. 2007 . Age of black coral (Antipathes dendrochristos) colonies, with notes on associated invertebrate species . Bulletin of Marine Science , 80 : 391 – 400 .
- Mastrototaro , F , D'Onghia , G , Corriero , G , Matarrese , A , Maiorano , P , Panetta , P , Gherardi , M , Longo , C , Rosso , A Sciuto , F . 2010 . Biodiversity of the white coral and sponge community off Cape Santa Maria di Leuca (Mediterranean Sea) . Deep Sea Research II , 57 : 412 – 430 .
- Millard , NAH. 1975 . Monograph on the Hydroida of southern Africa . Annals of the South African Museum , 68 : 1 – 513 .
- Millard , NAH and Bouillon , J. 1973 . Hydroids from the Seychelles (Coelenterata) . Annales du Musée Royale de l'Afrique Centrale, Sciences Zoologiques , 206 : 1 – 106 .
- Molodtsova , T and Budaeva , N. 2007 . Modifications of corallum morphology in black corals as an effect of associated fauna . Bulletin of Marine Science , 81 : 469 – 479 .
- Morri , C and Boero , F. 1986 . “ Marine fouling hydroids ” . In Catalogue of the main marine fouling organisms, 7, Hydroids. Comité international permanent pour la recherche sur la préservation de matériaux en milieu marin. Office d’études marines et atmosphériques 1 – 91 . Belgium
- Mullineaux , LS and Garland , ED. 1993 . Larval recruitment in response to manipulated field flows . Marine Biology , 116 : 667 – 683 .
- Nellis , P and Bourget , E. 1996 . Influence of physical and chemical factors on settlement and recruitment of the hydroid Tubularia larynx: Field and laboratory experiments . Marine Ecology Progress Series , 140 : 123 – 139 .
- Opresko , DM. 2001 . Revision of the Antipatharia (Cnidaria: Anthozoa) . Part I. Establishment of a new family, Myriopathidae. Zoologische Mededelingen Leiden , 75 : 343 – 370 .
- Oswald , RC and Seed , R. 1986 . Organization progression within the epifaunal communities macroalgae . Cahiers de Biologie Marine , 27 : 29 – 40 .
- Pagliara , P , Bouillon , J and Boero , F. 2000 . Photosynthetic planulae and planktonic hydroids: Contrasting strategies of propagule survival. In: Mills CE, Boero F, Migotto A, Gili JM . Trends in hydrozoan biology. Scientia Marina , 64 : 173 – 178 .
- Pantos , O and Bythell , JC. 2010 . A novel reef coral symbiosis . Coral Reefs , 29 : 761 – 770 .
- Petersen , KW. 1990 . Evolution and taxonomy in capitate hydroids and medusae . Zoological Journal of the Linnean Society , 100 : 101 – 231 .
- Puce , S , Calcinai , B , Bavestrello , G , Cerrano , C , Gravili , C and Boero , F. 2005 . Hydrozoa (Cnidaria) symbiotic with Porifera: A review . Marine Ecology , 26 : 73 – 81 .
- Puce , S , Cerrano , C , Di Camillo , CG and Bavestrello , G. 2008a . Hydroidomedusae (Cnidaria: Hydrozoa) symbiotic radiation . Journal of the Marine Biological Association of the United Kingdom , 88 : 1715 – 1721 .
- Puce , S , Di Camillo , CG and Bavestrello , G. 2008b . Hydroids symbiotic with octocorals from Sulawesi Sea, Indonesia . Journal of the Marine Biological Association of the United Kingdom , 88 : 1643 – 1654 .
- Riedl , R. 1966 . Biologie der Meereshohlen , 639 Hamburg und Berlin : Paul Parey .
- Roark , EB , Guilderson , TP , Dunbar , RB and Ingram , BL. 2006 . Radiocarbon based ages and growth rates: Hawaiian deep-sea corals . Marine Ecology Progress Series , 327 : 1 – 14 .
- Schuchert , P. 2010 . The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Capitata part 2 . Revue Suisse de Zoologie , 117 : 337 – 555 .
- Silveira , FL da and Migotto , AE . 1984 . Serehyba sanctisebastiani n. gen., n. sp. (Hydrozoa, Tubulariidae), symbiont of a gorgonian octocoral from the southeast coast of Brazil . gBijdragen Tot De Dierkunde , 54 : 231 – 242 .
- Stechow , E. 1909 . Beiträge zur Naturgeschichte Ostasiens: hydroid-polypen der japanischen Ostküste. Abhandlungen Kgl . Bayerischen Akademie der Wissenschaften , 1 : 1 – 111 .
- Tazioli , S , Bo , M , Boyer , M , Rotinsulu , H and Bavestrello , G. 2007 . Ecology of some common antipatharians from the Marine Park of Bunaken (North Sulawesi, Indonesia) . Zoological Studies , 46 : 227 – 241 .
- Vertino , A , Savini , A , Rosso , A , Di Geronimo , I , Mastrototaro , F , Sanfilippo , R , Gay , G and Etiope , G. 2009 . Benthic habitat characterization and distribution from two representative sites of the deep-water SML Coral Mound Province (Mediterranean) . Deep Sea Research II , 57 : 380 – 396 .
- Wasserthal , LT and Wasserthal , W. 1973 . Ökologische Bedeutung der Schleimsekretion bei den Planula-larven der Hydroidengattung Eudendrium . Marine Biology , 22 : 341 – 345 .
- Watson , JE. 1978 . New species and new records of Australian athecate hydroids . Proceedings of the Royal Society of Victoria , 90 : 301 – 314 .
- Watson , JE. 1980 . The identity of two tubularian hydroids from Australia with a description and observations on the reproduction of Ralpharia magnifica gen. et sp. nov . Memoirs of the National Museum of Victoria , 41 : 53 – 63 .
- Watson , JE. 1984 . Two new species of tubularian hydroids from southern Australia . Memoirs of the National Museum of Victoria , 45 : 7 – 12 .
- Zea , S. 1993 . Recruitment of demosponges (Porifera, Demospongiae) in rocky and coral reef habitats of Santa Marta, Colombian Caribbean . Marine Ecology , 14 : 1 – 21 .
- Zintzen , V , Norro , A , Massin , C and Mallefet , J. 2008a . Spatial variability of epifaunal communities from artificial habitat: Shipwrecks in the Southern Bight of the North Sea . Estuarine and Coast Shelf Science , 76 : 327 – 344 .
- Zintzen , V , Norro , A , Massin , C and Mallefet , J. 2008b . Temporal variation of Tubularia indivisa (Cnidaria, Tubulariidae) and associated epizoites on artificial habitat communities in the North Sea . Marine Biology , 153 : 405 – 420 .