Abstract
A new species of Uncispio Green, Citation1982 is described from a boulder clay habitat in the southern Irish Sea. Uncispio reesi n. sp. can be separated from Uncispio hartmanae Green, Citation1982 by the size and shape of the occipital antenna, the arrangement of the posterior parapodial lobes, the number of anal lobes and the position and shape of thickened anterior neuropodial chaetae. The other two species of Uncispionidae, Uncispio hartmanae and Uncopherusa bifida, are re-examined and their descriptions expanded. The family status of Uncispionidae is discussed with respect to the other families in Spionida.
Introduction
The spioniform family Uncispionidae was erected by Green (Citation1982) for the new genus and species Uncispio hartmanae. The family was mainly defined on the enlarged, modified neuropodial hooks present on the last two (Uncispio) or more (Uncopherusa Fauchald & Hancock, Citation1981) segments. The three small specimens of U. hartmanae were described from a hard clay sample in 222 m depth off Santa Cruz Island, California.
The genus Uncopherusa, reassigned to the new family, was originally placed within the family Flabelligeridae due to its perceived resemblance to Pherusa Oken, Citation1807. The only member of the genus, Uncopherusa bifida Fauchald & Hancock, Citation1981, was described from a single, whole specimen collected off Oregon in deep water (2860 m), sediment type unknown. No other accounts of this species have been found in the literature.
Other undescribed specimens, likely to be members of this family, have been mentioned sparsely in the literature. The first, a single posterior fragment identified as ?spionid, was identified by Hartman (Citation1965) from north of Dutch Guiana in 1500 m. A second specimen, from 4749 m depth northwest of Bermuda (no details of whether it was a fragment or whole), was also referred to the same description by Hartman and Fauchald (Citation1971). The description of the chaetae, including enlarged neuropodial bifid hooks on the last six segments, would place the specimen in Uncopherusa. Blake and Arnofsky (Citation1999) mentioned a new species of Uncispionidae, with neuropodial spines on chaetiger 3, from the northwest Atlantic, although no other description of the species or its locality was given. Blake (Citation2006) refined this description to Uncispio sp., although further details are still unpublished. Finally, Read (Citation2004) listed Uncopherusa sp. A in his ‘Checklist of New Zealand Polychaeta Species’. The specimens, fragments only, were originally published as ?Trochochaeta by Stull (Citation1979) from three stations, one muddy, from 124 to 254 m (Read, personal communication). Specimens have enlarged neuropodial hooks on the last five segments identifying them as Uncopherusa.
All of these undescribed specimens have been identified from stations in greater than 120 m depth in mud or clay habitats (where sediment type is described). Generally, only a single specimen, a very small number or fragments only were found at each locality. Until now, no members of the family have been identified from the northeast Atlantic.
The new species, Uncispio reesi, was identified in very large numbers from a boulder clay habitat in 120–171 m water to the west of Anglesey in the southern Irish Sea. The sediment – stiff, grey boulder clay – was a new habitat type, not previously sampled and had a patchy distribution. Several samples were taken at two different stations, some with the boulder clay sediment, some with a mixture of sand, gravel and clay (Robinson et al. Citation2009). Those samples with mixed sediment contained relatively few (2–13) specimens of U. reesi, while the boulder clay-only samples were generally dominated by the species. The two latter samples, 82d and 83c, contained 325 and 239 specimens respectively, of which nearly 150 were whole. Two posterior fragments were also identified from a sandy, shallow (29 m) station off Arklow on the east coast of Ireland, an apparently anomalous occurrence in terms of depth and sediment type.
The new species was immediately recognizable as a member of Uncispionidae due to the very large neuropodial hooks located on the posterior-most segments. As the large hooks are only found on the last two segments in all specimens, this placed them into Uncispio. Most specimens are larger than those described by Green (Citation1982). Whole specimens range in size from 31 to 60 segments, with the majority being over 40 segments in length.
The holotypes of both Uncispio hartmanae and Uncopherusa bifida were obtained from the Los Angeles County Natural History Museum and examined. In both cases, the specimens were particularly small and difficult to examine. Some features that had not been mentioned previously were investigated and detailed to enable a better comparison with the new species.
Materials and methods
Specimens of Uncispio reesi were collected from three stations in the southern Irish Sea sampled during a single survey in 2005 (Robinson et al. Citation2009). All stations were sampled using a 0.1 m2 modified Van Veen grab. Samples were processed onboard and sieved through a 0.5-mm mesh sieve and then fixed in a 10% formaldehyde solution with Rose Bengal to stain the specimens. Specimens were later sorted in the laboratory by hand and preserved in 80% alcohol with 2% propylene glycol (see Mackie & Oliver Citation1996).
The three specimens of Uncispio hartmanae were collected during a baseline study of the Southern California Bight during 1975–1978 (Green Citation1982). All were obtained from a single station located off Santa Cruz Island, California in 222 m. No details of the equipment used were listed.
The single specimen of Uncopherusa bifida was collected from a station at 2860 m depth, sampled in 1964 as part of a transect from Yaquina Bay, Oregon extending offshore (Fauchald & Hancock Citation1981). Samples were obtained using an anchor-box dredge (J. Blake, personal communication; details not given in the publication).
All drawings and measurements were made using a camera lucida attachment on a Nikon Labophot-2 compound microscope, Nikon Eclipse E400 or Wild M8 binocular microscope. Photographs were taken using AutoMontage™ software.
Material is deposited in Amgueddfa Cymru – National Museum Wales, Cardiff (NMWZ), Natural History Museum of Los Angeles County (LACM), Smithsonian Institute, Washington (USNM), Zoological Museum University of Copenhagen (ZMUC) and National Institute of Water & Atmospheric Research Ltd, Wellington (NIWA).
Taxonomic accounts
Family Uncispionidae Green, Citation1982
Diagnosis (emended)
Small, slender polychaetes with palps inserted dorsally at junction between pro- and peristomium (postectal prostomial margins). Occipital antenna present or absent. Parapodia biramous with reduced, simple lobes. Branchiae present on some antero-median segments, fused or not to notopodial lobes. Chaetae simple, including capillaries (smooth, haired or spinose) and hooded bidentate hooks. Capillaries long on chaetiger 1, forming a cephalic cage; notopodial spines may be present. Enlarged modified neuropodial hooks enlarged on two or more posterior segments. Anus terminal, surrounded by up to 8 digitate lobes.
Remarks
Diagnosis of the family is as originally published by Green (Citation1982) except for new observations in italics. The presence of an occipital antenna could not be confirmed on Uncopherusa due to the condition of the holotype. The original description did not state whether one was present, although Green (Citation1982) included presence as a diagnostic feature for the family. The family status of Uncispionidae is discussed at the end of the paper.
Genus Uncispio Green, Citation1982
Type species: Uncispio hartmanae Green, Citation1982
Diagnosis (emended)
Body somewhat flattened dorso-ventrally, cylindrical medially, with three distinct regions. Occipital antenna present. Cephalic cage formed by long, smooth capillaries of both noto- and neuropodia of chaetiger 1. Anterior chaetigers with short capillaries with one edge finely haired in noto- and neuropodia; mid-bundle capillaries of some anterior neuropodia more robust. Median notopodia with long, spinose capillaries in addition to short, haired capillaries. Median neuropodia with bidentate hooded hooks and inferior bundle of long, curved, smooth capillaries. Posterior chaetigers without long, spinose capillaries in notopodia; last two chaetigers with only neurochaetae, hooded hooks enlarged, modified. Anus terminal with up to eight anal lobes.
Remarks
The above definition is expanded following description of the new species and a re-examination of the holotype of Uncispio hartmanae.
Uncispio reesi n. sp.
(Figures , –C, 5)
Figure 1. Uncispio reesi n. sp. (A, B: NMWZ.2005.014.0088; C, F: NMWZ. 2005.014.0086; D: NMWZ.2005.014.000087; E: NMWZ.2005.014.0091). (A) Anterior end, dorsal view, notochaetae of chaetiger omitted; (B) anterior end, ventral view; (C) holotype, anterior end, lateral view; (D) head, frontal view; (E) head, proboscis everted; (F) posterior end, terminal view. Abbreviations: ml, mouth lobe(s); mp, medio-dorsal pad; oa, occipital antenna; p, palp; pel, peristomial lobe; lw, lateral wing.
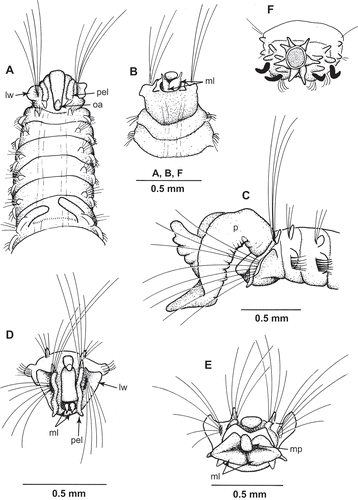
Figure 2. Uncispio reesi n. sp. (A–E: NMWZ.2005.014.0092; F,G: NMWZ. 2005.014.0089). (A) Chaetiger 1, anterior view; (B) chaetiger 2, anterior view; (C) chaetiger 3, anterior view; (D) chaetiger 7, anterior view; (E) chaetiger 19, anterior view; (F) posterior end, dorsal view; (G) posterior end, ventral view.
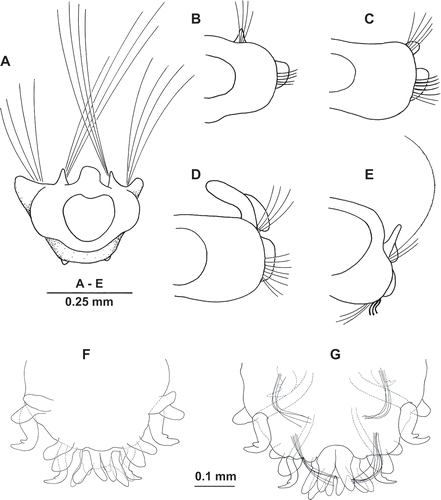
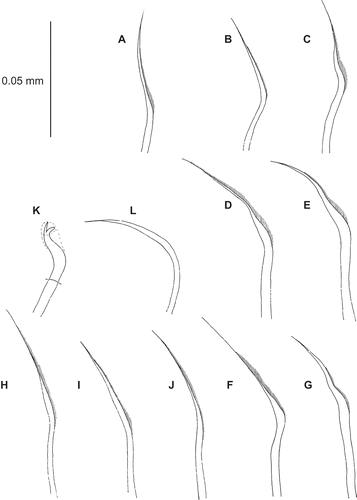
Figure 3. Uncispio reesi n. sp. (A,C–N: NMWZ.2005.014.0090; B,O–Q: NMWZ. 2005.014.0089). (A) Notochaeta, chaetiger 3; (B) spinose notochaeta, chaetiger 17, inset with detailed view; (C–E) chaetiger 3, upper, mid, lower neurochaeta; (F–H) chaetiger 4, upper, mid, lower neurochaeta; (I–K) chaetiger 5, upper, mid, lower neurochaeta; (L–N) chaetiger 6, upper, mid, lower neurochaeta; (O) bidentate hook, chaetiger 12; (P) smooth neurochaeta, chaetiger 12; (Q) penultimate hook, chaetiger 50; (R) terminal hook, chaetiger 51.
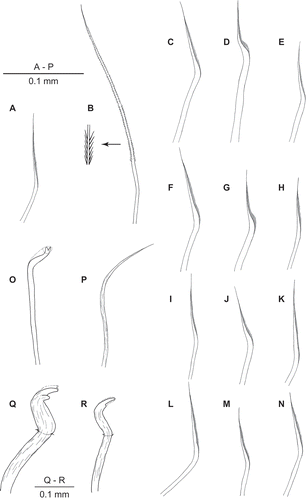
Figure 4. (A) Uncispio reesi n. sp., holotype (NMWZ.2005.014.0086); (B) Uncispio reesi n. sp. (NMWZ.2005.014.0088), antero-ventral view showing stained glandular patches; (C) Uncispio reesi n. sp. (NMWZ.2005.014.0093), egg; (D) Uncispio hartmanae, holotype (LACM-AHF Poly 1365); (E) Uncopherusa bifida, holotype (LACM-AHF Poly 1147).
Uncispio sp. Robinson et al. Citation2009:94
Uncispio n. sp. Mackie et al. Citation2010:25,
Material examined
West of Anglesey, Wales, Station 82d (53° 18.470ʹ N, 005° 05.430ʹ W), boulder clay, 171 m, holotype (NMWZ.2005.014.0086), 47 paratypes (NMWZ.2005.014.0087-0094; LACM-AHF POLY 2652; NIWA 70644; USNM 1151790; ZMUC-POL-2146) 07.08.2005; Station 83a (53° 18.600ʹ N, 005° 09.580ʹ W), gravelly sand over boulder clay, 139 m, 1 paratype (NMWZ.2005.014.0095), 07.08.2005; Station 83c (53° 18.590ʹ N, 005° 09.530ʹ W), boulder clay, 120 m, 97 paratypes (NMWZ.2005.014.0096), 07.08.2005; Station 83d (53° 18.610ʹ N, 005° 09.610ʹ W), gravelly sand over boulder clay, 137 m, 1 paratype (NMWZ.2005.014.0097), 07.08.2005.
Description
Holotype () complete with 50 chaetigers, 12.7 mm long, 0.47 mm wide at mid-body region. Complete paratypes with 31–60 chaetigers, up to 15.3 mm long, 0.59 mm wide. Body lacking pigmentation in alcohol, pale green when alive. Epidermis smooth, without papillae. Body flattened dorso-ventrally anterior and posterior, more cylindrical medially. Segmentation distinct. Animals are free-living, burrowing in the thick sediment.
Prostomium (,B,D) narrow, ovate, with anterior margin truncate, posterior margin rounded; with short, triangular occipital antenna arising at level of first notochaetae. Eyes absent. Conspicuous lateral wings on either side of prostomium extending to form post-chaetal lobe of first neuropodia. Palps (frequently lost) ventrally grooved and tapering (), directed forward, 5–7 chaetigers in length; inserted dorsally, lateral to and slightly anterior to occipital antenna. Long, peristomial (?) sinuose lobes (–D) either side of prostomium from first notopodia to ventrolateral edges of mouth terminating in short triangular projections.
Proboscis (,D) with two large, ciliated fleshy lobes, each with slender, distal process. When fully extruded, medio-dorsal pad apparent ().
Anus terminal (, ,G), surrounded by six to eight digitiform lobes.
Parapodia biramous. Notopodia of first two chaetigers (, ,B) elevated dorsally with conical post-chaetal lobes; lobes dorso-lateral, short, elliptical from chaetiger 3 () to 5–7 becoming fused to branchiae (starting chaetigers 6–8, usually 7; ) then absent in post-branchial region until last few chaetigers, larger on last two. Neuropodium chaetiger 1 anterior to notopodium. Neuropodial post-chaetal lobes low and rounded, becoming short, conical to digitiform in post-branchial region to end of body. Last two chaetigers with larger, equal-sized noto- and neuropodial lobes.
Branchiae, number variable (2–22 pairs; holotype with 22 pairs), strap-like with ciliated inner margins, almost meeting dorsally, last few pairs rapidly decreasing in size (,E). Band of cilia dorsally between branchiae ().
All chaetae simple, including capillaries and bidentate hooded hooks. Number of chaetae and presence on segments varies with body size. Descriptions are limited to specimens with 50–55 segments.
Chaetiger 1 with elongated smooth capillaries in noto- and neuropodia. Neurochaetae spreading fascicle, directed anteriorly, forming cephalic cage; long, thick chaetae alternating with shorter (half length or less), thinner chaetae (longer chaetae approximately 3–4 times length of following neurochaetae). Notochaetae, alternating long, thick and shorter, thinner forms, as long as but slightly thicker than neurochaetae; spreading fascicle as with neurochaetae, directed anteriorly over prostomium (,E, 2A). Approximately 14–16 notochaetae, 12–20 neurochaetae comprising cephalic cage.
Notochaetae from chaetiger 2 consisting of single row of short, haired (one edge only) capillaries () of similar length along body. Approximately 4–6 capillaries on chaetiger 2, increasing to 7–9 on chaetigers 4–6, reducing to 1–5 on posterior segments; capillaries absent from last two chaetigers. Long, spinose capillaries (1–2 per fascicle; ) present in notopodia from approximately chaetiger 13 (10–17; holotype 14), absent from about last eight chaetigers; 2–3 times length haired capillaries, easily broken.
Neurochaetae short, haired capillaries on anterior and posterior chaetigers (slightly shorter than notochaetae) to start of hooded hooks from chaetigers 8–12. Mid-bundle chaetae thickened, more curved on chaetigers 3–5, similar to notochaetae from chaetiger 6 (–N). Neurochaetae arranged in two rows from chaetiger 2, on all segments until start of hooded hooks from chaetiger 9 or 10, occasionally from 8 or 11, rarely 12, to end of body. Bidentate hooded hooks, strongly curved (). Hooks accompanied ventrally by 2–5, usually 3 or 4, smooth and highly curved capillaries (, ). Hooks of last two chaetigers single, greatly enlarged and highly modified (, R), penultimate hook largest; with remnant of hood present. Some with subdermal replacement hooks in place ().
Methyl blue staining revealed conspicuous glandular patches posterior () and ventral () to anterior parapodia.
Eggs approximately 100–120 μm diameter. Type 1 structure, as defined by Blake and Arnofsky (Citation1999), with thick honeycomb envelope containing prominent and numerous cortical alveoli ().
Etymology
Uncispio reesi is named after Ivor Rees in recognition of his willingness to share the extensive knowledge of the Welsh marine fauna that he gained from a lifetime's work on the biology and sediments of the Irish Sea and Liverpool Bay.
Habitat
The new species is prevalent in the boulder clay habitat, but can also be found in smaller numbers in sediments where boulder clay is mixed with coarser sediment; depths greater than 120 m. However, two fragments collected from a shallow and apparently sandy habitat off Arklow, east coast Ireland, indicate that the species may inhabit other sediments albeit in reduced numbers. Entire specimens only recorded from the southern Irish Sea region. A posteriorly incomplete fragment of Uncispio sp. has been identified from firm clay, 160 m, southern Norway (Station C160, Norwegian coastal monitoring programme) by Eivind Oug. We have examined the specimen and it agrees with the above description of U. reesi.
Morphometrics
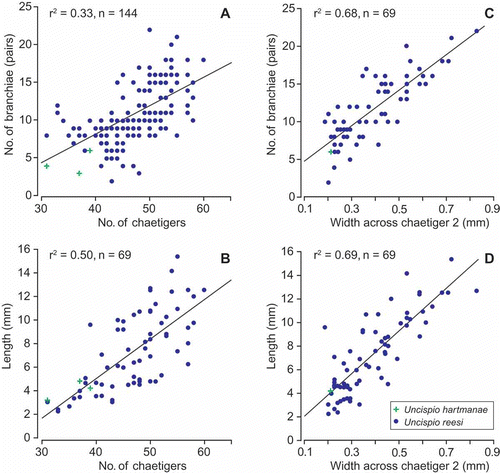
The relationships between various morphological attributes (chaetiger number, width, length, branchial pairs and commencement of neuropodial hooks and spinose notochaetae) were investigated. Only entire specimens exhibiting no obvious signs of anterior or posterior regeneration were included. Although positive relationships were observed between most attributes, coefficients of determination (r 2) involving number of chaetigers and number of branchial pairs or length were poor (,B). The best relationships were between width at chaetiger 2 (,D) and number of branchial pairs, length, and mid-body width (0.68, n = 69; not shown). No meaningful relationships between the size attributes and the commencement of the chaetal forms were found.
Figure 5. Size-related morphological variability in Uncispio species. (A) Number of branchial pairs relative to number of chaetigers, (B) length relative to number of chaetigers, (C) number of branchial pairs relative to width of chaetiger 2, and (D) length relative to width of chaetiger 2. Linear regression lines included for reference.
Remarks
With only one other described species in the genus, Uncispio reesi can be differentiated from U. hartmanae primarily by the nature of the posterior region. Uncispio hartmanae has four digitate anal lobes (plus an additional cirrus, possibly aberrant as there was not one on either of the paratypes), while U. reesi has six to eight anal lobes; the last two chaetigers of U. reesi both have a pair of dorsal lobes above the neuropodial hooks while U. hartmanae has a single pair of lobes dorsal to each neuropodial hook on both of the last two chaetigers and a single pair of lobes ventral to the neurochaetae on the last chaetiger only. In addition, the occipital antenna of U. hartmanae is much larger and more erect relative to body size.
Evidence of regeneration is apparent on many specimens of U. reesi either in the reduced appearance of one end of an animal or in discrepancies in the morphology such as misaligned segments or bifurcate anal cirri. Variation in the number of anal lobes may be due to regeneration although specimens investigated always had more than four lobes.
Morphometric analyses of polychaetes often reveal strong patterns between size measures and other attributes, with high proportions of the observed variation explained (e.g. r 2>0.9; see Mackie Citation1984, Citation2000). The relatively poor relationships evident for Uncispio reesi may well be due to a high frequency of regeneration within the population. This was highlighted by the greater spread of values in the graphs involving chaetiger number compared to those involving size (width). In particular, there were examples of specimens with either noticeably few or more branchial pairs compared to other similarly sized specimens (). Furthermore, close examination of apparently ‘normal’ specimens exposed signs of possible earlier regeneration. For example, a number of specimens (including the holotype) showed six prebranchial chaetigers on one side of the body and five on the other. Other specimens had few or no chaetae on one side of chaetiger 1. We believe these observations are likely indications of frequent sublethal predation through cropping (see Woodin Citation1982; Bely Citation2006; Lindsay et al. Citation2007). No evidence of schizotomy was found.
Uncispio hartmanae Green, Citation 1982
(, )
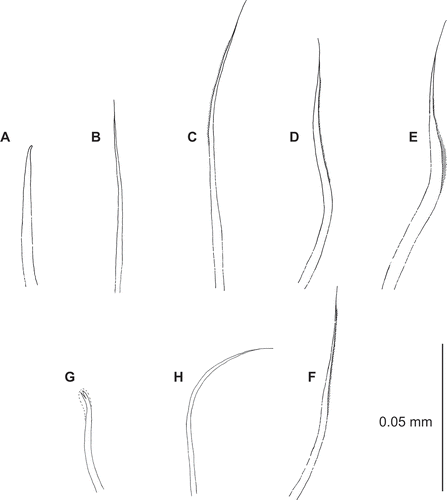
Figure 6. Uncispio hartmanae, holotype (LACM-AHF Poly 1365). (A) Notochaeta, chaetiger 6; (B,C) chaetiger 2, upper, mid neurochaeta; (D,E) chaetiger 3, upper, mid neurochaeta; (F,G) chaetiger 4, upper, mid neurochaeta; (H,I) chaetiger 5, upper, mid neurochaeta; (J) chaetiger 6, mid neurochaeta; (K) bidentate hook, chaetiger 10; (L) smooth neurochaeta, chaetiger 10.
Uncispio hartmanae Green, Citation1982:530–536, figs .
Material examined
Offshore Santa Cruz Island, California, USA, BLM Station 80901, light brown hard clay with pebbles, winter sample, 222 m, holotype (LACM-AHF Poly 1365), 1975–1976.
Observations
Holotype () complete with 39 chaetigers, 4.2 mm long, 0.25 mm wide. Body lacking pigmentation in alcohol. Description as originally published by Green (Citation1982) with some additional details.
Epidermis smooth, no papillae. Body somewhat flattened dorso-ventrally anterior and posterior, more cylindrical medially. Segmentation distinct. Occipital antenna very large, triangular (as in Green Citation1982: figs. ,B).
Parapodia biramous. Notopodia of chaetigers 1–2 elevated dorsally, although left chaetiger 2 missing, probably due to regeneration following non-lethal predation (cropping). Remaining notopodia all lateral.
Post-chaetal lobes present on notopodia to chaetiger 5 then fused to branchiae, specimen too small to accurately determine shape and relative size. Neuropodial lobes present to anterior branchial region, again specimen too small to positively determine shape and size or presence posteriorly. Rounded lobe present dorsally on final two chaetigers (as in Green Citation1982: fig. ), similar lobes present ventrally on last chaetiger only. Pygidium with four digitate lobes; possibly regenerative (?).
Notochaetae short, haired capillaries, more finely pointed than originally described (; Green Citation1982: fig. ). Neurochaetae short, haired capillaries to start of hooded hooks. Mid-bundle chaetae thickened, double curved on chaetigers 2–4 (–G), similar to notochaetae from chaetiger 5 (–J). Neurochaetae arranged in two rows from chaetiger 2 to start of hooded hooks on chaetigers 8–9; hooks strongly curved, hooded, bidentate (), continuing to end of body. Hooks accompanied by two inferior, smooth and highly curved capillaries (). Greatly enlarged, posterior modified hooks as originally described.
Morphometric analyses on U. hartmanae were limited as data exists for only three specimens. However, from this limited information U. hartmanae was placed at the lower end of all the analyses performed (). Whether this is because U. hartmanae is a smaller species than U. reesi or whether only small specimens of the former have as yet been identified cannot be determined until more material is collected.
Genus Uncopherusa Fauchald & Hancock, Citation1981
Type species: Uncopherusa bifida Fauchald & Hancock, Citation1981
Diagnosis (emended)
Cephalic cage formed from neurochaetae of first chaetiger; notopodia on first chaetiger with acicular and capillary chaetae. Chaetae include smooth and haired capillaries, acicular chaetae, bifid hooded hooks, and greatly expanded, curved hooded hooks in several chaetigers of the posterior end. Branchiae present; body smooth.
Uncopherusa bifida Fauchald & Hancock, Citation 1981
(, )
Figure 7. Uncopherusa bifida, holotype (LACM-AHF Poly 1147). (A) Chaetiger 1, acicular notochaeta; (B) chaetiger 1, capillary notochaeta; (C) chaetiger 8, notochaeta; (D,E) chaetiger 3, upper, mid neurochaeta; (F) chaetiger 4, mid neurochaeta; (G) bidentate hook, chaetiger 10; (H) smooth neurochaeta, chaetiger 10.
Uncopherusa bifida Fauchald & Hancock, Citation1981:36–37, pl. 6, figs e–h.
Material examined
Off central Oregon, USA, Station AD-89, NAD-22A (44°38.5ʹ N, 126°16.1ʹ W–44°38.1ʹ N, 126°16.4ʹ W), 2860 m, holotype (LACM-AHF Poly 1147), 20.05.1964.
Observations
Holotype () complete with 30 chaetigers, 4.5 mm long, 0.3 mm wide. Description as originally published by Fauchald and Hancock (Citation1981) with some additional details.
Body lacking pigmentation in alcohol. Epidermis smooth, no papillae. Body cylindrical; segmentation distinct. Anterior end unlikely to be retractable as originally stated, however prostomial shape not determinable due to size and condition of specimen. An additional prostomial process present ventrally close to mouth but further determination proved impossible.
Occipital antenna not observed. Notopodia of chaetiger 2 present (not reduced as in original account), elevated dorsally; capillaries directed forward, few in number compared to following chaetigers. Remaining notopodia all placed laterally, chaetae directed posteriorly. Posterior segments ventrally concave with enlarged neuropodial hooks arising from margins.
Post-chaetal lobes present on notopodia to chaetiger 5, larger on chaetigers 1–2. Neuropodial post-chaetal lobes low, rounded, present to at least chaetiger 7. Additional lobes variably apparent further along body, but too difficult to accurately determine position due to size and condition of specimen (post-chaetal notopodial lobe?). Single small, conical lobes present dorsal to neuropodial hooks on posterior-most segments, no lobes apparent ventral to hooks; notochaetae absent.
Branchiae small, strap-like, present on chaetiger 5 only, possibly fused basally to short notopodial post-chaetal lamella.
Neuropodial cage chaetae long, thick capillaries alternating with shorter, thinner ones; notopodial chaetae short, acicular (), alternating with thin capillary chaetae () of the same length as shorter neuropodial chaetae. Notochaetae from chaetiger 2 long, haired capillaries (); absent from last five chaetigers. Neurochaetae shorter, haired capillaries to start of hooded hooks. Mid-bundle chaetae thickened on chaetiger 3 (,E) only, similar to notochaetae from chaetiger 4 (). Neurochaetae arranged in two rows from chaetiger 2 to start of hooded hooks. Hooded, bidentate hooks () from chaetiger 8 to end of body. Hooks accompanied ventrally by one or a few smooth and highly curved capillaries (). Posterior modified neuropodial hooks present on last six chaetigers, last hook reduced in size.
Conclusive remarks
Only a single animal representing Uncopherusa and three specimens identified for Uncispio are known, and all are small and in poor condition. Classifying the family Uncispionidae definitively has been difficult.
Uncispionidae was placed within Spionida by Rouse and Fauchald (Citation1997), along with Apistobranchidae, Chaetopteridae, Longosomatidae, Magelonidae, Poecilochaetidae, Spionidae and Trochochaetidae. However, Uncispionidae were mostly excluded from the cladistic analyses due to insufficient knowledge of the group.
Most recently, Blake and Arnofsky (Citation1999; re-examined in Blake Citation2006) published a detailed phylogenetic analysis of the Spionida, using 38 characters relating to both reproduction and morphology. In their analyses, Chaetopteridae and Magelonidae were excluded and some gaps in data for other groups (including Uncispionidae) were filled using museum and personal collections, although details of the latter have not been published. Following these investigations, it was suggested that Spionidae be reclassified to include the members of Longosomatidae, Poecilochaetidae, Trochochaetidae and Uncispionidae as genera within the subfamily Nerininae (Blake Citation2006). The reclassification was not stated as a definitive outcome, but rather as a step towards a larger, more complete analysis later.
Certain morphological features of both Uncopherusa and Uncispio bear resemblance to species not just of Spionidae, but to Poecilochaetidae and Trochochaetidae also. The long, spinose notopodial capillaries of Uncispio are structurally similar to those found in Poecilochaetidae (see Mackie Citation1990: figs. ,C). Furthermore, their cephalic cages are very reminiscent of those found in Poecilochaetids.
The neuropodial hooded hooks in Uncispionidae are similar to those generally used as a defining feature of the Spionidae and the form and position of the Uncispionid branchiae are also similar to species found within the latter group. The grooved, forwardly-directed palps of Uncispio (palps unknown for Uncopherusa) are comparable with those of the Spionidae, but are also found in the Trochochaetidae, Poecilochaetidae and Longosomatidae (Rouse & Pleijel Citation2001).
Thickened neuropodial capillaries, found on anterior chaetigers 2–4 or 3–5 in Uncispio and chaetiger 3 in Uncopherusa, resemble neurochaetae described within the Trochochaetidae, particularly for Trochochaeta watsoni (Fauvel Citation1916; see Pettibone Citation1976: fig. ) and T. diverapoda (Hoagland Citation1920; see Pettibone Citation1976: fig. ). The position of these modified neurochaetae is also comparable to the position of acicular chaetae on chaetigers 2 and 3 of Trochochaeta.
Blake and Arnofsky (Citation1999) described three types of egg structure for the spionid genera. The first type, with a thick, honeycomb envelope and numerous cortical alveoli, was also attributed to Poecilochaetus, Heterospio, Trochochaeta and Uncispio, the latter being confirmed in this article.
Uncispionidae also exhibit modified, enlarged neuropodial hooks in the final two (Uncispio) or more (Uncopherusa) posterior chaetigers. In Uncopherusa, this last section of the body becomes concave with the hooks and parapodia directed ventrally. Although the enlarged neuropodial hooks are unique to the family, the posterior concave appearance in Uncopherusa would seem analogous to the dorsal ‘concavity’ formed by the notopodia and curved notopodial spines in the posterior region of Poecilochaetus serpens and related species (see Allen Citation1904: Pl. 8, fig. ).
The large number of specimens collected by the HABMAP survey (Robinson et al. Citation2009) is unrivalled in its contribution to the knowledge of the Uncispionidae. With multiple specimens, both whole and fragmented, multiple dissections were possible. Several specimens were also ripe with eggs enabling the first published description and images of Uncispio eggs. Together with a re-examination of the holotypes of both Uncispio hartmanae and Uncopherusa bifida, the descriptions of the three species are now more complete and comparable than previously available. However, the expanded descriptions for the two earlier described species are still incomplete due to the size and condition of the material. More material is essential in order to answer questions on whether these specimens are simply small representatives of their species (the smallest specimens of Uncispio reesi are a comparable size to the specimens of Uncopherusa bifida and Uncispio hartmanae) or if all specimens are naturally small, as well as several other morphological questions the answers to which were impossible to determine with the specimens available.
Although we recognize the similarities between Uncispionidae and Spionidae, we choose to maintain the separation pending further analysis and molecular investigation.
Acknowledgements
We would like to thank the Captain and crew of the R.V. Celtic Voyager for their help and work during the HABMAP survey in 2005, the EU European Regional Development Fund INTERREG IIIA Ireland/Wales Community Initiative Programme 2000–2006 for funding the project and Countryside Council for Wales for administering the project. Also, Leslie Harris from the Natural History Museum of Los Angeles County for the loan of the important holotype material, Geoff Read for the details of the Uncopherusa sp. A identified from New Zealand, Eivind Oug and the Norwegian Institute for Water Research (NIVA) for the Norwegian specimen of Uncispio and Jim Turner for his photographic work with the specimens.
References
- Allen , EJ. 1904 . The anatomy of Poecilochaetus, Claparède . Quarterly Journal of Microscopical Science , 48 : 79 – 151 . pls 157–110
- Bely , AE. 2006 . Distribution of segment regeneration ability in the Annelida . Integrative and Comparative Biology , 46 : 508 – 518 .
- Blake , JA. 2006 . “ Spionida ” . In Reproductive biology and phylogeny of Annelida , Edited by: Rouse , GW and Pleijel , F . 565 – 638 . Enfield, NH : Science Publishers .
- Blake , JA and Arnofsky , PL. 1999 . “ Reproduction and larval development of the spioniform Polychaeta with application to systematics and phylogeny ” . In Reproductive strategies and developmental patterns in annelids Edited by: Dorresteijn , AWC and Westheide , W . 57 – 106 . Hydrobiologia 402
- Fauchald , K and Hancock , DR. 1981 . Deep-water polychaetes from a transect off central Oregon . Allan Hancock Monographs in Marine Biology , 11 : 1 – 73 .
- Fauvel , P. 1916 . Deux polychètes nouvelles (Disoma watsoni n. sp. et Hyalinoecia brementi n. sp.) . Bulletin de l'Institut Océanographique de Monaco , 316 : 1 – 10 .
- Green , KD. 1982 . Uncispionidae, a new polychaete family (Annelida) . Proceedings of the Biological Society of Washington , 95 : 530 – 536 .
- Hartman , O. 1965 . Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic areas . Occasional Papers of the Allan Hancock Foundation , 28 : 1 – 378 .
- Hartman , O and Fauchald , K. 1971 . Deep-water benthic polychaetes off New England to Bermuda and other North Atlantic areas . Allan Hancock Monographs in Marine Biology , 6 : 1 – 327 .
- Hoagland , RA. 1920 . Polychaetous annelids collected by the United States Fisheries Steamer ‘Albatross’ during the Philippine Expedition of 1907–1909 . Bulletin of the United States National Museum , 1 : 603 – 635 .
- Lindsay , SM , Jackson , JL and He , SQ. 2007 . Anterior regeneration in the spionid polychaetes Dipolydora quadrilobata and Pygospio elegans . Marine Biology (Berlin) , 150 : 1161 – 1172 .
- Mackie , ASY. On the identity and zoogeography of Prionospio cirrifera Wirén, 1883 and Prionospio multibranchiata Berkeley, 1927 (Polychaeta; Spionidae) . Proceedings of the First International Polychaete Conference . 1983 , Sydney. Edited by: Hutchings , PA . pp. 35 – 47 . Linnean Society of New South Wales .
- Mackie , ASY. 1990 . “ The Poecilochaetidae and Trochochaetidae (Annelida: Polychaeta) of Hong Kong ” . In The marine flora and fauna of Hong Kong and southern China II , Edited by: Morton , B . 337 – 362 . Hong Kong : Hong Kong University Press .
- Mackie , ASY. Micronephthys oculifera (Polychaeta: Nephtyidae), a remarkable new species from Hong Kong, China . Proceedings of the Sixth International Polychaete Conference . 1998 , Curitiba, Brazil. Edited by: Reish , DJ . pp. 517 – 527 . Bulletin of Marine Science 67
- Mackie , ASY and Oliver , PG. 1996 . “ Marine macrofauna: Polychaetes, molluscs and crustaceans ” . In Methods for the examination of organismal diversity in soils and sediments , Edited by: Hall , GS . 263 – 284 . Wallingford : CAB International .
- Mackie , ASY , Darbyshire , T , Bamber , RN and Turner , JA. 2010 . Notes on new benthic invertebrates from the southern Irish Sea . Porcupine Marine Natural History Society Newsletter , 27 : 24 – 27 .
- Oken , L. 1807 . Göttingische gelehrte Anzeigen , 2 : 1161 – 1168 .
- Pettibone , MH. 1976 . Contribution to the polychaete family Trochochaetidae Pettibone . Smithsonian Contributions to Zoology , 230 : 1 – 21 .
- Read GB. 2004. Checklist of New Zealand Polychaeta species. Available online at http://www.annelida.net/nz/Polychaeta/References/NZPolySpeciesListV2.htm (http://www.annelida.net/nz/Polychaeta/References/NZPolySpeciesListV2.htm)
- Robinson , KA , Darbyshire , T , Van Landeghem , K , Lindenbaum , C , McBreen , F , Creaven , S , Ramsay , K , Mackie , ASY , Mitchell , NC , Wheeler , A , Wilson , JG and O'Beirn , F. 2009 . Habitat mapping for conservation and management of the southern Irish Sea (HABMAP) . I: Seabed surveys. Studies in Marine Biodiversity and Systematics from the National Museum of Wales. BIOMÔR Reports , 5 : 1 – 234 .
- Rouse , GW and Fauchald , K. 1997 . Cladistics and polychaetes . Zoologica Scripta , 26 : 139 – 204 .
- Rouse , GW and Pleijel , F. 2001 . Polychaetes , Oxford : Oxford University Press . 354 pages
- Stull , J. 1979 . Some benthic polychaetes from New Zealand . NZOI Records , 4 : 25 – 43 .
- Woodin , SA. 1982 . Browsing: important in marine sedimentary environments? Spionid polychaete examples . Journal of Experimental Marine Biology and Ecology , 60 : 35 – 45 .