Abstract
Investigation of trout (subf. Salmoninae) for their continuous osteological characters of head that included analyses of elements from skull and visceral (jaws, gills and gill covers) bones revealed the unambiguous distinction between the autochthonous and admixed brown trout Salmo trutta stocks of the Danube River drainage area in Serbia for the characters on vomer, skull height, pterotic and premaxilar bones. Rainbow trout Oncorhynchus mykiss and Ohrid Lake's belvica trout Salmo (Salmothymus) ohridanus were also examined as outgroup taxa in order to understand better the overall variability and to reduce bias in the methodology applied. Belvica trout were more similar to the admixed than to the autochthonous brown trout populations, especially concerning the characters on the bones of the skull. Rainbow trout appeared clearly distinct from all brown trout (autochthonous and admixed) on their elements of skull (its height and width at the sphenotic level), being similar to the autochthonous brown trout on the elements of visceral skeleton, whereas on the elements of visceral skeleton, belvica trout were the most distinct from the brown trout and the rainbow trout, mainly due to the difference in the length of the premaxilar and maxilar bones.
Introduction
Phylogenetic relationships within the family Salmonidae were primarily studied upon the sets of morphological (primarily osteological) characters, and recently by molecular genetic characters. Results of phylogenetic analysis based on morphological (Norden Citation1961; Shaposhnikova Citation1975; Kendall & Behnke Citation1984; Dorofeeva Citation1989; Sanford Citation1990; Stearley & Smith Citation1993; Glubokovsky Citation1995; Simonović et al. Citation2005, 2007) and molecular genetic data (Ferguson & Fleming Citation1983; Grewe et al. Citation1990; Phillips & Pleyte Citation1991; McKay et al. Citation1996; Shed'ko et al. Citation1996; Oleynik Citation1997; Phillips & Oakley Citation1997; Oakley & Phillips Citation1999; Phillips et al. Citation2000; Snoj et al. Citation2002, Snoj Citation2003; Crespi & Fulton Citation2004) are conflicting. The majority of authors agree that the existing uncertainties regarding phylogeny of genera and species of the family Salmonidae could be resolved only by combining the results obtained through morphological and molecular genetic analyses (Bernatchez et al. Citation1992; Delling Citation2003). Morphological studies, namely the external morphology, as well as the osteological ones, are considered as the main sources for analyses of phylogenetic relationships, regardless of the classification level (Klyukanov Citation1969, Citation1970, Citation1975; Shaposhnikova Citation1975) aiming to determine the status of traits and their frequencies in studied taxa. The individual states of characteristics from the phylogenetic aspect were resolved, i.e. whether their state in particular salmonid taxa are ancestral (plesiomorphic), or derived (apomorphic) sensu Hennig (Citation1966). Reconstruction of phylogenetic relationships among genera and species that leads to the setting of the stable classification in the family Salmonidae is important for conservation of biodiversity, since many species of the family are considered threatened (Crivelli Citation1996).
According to analyses of the osteological characters, three subfamilies: Salmoninae, Coregoninae and Thymallinae were classified into the family Salmonidae (Shaposhnikova Citation1975). The research accomplished on the six genera Salvelinus, Hucho, and Brachymystax of the subfamily Salmoninae and Stenodus, Prosopium and Coregonus of the subfamily Coregoninae, raised the knowledge about osteological characters important for their taxonomy at the generic level.
Ivanović et al. (Citation2009) suggested that the skull, or parts of it, represents developmentally and functionally complex morphological structures, which has been frequently used to estimate the strength of the phylogenetic signal in morphological data. Both Sanford (Citation1990) and Dorofeeva (Citation1991, Citation1998) reconstructed phylogeny and considered taxonomic status and phylogeography of salmonid fishes of the genus Salmo on analysis of osteological characters, which emphasizes a leading role of osteological characters in salmonid systematics.
The problem of identification of autochthonous and allochthonous populations of trout is increasingly popular in research. Several studies (Mořan et al. Citation1989; Lahnsteiner & Jagsch Citation2005; Sušnik et al. Citation2006; Razpet et al. Citation2007) showed that the most reliable results were obtained by combining morphological and genetic methods. Marić et al. (2004) revealed that certain longitudinal characters on the dorsal side of the head (dorsal head length) and body (predorsal body length without the head, as well as the distance between the end of base of dorsal and the beginning of base of adipose fins) and vertical distance between the end of base of the dorsal fin and beginning of base of the anal fin can serve to distinguish indigenous brown trout Salmo trutta populations from those subject to fish stocking in the past period. On the characteristics of external morphology, the a-priori drawn hypothesis that two brown trout populations (Godljevačka River and Buk Stream) in the Danube River drainage area of Serbia are autochthonous was confirmed, whereas other populations were admixed by stocking with the allochthonous material (Marić et al. Citation2004). The preliminary genetic data also revealed that brown trout on those two locations are autochthonous (Marić et al. 2006). The results obtained in the investigation of external morphology (Marić et al. 2004) served to set the main goal of this study to define diagnostic osteological characters of the head and to verify their applicability for distinguishing autochthonous and admixed populations of the brown trout in the Danube River drainage area of Serbia.
Materials and methods
Sample collection
Samples of trout (n = 87) were collected between May 1996 and October 1999. Sampling was carried out at sites in FYR of Macedonia (belvica trout Salmo (Salmothymus) ohridanus in the Adriatic Sea basin in the Lake Ohrid) and in Serbia (brown trout Salmo trutta and rainbow trout Oncorhynchus mykiss in streams and rivers of the Danube River drainage area) (). Most fish were caught by angling, the rest by electro-fishing.
Table I. Samples of trout taxa. Asterisks represent non-stocked locations
Preparation of samples for analysis
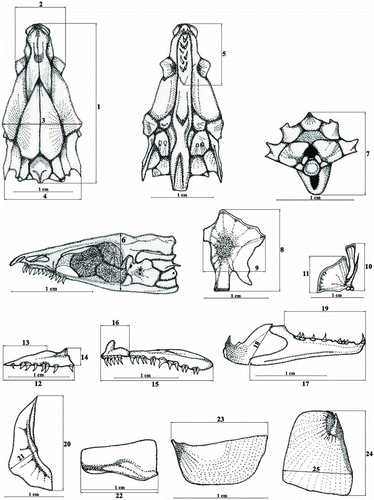
Preparation of samples for analysis included decapitation of specimens, drying of heads and removal of flesh and brain tissues with necrophagous insects (Dermestres lardarius). The head skeletons were then further cleaned and disassembled into two complements of bones. The skeleton of the skull (comprising the neurocranium and dermal bones of the roof and the base of skull) was processed completely, while the bones supporting the jaws, mouth, gills and gill covers (which includes the viscerocranium and the remaining bones of the dermatocranium), to which we refer further on as visceral bones, were disassembled into particular elements.
Analysed characters
Twenty-five morphometric traits were used; 7 were on the skull (1, Lcranium – the length of the skull from the crest of supraoccipital to the top of nasal; 2, Wexeth – the width of the skull at the level of exoethmoid; 3, Wsphen – the width of the skull at the level of sphenotic; 4, Wptero – the width of the skull at the level of pterotic; 5, Lvom – the length of vomer; 6, H1 – the height of skull at the level of joint between parasphenoid and basioccipital; 7, H2 – height of the skull at the level between the top of the crest of the supraoccipital and posterior part of the basioccipital), and 18 were characters of the visceral bones (8, Lhyo – the length of hyomandibular; 9, Whyo – the width of hyomandibular; 10, Lqua – the length of quadrate; 11, Wqua – the width of quadrate; 12, Lmax – the length of maxilla; 13, Lnmax – the length of downward distance of maxilla; 14, Smax – the width of maxilla; 15, Lpmax – the length of praemaxilla; 16, Lpdpmax – the length of the anterior part of praemaxilla; 17, Lda – the length of angular + dental; 18, Wdaa – the width of lower jaw; 19, Ldd – the length of dental – part covered with teeth; 20, Lprop -–the length of praeopercular; 21, Wprop – the width of praeopercular; 22, Linop – the length of interopercular; 23, Lsubop – the length of subopercular; 24, Loper – the length of opercular; 25, Woper – the width of opercular) ().
In the cases of bilateral traits, only measurements of the right side of the head skeleton were taken, except in cases of loss or damage of certain elements. An adjustable digital caliper was used for measuring.
The morphometric data were tested for normality of distribution by Kolmogorov–Smirnov normality test (Lilliefors Citation1967) and analysed with multivariate and univariate statistical methods.
All analysed morphometric traits were size-corrected by using PCA scores on correlation matrices for all specimens in order to avoid body-size biases on variability as much as possible. The morphometric data set of PCA scores was analysed by MANOVA (Sneath & Sokal Citation1973) that tested the significance of difference in morphological variability among nominal taxa as well as among stocks of brown trout samples. Canonical Discriminant Analysis on PCA scores was also used in order to define multivariate morphological difference between a-priori groups (Manly Citation1986), while UPGMA Cluster Analysis (Sneath & Sokal Citation1973) on Mahalanobis D 2 distances (Sokal & Rohlf Citation1995) was used for grouping the most similar groups and populations.
The discriminating power of the most discriminative morphometric characters was a-posteriori tested univariately for the statistical significance by using ANOVA and Scheffe post-hoc test (Sokal & Rohlf Citation1995).
STATISTICA v. 5.1 for Windows 95 was used for statistical analyses (Statsoft Inc. Citation1997).
Results
Kolmogorov–Smirnov test showed the normal distribution of data for all characters of skull and visceral bones (values of range 0.055–0.25, Lillefors P > 0.05).
Significant differences for characters of skull were shown also by MANOVA, among belvica, rainbow and brown trout (Wilks λ = 0.46, P = 5.32, P < 0.001) as well as among different populations of the brown trout (Wilks λ = 0.58, P = 6.42, P < 0.001). In addition, the significant differences in characters of visceral bones were shown for both nominal taxa (Wilks λ = 0.46, P = 1.79) and stocks of brown trout samples (Wilks λ = 0.44, P = 3.64, P < 0.001).
Discriminant Canonical Analysis of samples from different localities showed that five characters (Lcranium, Wsphen, Lvom, H1 and H2) out of seven analysed characters of skull had strong discriminating power; while the results of analysed trout groups (belvica trout, rainbow trout and brown trout – autochthonous and admixed), emphasized six discriminative characters (Lcranium, Wptero, Wsphen, Lvom, H1 and H2). In addition, Discriminant Canonical Analysis of samples from different localities indicated seven characters (Whyo, Lqua, Lpmax, Lda, Wdaa, Ldd and Lprop) of strong discriminating power (out of set of 18 characters of visceral bones). Regarding trout groups, four characters from complements of visceral bones were discriminative (Lmax, Lnmax, Lpmax and Wdaa).
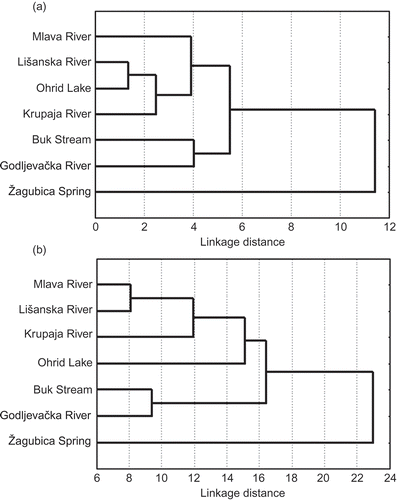
The Mahalanobis D 2 distances as a measure of difference among different populations of brown trout and nominal taxa (characters of skull and visceral bones) revealed that samples of autochthonous brown trout (collected from Godljevačka River and Buk Stream) were the most similar. The samples from the populations containing the stocked material of brown trout (Lišanska, Mlava and Krupaja Rivers) were clustered separately ().
Figure 2. Phenogram of relationship among different populations based on UPGMA Cluster Analysis of Squared Mahalanobis Distances (characters of skull (a) and visceral bones (b) ).
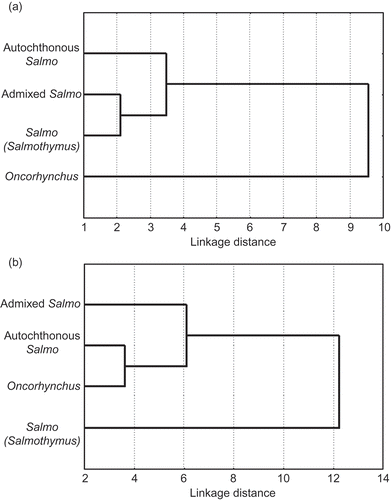
The phenetic differentiation among particular groups of salmonids (brown, rainbow and belvica trout) based on characters of skull showed that rainbow trout genus Oncorhynchus was clearly discriminated from all other analysed groups; the most similar groups were the belvica trout and group of admixed brown trout populations, phenetically similar to populations with the autochthonous brown trout (). The phenogram of the studied groups upon the characters of visceral bones showed that the belvica trout was clearly discriminated from the rainbow trout and the autochthonous group of brown trout populations, which made the distinct group linked to the phenetically similar group of admixed populations of brown trout ().
Figure 3. Phenogram of relationship among different groups based on UPGMA Cluster Analysis of Squared Mahalanobis Distances (characters of skull (a) and visceral bones (b)).
The statistical significance of the most discriminative morphometric characters (skull and visceral bones) among groups was proved with ANOVA tests ( and ).
Table II. ANOVA tests between analysed groups of salmonids (discriminative characters of skull)
Table III. ANOVA tests between groups of salmonids (discriminative characters of visceral bones)
The post-hoc Scheffe test for discriminative characters clarified that all characters of skull and all characters of visceral bones, except Lpmax (it was on the limited value), can be used for distinguishing between indigenous and non-indigenous populations ( and ).
Table IV. Post-hoc tests (Scheffe test) among the brown trout groups (discriminative characters of skull)
Table V. Post-hoc tests (Scheffe test) among the brown trout groups (discriminative characters of visceral bones)
Discussion
Results obtained on characters of skull and visceral bones () were completely identical with those obtained using the characters of external morphology, which is in accordance with a-priori set hypothesis that samples from the locality Godljevačka River and Buk Stream belong to autochthonous populations of the brown trout, while the other analysed brown trout populations were stocked (Marić et al. Citation2004). Analysis of mtDNA in brown trout from Serbia revealed that the Da-s1 haplotype, which was discovered in brown trout at localities Godljevačka River and Buk Stream, as well, is the most frequent autochthonous brown trout haplotype in the Danube River drainage area of Serbia (Marić et al. Citation2006). The genetic structure of brown trout from the rest of the localities where the stocking was accomplished has not been studied so far. However, it is most probable that they were stocked with material of Atlantic lineage, already recorded at two localities in Serbia (Marić et al. Citation2006) and many others in the region (Povž et al. Citation1996; Razpet et al. Citation2007).
Characters of skull and visceral bones considered as the most significant for the discrimination of autochthonous brown trout populations from the admixed were: Lvom, H1, Wptero, Lcranium and Lpmax. The most distinguishing characters were those of the skull (i.e. neurocranium) (all except character Lpmax), which corresponded to the strong phylogenetic conservativeness of that part of the head skeleton (Cardini & Elton Citation2008; Ivanović et al. Citation2009).
At the generic level, characters Lcranium and H2 clearly discriminated the belvica trout with its proportionally shorter skull, higher in its rear part, while the rainbow trout with its narrower and higher front part of the skull was different in characters H1 and Ssphen when comparing with the other two trout taxa. Regarding the characters of visceral bones, the belvica trout was discriminated by characters Lpmax and Lmax, having in total the shorter upper jaw than other trout taxa, probably due to its specialization to the planktivorous feeding niche in the lake habitat. Character Lpmax also discriminated the group of autochthonous brown trout with its proportionally shorter upper jaw from the group of admixed populations and from the rainbow trout. The rainbow trout was different in characters Lnmax and Ldaa describing the greater longitudinal dimension in the rear part of both upper and lower jaws, respectively, when compared with the other two trout taxa.
The set of skull characters clearly separated the rainbow trout genus and group of autochthonous brown trout populations (). The group of admixed brown trout populations was grouped with the belvica trout, which is concordant both to the recent genetic studies that proposed revision of the former genus Salmothymus (Snoj et al. Citation2002) and to the close relationship reconstructed by Simonović et al. (Citation2007), using the characters of external morphology. Phenetical analyses of different groups based on character of visceral bones () showed the separation of the belvica trout, as well as the separation of admixed brown trout populations. Autochthonous brown trout populations grouped unexpectedly with the rainbow trout. The cause of those relationships might be either the much smaller sample size (n = 5) of belvica trout in comparison with those of other trout species, the difference in feeding habits occurring between, for example, planktivorous belvica and benthivorous brown trout (Liem Citation1993; Westneat et al. Citation2005), or a lesser stability of the characters of visceral bones in relation to the characters of the neurocranium (Cardini & Elton, Citation2008; Ivanović et al. Citation2009), probably due to their greater exposure to the selective pressure and adaptive responses occurring in those regions of the head skeleton.
Both multivariate (MANOVA) and univariate (ANOVA) analysis of variance of characters of skull and visceral bones among the studied groups showed significant differences, implying that the remarkable amount of overall variability occurs in the sample. However, results of post-hoc (Scheffe) tests showed a significant amount of differences occurring only between autochthonous and admixed populations, but not among trout taxa (e.g. between rainbow and belvica trout), probably because of their resolved derived character in the subfamily Salmoninae (Dorofeeva Citation1989; Phillips et al. Citation2000).
The morphological and osteological differentiation between the admixed and indigenous brown trout populations can be used for the conservation of autochthonous populations, especially if future comprehensive research on the molecular genetic level confirms whether the diagnosed morphological differences between autochthonous and admixed populations of brown trout are genetically determined.
Acknowledgements
This work was supported by Ministry of Science and Technological Development of the Republic of Serbia (Grant No. 173025).
References
- Bernatchez , L , Guyomard , R and Bonhomme , F. 1992 . DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout Salmo trutta populations . Molecular Ecology , 1 : 161 – 173 .
- Cardini , A and Elton , S. 2008 . Does the skull carry a phylogenetic signal? Evolution and modularity in the guenons . Biological Journal of the Linnean Society , 93 : 813 – 834 .
- Crespi , BJ and Fulton , MJ. 2004 . Molecular systematics of Salmonidae: Combined nuclear data yields a robust phylogeny . Molecular Phylogenetics and Evolution , 31 : 658 – 679 .
- Crivelli , AJ. 1996 . The freshwater fish endemic to the northern Mediterranean region . Le Sambuc, Arles, France. , : 171
- Delling , B. 2003 . Species diversity and phylogeny of Salmo with emphasis on southern trouts (Teleostei, Salmonidae) . PhD thesis. Department of Zoology, Stockholm University. , : 142
- Dorofeeva , EA. 1989 . “ The basic principles of classification and phylogeny of the salmonid fishes (Salmoniformes, Salmonoidei, Salmonidae) ” . In Biology and phylogeny of fishes. St. Petersburg , Edited by: Korovina , VM . 5 – 16 . in Russian : Proceedings of Zoologikal Institute, USSR Academy of Sciences .
- Dorofeeva , EA. 1991 . Osteological features of the endemic Balkan marbled trout, Salmo marmoratus Cuv. (Salmonidae) . Journal of Ichthyology , 31 : 113 – 121 .
- Dorofeeva , EA. 1998 . Systematics and distribution history of European salmonid fishes of the genus . Journal of Ichthyology , 38 : 419 – 429 .
- Ferguson , A and Fleming , CC. 1983 . “ Evolutionary and taxonomic significance of protein variation in the brown trout (Salmo trutta L.) and other salmonid fishes ” . In Protein polymorphism: Adaptive and taxonomic significance , Edited by: Oxford , GS and Rollinson , D . 84 – 99 . London : Academic Press .
- Glubokovsky , MK. 1995 . Evolutionary biology of Salmonid fishes , Moscow : Nauka (in Russian) .
- Grewe , PM , Billington , N and Hebert , PDN. 1990 . Phylogenetic relationships among members of Salvelinus inferred from mitochondrial DNA divergence . Canadian Journal of Fisheries and Aquatic Sciences , 47 : 984 – 991 .
- Hennig , W. 1966 . Phylogenetic systematics , 263 Urbana, IL : University of Illinois Press .
- Ivanović , A , Sotiropoulos , K , Džukić , G and Kalezić , LM. 2009 . Skull size and shape variation versus molecular phylogeny: A case study of alpine newts (Mesotriton alpestris, Salamandridae) from the Balkan Peninsula . Zoomorphology , 128 : 157 – 167 .
- Kendall , AW and Behnke , RJ. 1984 . Salmonidae: Developments and relationships. In: Moser HG, editor. Ontogeny and systematics of fishes . American Society of Ichthyologysts and Herpetologists Special Publication , 1 : 142 – 149 .
- Klyukanov , VA. 1969 . Morphological bases of classification of smelts of the genus Osmerus (Osmeridae) . Zoologicheskii Zhurnal , 48 : 99 – 109 .
- Klyukanov , VA. 1970 . Morphological basis of the classification of smelts of the genus Hypomesus . Zoologicheskii Zhurnal , 49 : 1534 – 1542 .
- Klyukanov , VA. 1975 . The systematic position of the Osmeridae in the order Salmoniformes . Journal of Ichthyology , 15 : 1 – 17 .
- Lahnsteiner , F and Jagsch , A. 2005 . Changes in phenotype and genotype of Austrian Salmo trutta populations during the last century . Environmental Biology of Fishes , 74 : 51 – 65 .
- Liem , KF. 1993 . “ Ecomorphology of the teleostean skull ” . In The skull , Edited by: Hanken , J and Hall , BK . Vol. 3 , 422 – 452 . Chicago, IL : University of Chicago Press . Functional and evolutionary mechanisms
- Lilliefors , HW. 1967 . On the Kolmogorov–Smirnov test for normality with mean and variance unknown . Journal of the American Statistical Association , 64 : 399 – 402 .
- Manly , FJB. 1986 . Multivariate statistical methods – A primer , New York, NY : Chapman & Hall .
- Marić , S , Nikolić , V and Simnović , P. 2004 . Pilot study on the morphological identity of wild brown trout (Salmo trutta) stocks in the streams of the Danube river basin (Serbia) . Folia Zoologica , 53 : 411 – 416 .
- Marić , S , Sušnik , S , Simonović , P and Snoj , A. 2006 . Phylogeographic study of brown trout from Serbia, based on mitochondrial DNA control region analysis . Genetics, Selection, Evolution , 38 : 411 – 430 .
- McKay , SJ , Devlin , RH and Smith , MJ. 1996 . Phylogeny of Pacific salmon and trout based on growth hormone type-2 and mitochondrial NADH dehydrogenase subunit 3 DNA sequences . Canadian Journal of Fisheries and Aquatic Sciences , 53 : 1156 – 1176 .
- Mořan , P , Pendás , AM , García-Vázquez , E and Linde , AR. 1989 . Chromosomal and morphological analysis of two populations of Salmo trutta sbp . fario employed in repopulation. Journal of Fish Biology , 35 : 839 – 843 .
- Norden , CR. 1961 . Comparative osteology of representative salmonid fish with particular reference to the grayling (Thymallus arcticus) and phylogeny . Journal of the Fisheries Research Board of Canada , 18 : 679 – 791 .
- Oakley , TH and Phillips , RB. 1999 . Phylogeny of salmonine fishes based on growth hormone introns: Atlantic (Salmo) and Pacific (Oncorhynchus) salmon are not sister taxa . Molecular Phylogenetics and Evolution , 11 : 381 – 393 .
- Oleynik , AG. 1997 . Molecular phylogeny of salmonid fishes: Concordance of results from nuclear and mitochondrial DNA analyses . Genetika , 33 : 229 – 234 . in Russian
- Phillips , RB , Matsuoka , MP , Konon , I and Reed , KM. 2000 . Phylogenetic analysis of mitochondrial and nuclear sequences supports inclusion of Acantholingua ochridana in the genus Salmo . Copeia , 2 : 546 – 550 .
- Phillips , RB and Oaakley , TH. 1997 . “ Phylogenetics relationships among the Salmoninae based on nuclear and mitochondrial DNA sequences ” . In Molecular systematics of fishes , Edited by: Kocher , TD and Stepien , CA . 145 – 162 . San Diego, CA : Academic Press .
- Phillips , RB and Pleyte , KA. 1991 . Nuclear DNA and salmonid phylogenetics . Journal of Fish Biology , 39 ( Suppl. A ) : 259 – 275 .
- Povž , M , Jesenšek , D , Berrebi , P and Crivelli , A. 1996 . The marble trout, Salmo trutta marmoratus, Cuvier 1817, in the Soča River basin, Slovenia . La Tour du Valat, Le Sambuc , : 65
- Razpet , A , Marić , S , Parapot , T , Nikolić , V. and Simonović , P. 2007 . Re-evaluation of Salmo data by Gridelli (1936) – Description of stocking, hybridization and repopulation in the River Soča basin . Italian Journal of Zoology , 74 : 63 – 70 .
- Sanford , CPJ. 1990 . The phylogenetic relationship of salmonid fishes . Bulletin of the British Museum of Natural History (Zoology) , 56 : 145 – 153 .
- Shaposhnikova , GCH. 1975 . Systematic relation of some representatives of the family Salmonidae . Ichthyologia , 7 : 61 – 70 .
- Shed'ko , SV , Ginatulina , LK , Parpura , IZ. and Ermolenko , AV. 1996 . Evolutionary and taxonomic relationships among Far-Eastern salmonid fishes inferred from mitochondrial DNA divergence . Journal of Fish Biology , 49 : 815 – 829 .
- Simonović , P , Marić , S and Nikolić , V. 2005 . Morphological differentiation of salmonines (subfamily Salmoninae) with emphasis on trout Salmo spp . stocks in Serbia and adjacent regions. Acta Zoologica Bulgarica , 57 : 341 – 362 .
- Simonović , P , Marić , S and Nikolić , V. 2007 . Trout Salmo spp. complex in Serbia and adjacent regions of western Balkans: Reconstruction of evolutionary history from external morphology . Journal of Fish Biology 70 (Supplement C) , : 359 – 380 .
- Sneath , PHA and Sokal , RR. 1973 . Numerical taxonomy , San Francisco, CA : W.H. Freeman .
- Snoj , A. 2003 . Molecular phylogeny of ‘archaic trouts’ and their taxonomic classification. 3rd congress of the genetics society of Slovenija, Bled . Book of Abstracts. , : 73 – 74 .
- Snoj , A , Melkič , E , Sušnik , S , Muhamedagić , S and Dovč , P. 2002 . DNA phylogeny supports revised classification of Salmothymus obtusirostris . Biological Journal of the Linnean Society , 77 : 399 – 411 .
- Sokal , R and Rohlf , FJ. 1995 . “ Biometry ” . In The principles and practice of statistics in biological research , 776 New York, NY : W.H. Freeman & Comp .
- Statsoft Inc. 1997. Statistica for Windows (computer program manual). Tulsa, OK: Statsoft Inc. http://www.statsoft.com (http://www.statsoft.com) (Accessed: 18 November 2004 ).
- Stearley , RF and Smith , GR. 1993 . Phylogeny of the Pacific trouts and salmons (Oncorhynchus) and genera of the family Salmonidae . Transactions of the American Fisheries Society , 122 : 122 – 133 .
- Sušnik , S , Knizhin , I , Snoj , A and Weiss , S. 2006 . Genetic and morphological characterization of a Lake Ohrid endemic, Salmo (Acantholingua) ohridanus with a comparison to sympatric Salmo trutta . Journal of Fish Biology 68 (Supplement A) , : 2 – 23 .
- Westneat , MW , Alfaro , ME , Wainwright , PC , Bellwood , DR , Grubich , JR Fessler , JL . 2005 . Phylogenetic relationships and evolutionary history of the reef fish family Labridae . Molecular Phylogenetics and Evolution , 36 : 370 – 390 .