Abstract
Although most of the Eunicidae (Polychaeta) of the Western Caribbean Sea appear to be well studied, the taxonomic status of Lysidice has yet to be evaluated. A first attempt to revise the taxonomy of this genus is here presented, based on material collected at Carrie Bow Cay (CBC, Belize), as well as at other sites along the Yucatan Peninsula, representing a variety of habitat types (coralline rock–coral rubbles, sponges, coralline sands, Thalassia testudinum meadows) and depths (0.5–20 m). The collected new taxa found were described and compared with literature description of other known species, as well as with museum specimens of the type-species of the genus, Lysidice ninetta Audouin & Milne Edwards, which has been confused with and synonymized with many Caribbean morphotypes of Lysidice. The analysis reveals the presence of five previously undescribed species of Lysidice: L. caribensis n. sp. and L. adrianae n. sp., preferentially associated with coralline rock; L. thalassicola n. sp. associated with Thalassia testudinum meadows, as a borer in the seagrass sheaths, and representing the only polychaete species strictly associated with Thalassia; lastly, L. carriebowensis n. sp. and L. phyllisae n. sp. are less common and collected only at CBC in association with coralline rock (coral rubble). Several specimens of the three more common new species, L. caribensis, L. thalassicola and L. adrianae were found mature, both males and females, with an epitokous transformation in which nearly two-thirds of the body is swollen and full of gametes, and with eyes extraordinarily enlarged. The ultrastrucure analysis of mature spermatozoa of L. caribensis and L. adrianae shows a typical ect-aquasperm structure. These show that Lysidice is a highly diversified genus in the Western Caribbean, with the different species showing clear habitat and geographic separation.
Introduction
The Eunicidae of the Western Caribbean Sea have been relatively well studied (Fauchald Citation1970, 1992; Salazar-Vallejo Citation1996; Carrera-Parra & Salazar-Vallejo Citation1998; Salazar Vallejo & Carrera Parra 1998), with the exception of two genera, Lysidice and Nematonereis (Gambi et al. Citation2006; Fauchald Citation2010). The taxonomic status of Lysidice in particular needs a careful revision, since this genus, more than other eunicids and despite the complex jaw apparatus, has a relatively simple body with few of the characters normally used in eunicid systematics to separate species.
In the Mediterranean Sea, a recent genetic analysis of the genus type species, Lysidice ninetta Audouin & Milne-Edwards, shows the presence of at least two cryptic forms (Iannotta et al. Citation2007, Citation2009), both associated with the seagrass Posidona oceanica as borers of the sheaths of this plant (bases of the old leaves that persist in the rhizomes; Gambi Citation2002). One of these cryptic forms was hypothesized to be the type-species of the genus L. ninetta, and the redescription of this form was highly recommended, but not undertaken by Iannotta et al. (Citation2009). Other Lysidice species, synonymized with L. ninetta, have been also recently been validated and redescribed in the Mediterranean (Iannotta et al. Citation2007; Kurt Sahin & Cinar Citation2009). These recent findings confirm that Lysidice is much more diversified and shows much more detailed local ecological adaptation than previously suspected. The tropical Caribbean areas might show a similar pattern, considering that tropical habitats usually have high diversity of Eunicidae and a high potential for microhabitat differentiation. Previous records from the Caribbean have already shown the occurrence of the genus Lysidice in various habitats, such as dead rubble corals, seagrass and sandy or muddy bottoms.
Up to now, only three species of Lysidice have been reported for the Western Caribbean (Salazar-Vallejo Citation1996; Salazar Vallejo & Carrera Parra 1998): L. ninetta, L. notata Ehlers, 1818 and L. tortugae Treatwell, 1921. The latter was described from a single, incomplete specimen which has turned out to be a juvenile form of a different genus (Carrera-Parra L.F., personal observation). Previous observation on specimens associated with the seagrass Thalassia testudinum suggested the possible occurrence of two new species (Gambi et al. Citation2003), that in order to limit further confusion with existing species had been indicated as L. cf ninetta and L. cf collaris (Gambi et al. Citation2003). Consequently, for the whole Caribbean we expected only a few potentially undescribed species of Lysidice to be present (Carrera-Parra et al. Citation2007), but none had been described and compared with known material.
The aim of this article is to provide a first revision of the genus Lysidice in the Western Caribbean Sea, based on material collected mainly from Belize (Carrie Bow Cay, CBC) and the Mexican Yucatan Peninsula, resulting in the discovery and description of five new species. The new species were described and compared with literature descriptions of other known species, as well as with museum specimens. The taxon identified as L. ninetta Audouin & Milne Edwards was included mainly to function as a proxy for a redescription of the type species of the genus, which has been confused and synonymized with many Caribbean morphotypes of Lysidice. In addition to the morphological description, notes on reproductive features and ecological habits of some of these new taxa are provided.
Materials and methods
Materials collected along the Mexican coast of Campeche and Yucatán (Gulf of Mexico), Quintana Roo (Mexican Caribbean) and at Carrie Bow Cay, Belize were studied. The specimens were collected in a variety of habitat types (coralline rocks, sponges, coralline sands, Thalassia testudinum meadows) and depths (0.5–20 m), mainly by SCUBA diving. All specimens collected were fixed in 10% formalin–seawater for a minimum of 24 h. Specimens were washed in the lab with tap water for 24 h, and transferred to 70% ethanol for long-term preservation.
All specimens were examined from prostomium to pygidium under the microscope to describe the different shapes and distributions along the body of acicula, chaetae, subacicular hooks and parapodial lobes. When the maxillary apparatus was not exposed, an antero-dorsal dissection was made to extract it; the apparatus was mounted on a glass slide to be examined. After examination the maxillary apparatus was returned to the original position in the specimen. The morphological details follow previous formats (Fauchald Citation1992; Carrera-Parra & Salazar-Vallejo Citation1998). Because many of the specimens are incomplete, the measurements were standardized for length through chaetiger 10 (L10), and width at chaetiger 10 excluding parapodia (W10). Photographs were taken by a digital camera attached to stereo- and compound microscopes; a set of photographs were fused to obtain a better definitions of each features illustrated. Some individuals were photographed with a digital camera while still alive to document different coloration of live material; the coloration disappears after fixation. Specimens of the newly described species were found in a reproductive phase full of gametes (eggs or sperms); eggs of female specimens were measured under an optic microscope using an ocular micrometer; one mature male of Lysidice caribensis n. sp and one of L. adrianae n. sp. were fixed for analysis in the Transmission Electron Microscopy (TEM) to demonstrate the morphology of mature sperm. Worms for TEM analysis were fixed for 2 h with 2.5% glutaraldehyde, washed in filtered seawater and preserved in 70% alcohol. The samples were post-fixed in 1% osmium tetroxide for 1 h. All fixation steps were performed at 4°C. Fixed material was dehydrated through graded concentrations of ethanol (20 min for each concentration) followed by propylene oxide (10 min), and a mixture of epoxy resin and propylene oxide (1:1, for 5 h in a drier), and finally embedded in epoxy resin (2 days at 60°C). Silver-gray sections were stained with alcoholic uranyl acetate followed by lead citrate. Micrographs were taken with a Phillips 400 TEM at the Microscopy Service of the Stazione Zoologica Anton Dohrn of Naples.
Holotypes and paratypes were deposited at the collections of the National Museum of Natural History Smithsonian Institution, Washington (USMN); the Collection Reference of El Colegio de la Frontera Sur, Chetumal (ECOSUR) and the Stazione Zoologica Anton Dohrn of Naples (SZN).
Results
Taxonomic account
Class Polychaeta Grube, 1850
Order Eunicida Dales, 1962
Family Eunicidae Berthold, 1827
Lysidice Lamarck, 1818
Lysidice adrianae n. sp.
Figures 1A–H
Lysidice ninetta Salazar-Vallejo & Carrera-Parra Citation1998:1502, –l (partim, non Audouin & Milne-Edwards, 1833).
Material examined
Type material
Holotype ECOSUR-0109, Gulf of Mexico, Yucatan, off Ria Lagartos, 21°37ʹ16.7ʹʹ N, 88°10ʹ32.6ʹʹ W, in coralline rock, 30 May 2005, 3 m. Paratypes USNM; SZN-0015, same data as holotype. Additional materials: ECOSUR HUE1(2), Gulf of Mexico, Campeche, El Hueso, 50 m off coast in coralline rock, 25 May 2005, 2 m. ECOSUR XEN1(4), Gulf of Mexico, Campeche, Sea Turtle Camp ‘Punta Xen’ (between Punta Xen and Champotón), 20 m off coast in coralline rock, 24 May 2005, 1 m. ECOSUR HCH3(1), Mexican Caribbean, Quintana Roo, Hualalpich, Ascensión Bay, 19 June 1986. ECOSUR AVE4(3) Mexican Caribbean, Quintana Roo, Aventuras DIF, QR5, in coralline rock, 22 March 1992. Mexican Caribbean, Quintana Roo, CARICOMP-UNAM (7), Puerto Morelos, in coral rubbles, 3 m, 5 August 2008.
Description
Holotype complete with 220 chaetigers, L10 = 2.0 mm, W10 = 1.0 mm. Prostomium slightly bilobed, median sulcus shallow. Lateral antennae to second peristomial ring; median antenna to chaetiger 1; all antennae never outreaching prostomium when stretched forward. All ceratophores short, ceratostyles without articulations (). Eyes reniform, lateral to the base of lateral antennae.
Figure 1. Lysidice adrianae n. sp. A, Anterior end, dorsal view. B, Chaetiger 3, frontal view. C, Chaetiger 22, frontal view. D, Chaetiger 112. E, Compound falcigers, chaetiger 3. F, Compound falcigers, chaetiger 200. G, Subacicular hook, chaetiger 30. H, Subacicular hook, chaetiger 112. Scale bars: A, 0.5 mm; B–D, 0.05 mm; E–H, 0.01 mm.
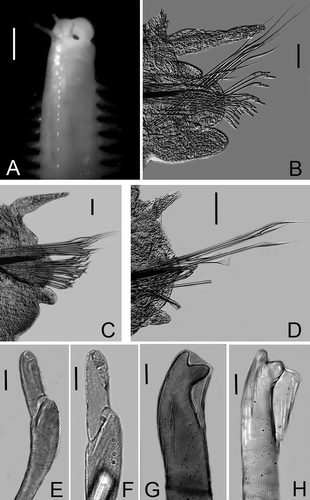
Peristomium with first ring twice as long as second ring, separation between rings distinct on all sides (). Maxillary apparatus with four paired and one single maxillae; MF = 1+1, 4+5, 5+0, 3+7, 1+1, MIII part of distal arc. Mandibles scoop-shaped.
All parapodia with inconspicuous prechaetal lobes; postchaetal lobes rounded, best developed from parapodia 1 to 29, inconspicuous in posterior parapodia. Notopodial cirri without articulations, tapering, best developed in parapodia 4 to 18, decreasing gradually in size in posterior parapodia; always longer than ventral cirri. Ventral cirri in parapodia 1–3 short, thick; from parapodia 4 to 120 with globular swollen bases and digitiform tips, best developed in parapodia 8 to 24; posterior to parapodia 121 conical (,C).
Supracicular chaetae limbate and pectinate; the latter anodonts of two sizes, either slender with up to 17 teeth, present in all chaetigers, or thicker with up to 25 teeth; thick pectinates present in median and posterior chaetigers. Compound falcigers bidentate, in anterior chaetigers with proximal tooth slightly larger and thicker than distal one, both directed laterally (). Falcigers in posterior chaetigers with short, wide blades, proximal teeth much thicker than distal teeth, both directed laterally (). Anterior chaetigers with up to 17 falcigers; posterior ones with up to 3 falcigers (,D). Acicula black, blunt; one per chaetiger, thicker in posterior chaetigers than in anterior ones. Subacicular hooks present from chaetiger 22, one per chaetiger; bidentate, proximal teeth larger and thicker than distal one, distal teeth directed upward. Coloration of subacicular hooks changing from black to translucent along body, black from chaetiger 22 to 45, black with distal end translucent from chaetiger 46 to 51, and translucent from chaetiger 52 to the last one (,H).
Pygidium with two pairs of anal cirri without articulations; dorsal cirri longer than ventral ones.
Variation
L10 = 1.0–2.0 mm, W10 = 1.0–1.5 mm. The start of subacicular hooks is size-dependant and varies from chaetiger 17 to 22.
Type locality
Ria Lagartos, Yucatan (Gulf of Mexico).
Etymology
This species is named in honor of Dr. Adriana Giangrande, in recognition of her many contributions to the knowledge of taxonomy and ecology of polychaetes.
Distribution
Gulf of Mexico (Campeche and Yucatán), Mexican Caribbean. Some previous records of L. ninetta in the Grand Caribbean Region may belong to this species.
Remarks
Lysidice adrianae n. sp. resembles L. bilobata Verrill, 1900 and L. notata Ehlers, 1818 by having dark subacicular hooks at least in part of the body. L. adrianae n. sp. differs from the other two species in having the color of subacicular hooks change from black to translucent, while the other species have dark reddish acicula. Other differences can be seen in chaetae; L. adrianae n. sp. has anodont pectinates, whereas the two other species have heterodont pectinates. Lysidice notata has a rounded prostomium, and is restricted to deep water (+200 m), while L. adrianae n. sp. has a bilobed prostomium and seems restricted to shallow water (1–3 m). This species was identified as L. ninetta Audouin & Milne Edwards, 1833 in previous studies. However, L. adrianae differs from L. ninetta mainly in the color of subacicular hooks, which are always black in L. ninetta and change from black to translucent along the body in L. adrianae. In L. adrianae, the body region with basally inflated ventral cirri ends at about chaetiger 120, and in L. ninetta it ends at about chaetiger 49.
Lysidice caribensis n. sp.
Figures 2A–G
Lysidice ninetta Salazar-Vallejo & Carrera-Parra Citation1998:1502, –l (partim, non Audouin & Milne-Edwards, 1833).
Lysidice cf collaris Gambi, van Tussenbroek & Brearley Citation2003:67.
Material examined
Type material
Holotype ECOSUR-0110, Mexican Caribbean, Quintana Roo, Punta Nizuc, Cancún, 21°1ʹ33.86ʹʹ N, 86°46ʹ45.22ʹʹ W, in coralline rock, 31 August 1997, 2.6 m. Paratypes USNM; SZN-0016, same data as holotype. Additional materials: ECOSUR YA28(2), Mexican Caribbean, Quintana Roo, Yalahau lagoon, P. Vista Alegre, M28, 18 January 1991. ECOSUR NC2E6(46), Mexican Caribbean, Quintana Roo, Nichupte lagoon, 2 February 1988. ECOSUR NC2E7(14), Mexican Caribbean, Quintana Roo, Nichupte lagoon, 2 February 1988. ECOSUR PN-R9(34), Mexican Caribbean, Quintana Roo, Punta Nizuc, Cancún, in coralline rock, 1 September 1997, 4 m. ECOSUR CARICOMP-UNAM (22), Mexican Caribbean, Quintana Roo, Puerto Morelos, in coral rubbles, 3 m, 5 August 2008. ECOSUR PM3(3), Mexican Caribbean, Quintana Roo, Puerto Morelos, 01 October 1986. ECOSUR PAC1(2), Mexican Caribbean, Quintana Roo, Paraíso, Cozumel, 5 June 1995. ECOSUR CHA1, Mexican Caribbean, Quintana Roo, Chankanaab, Cozumel, QR7, 2 April 1987. ECOSUR XCA1(12), Mexican Caribbean, Quintana Roo, Xcacel, in coralline rock, 3 June 1995. ECOSUR PA1(2), Mexican Caribbean, Quintana Roo, Punta Allen, Ascensión Bay, 24 February 1986. ECOSUR VCH1(2), Mexican Caribbean, Quintana Roo, Vigia Chico, Ascensión bay, 27 February 1986. ECOSUR R2(20), Mexican Caribbean, Quintana Roo, Xahuayxol, 40 m off coast in coralline rock, 26 September 1996, 1.5 m. ECOSUR R4(9), Mexican Caribbean, Quintana Roo, Rancho Buenavista, 100 m off coast 40 m in coralline rock, 27 September 1996, 2 m. ECOSUR R7(12), Mexican Caribbean, Quintana Roo, Punta Herradura, 100 m off coast in coralline rock, 28 September 1996, 2 m. ECOSUR A46(17), Mexican Caribbean, Quintana Roo, Xahuayxol, 80 m off coast in prairies of Thalassia testudinum and Syringodium filiforme, 18°30ʹ15ʹʹ N 87°45ʹ34ʹʹ W, 1 June 1997, 1.7 m. ECOSUR E54(2), Mexican Caribbean, Quintana Roo, Xahuayxol, 80 m off coast in prairies of Thalassia testudinum and Syringodium filiforme, 18°30ʹ15ʹʹ N 87°45ʹ34ʹʹ W, 2 October 1996, 1.8 m. ECOSUR CHI1(2), Mexican Caribbean, Quintana Roo, Chinchorro Bank, in coralline rock, 3 October 1983. ECOSUR PGA1(2), Mexican Caribbean, Quintana Roo. Punta Gavilán, 2 April 1992.
Description
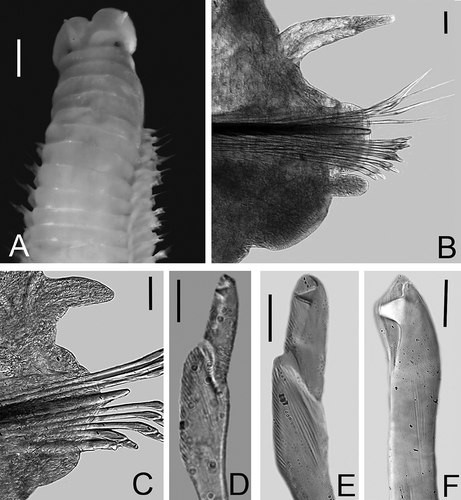
Holotype complete with 170 chaetigers; L10 = 2.0 mm, W10 = 1.0 mm. Prostomium slightly bilobed, shorter than peristomium; median sulcus shallow. Lateral antennae to posterior edge of first peristomial ring; median antenna to second peristomial ring; all antennae outreaching prostomium when stretched forward. All ceratophores short, ceratostyles without articulations. Eyes reniform, small, located lateral to the base of lateral antennae ().
Figure 2. Lysidice caribensis n. sp. A, Anterior end, dorsal view. B. Anterior end, dorsal view, live specimen with a peculiar coloration (reddish chaetigers). C, Chaetiger 3, frontal view. D, Chaetiger 160, frontal view. E, Compound falcigers, chaetiger 3. F, Compound falcigers, chaetiger 158. G, Subacicular hook, chaetiger 30. Scale bars: A, B, 0.5 mm; C, D, 0.05 mm; E–G, 0.01 mm.
Peristomiun with first ring twice as long as second ring, separation between rings distinct on all sides. Maxillary apparatus with four paired and one single maxillae; MF = 1+1, 5+5, 5+0, 4+8, 1+1, MIII part of distal arc. Mandibles scoop-shaped.
All parapodia with inconspicuous prechaetal lobes; postchaetal lobes from parapodia 1 to 26 short and rounded, inconspicuous in posterior parapodia. Notopodial cirri without articulations, tapering, best developed in parapodia 1 to 22, decreasing gradually in size; very short in posterior parapodia but always longer than ventral cirri. Ventral cirri in parapodia 1–3 short, thick; from parapodia 4 to 50 with globular swollen bases and digitiform tips; from parapodia 51 ventral cirri conical and very short (,D).
Supracicular chaetae limbate; pectinate chaetae anodont of two sizes, slender with up to 15 teeth in anterior chaetigers, and thicker with up to 26 teeth in posterior chaetigers. Compound falcigers bidentate, in anterior chaetigers with long and slender blades, proximal and distal teeth of similar size, both directed laterally (). Falcigers in posterior chaetigers with shorter blades, proximal teeth slightly thicker and larger than distal teeth, both directed laterally (). Anterior chaetigers with up to 16 falcigers; posterior chaetigers with up to 3 falcigers. Acicula yellow, blunt, one per chaetiger, thicker in posterior chaetigers. Subacicular hook present from chaetiger 20, yellow, bidentate; proximal teeth larger and thicker that distal ones, directed laterally; distal teeth directed upward (); always single in each chaetiger.
Pygidium with two pairs of anal cirri without articulations; dorsal cirri as long as the last two chaetigers, ventral cirri very short.
Variations
L10 = 0.5–4.0 mm, W10 = 0.5– 2.0 mm. The start of the subacicular hooks is size-dependant and varies from chaetiger 16 to 19. Coloration in several living specimens characterized by a red band on the peristomium and first chaetiger or second peristomial ring and first chaetiger, followed by a clear whitish band (like a collar) in chaetigers 2–4, followed by 2 or 3 red chaetigers (). In preserved specimens, this color pattern progressively fades until it is imperceptible (). A similar coloration, although consisting of a darker red, and with a very obvious clear–whitish band is present also in L. ninetta (Martin Citation1987).
Type locality
Punta Nizuc, Cancun, Quintana Roo (Mexican Caribbean).
Etymology
The name of the species refers to the wide geographic region where specimens were collected.
Distribution
Mexican Caribbean. Some records of L. ninetta in the Grand Caribbean Region may belong to this newly described species.
Remarks
Lysidice caribensis n. sp. resembles L. collaris Grube, 1870 and L. ninetta in having yellow subacicular hooks. The latter species differs mainly in having dark acicula; the other two species have yellow acicula. Furthermore, L. caribensis has two different sizes of anodont pectinate chaetae, while L. collaris has only wide anodont pectinate chaetae and L. ninetta has heterodont pectinate chaetae in anterior chaetiger and anodont ones in median and posterior chaetigers. Lysidice caribensis has short rounded postchaetal lobes in anterior parapodia, whereas L. ninetta has large auricular postchaetal lobes in anterior chaetigers.
Lysidice carriebowensis n. sp.
Figures 3A–F
Material examined
Type material
Holotype USNM, Carrie Bow Cay, Belize. CBC lagoon, in coral rubbles, 1 m, 9 October 2007. Paratypes ECOSUR-0111, SZN-0017, same data as holotype.
Additional materials
Carrie Bow Cay, Belize. CBC lagoon (2), in coral rubbles, 1 m, 12 October 2007. Carrie Bow Cay, Belize. CBC lagoon (7), in coral rubbles, 1 m, 15 October 2007. Carrie Bow Cay, Belize. CBC Sand Bore (2), in coral rubbles, 6 m, 13 October 2007.
Description
Holotype complete with 164 chaetigers; L10 = 1.7 mm, W10 = 0.9 mm. Prostomium bilobed, median sulcus shallow. Lateral antennae to second peristomial ring; median antenna to chaetiger 1; only median antenna outreaching prostomium when stretched forward. All ceratophores short, ceratostyles without articulations. Eyes reniform, lateral to the base of lateral antennae ().
Figure 3. Lysidice carriebowensis n. sp. A, Anterior end, dorsal view. B, Chaetiger 15, frontal view. C, Chaetiger 154, frontal view. D, Compound falcigers, chaetiger 3. E, Compound falcigers, chaetiger 154. F, Subacicular hook, chaetiger 30. Scale bars: A, 0.5 mm; B, C, 0.05 mm; D–F, 0.01 mm.
Peristomium slightly longer than prostomium, first peristomial ring slightly longer than second ring, separation between rings distinct on all sides. Maxillary apparatus with four paired and one single maxillae; MF = 1+1, 4+4, 4+0, 3+5, 1+1, MIII part of distal arc. Mandibles scoop-shaped.
All parapodia with inconspicuous prechaetal lobe; postchaetal lobe rounded, best developed in parapodia 1 to 18; inconspicuous in posterior parapodia. Notopodial cirri without articulations, tapering, best developed in parapodia 3 to 14, decreasing gradually in length throughout posterior parapodia; always longer than ventral cirri. Ventral cirri in parapodia 1–3 thick, digitiform; from parapodia 4 to 62 with a globular swollen base and digitiform tip, posterior ventral cirri conical (,C).
Supraciculars chaetae limbate and pectinate. Pectinate chaetae anodont with up to 23 teeth. Compound falcigers bidentate, in anterior chaetigers with both teeth of similar size, directed laterally (). Falcigers in posterior chaetigers with shorter blade, proximal teeth thicker and larger than distal teeth, both directed laterally (). Anterior chaetigers with up to 8 falcigers; posterior ones with up to 2 falcigers. Acicula black, blunt, thicker in posterior chaetigers. Subacicular hooks bidentate, present from chaetiger 19, yellow, proximal teeth larger than distal ones; distal teeth directed upward (); always single in each chaetiger.
Pygidium with two pairs of anal cirri without articulations; dorsal cirri longer than ventral one.
Variations
L10 = 1.5–2.3 mm, W10 = 0.6–1.1 mm. The start of subacicular hooks is size-dependant and varies from chaetiger 16 to 21.
Type locality
Carrie Bow Cay, Belize (Caribbean Sea).
Etymology
The name of the species refers to Carrie Bow Cay (Belize), where the Smithsonian Institution has a field-station and where the material was collected.
Distribution
Belize. Some previous records of L. ninetta in the Grand Caribbean Region may belong to this species.
Remarks
Lysidice carriebowensis n. sp. resembles L. ninetta in having black acicula and yellow subacicular hooks throughout the body. However, L. carriebowensis n. sp. differs from L. ninetta in having rounded postchaetal lobes in anterior chaetigers and anodont pectinate chaetae in all chaetigers instead of having auricular postchaetal lobes and heterodont pectinate chaetae in anterior chaetigers and anodont ones in median and posterior chaetigers.
Lysidice phyllisae n. sp.
Figures 4A–G
Material examined
Type material
Holotype USNM, Carrie Bow Cay, Belize. CBC North outer reef, in coralline rock, 18 m, 5 October 2007. Paratypes ECOSUR-0112, SZN-0018, same data as holotype.
Additional materials
Carrie Bow Cay, Belize. CBC Lagoon (3), in coralline rock, 1 m, 15 October 2007. CBC North outer reef (10), in coralline rock, 18 m, 5 October 2007.
Description
Holotype complete with 115 chaetigers; L10 = 1.8 mm, W10 = 0.8 mm. Prostomium bilobed, median sulcus shallow. Lateral antennae and median antenna reaching second peristomial ring; all antennae outreaching prostomium when stretched forward. All ceratophores short, ceratostyles without articulations. Eyes reniform, lateral to the base of lateral antennae ().
Figure 4. Lysidice phyllisae n. sp. A, Anterior end, dorsal view. B, Chaetiger 3, frontal view. C, Chaetiger 19, frontal view. D, Chaetiger 107, frontal view. E, Compound falcigers, chaetiger 3. F, Compound falcigers, chaetiger 100. G, Subacicular hook, chaetiger 30. Scale bars: A, 0.5 mm; B–D, 0.05 mm; E–G, 0.01 mm.
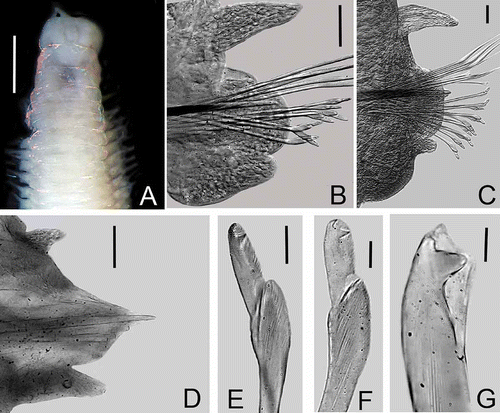
Peristomium twice as long as prostomium, both peristomial rings of similar size, separation between rings distinct on all sides. Maxillary apparatus with four paired and one single maxillae; MF = 1+1, 4+4, 4+0, 2+6, 1+1, MIII part of distal arc. Mandibles scoop-shaped.
All parapodia with inconspicuous prechaetal lobes; postchaetal lobes rounded, best developed in parapodia 1 to 15, inconspicuous in posterior parapodia. Notopodial cirri without articulations, tapering, best developed in parapodia 1 to 17, decreasing gradually in length in posterior parapodia; always longer than ventral cirri. Ventral cirri in parapodia 1–3 thick, digitiform; from parapodia 4 to 37 with a globular swollen base and digitiform tip; posterior ventral cirri conical (–D).
Supracicular chaetae limbate and pectinate. Pectinate chaetae anodont of two sizes, slender with up to 10 teeth in anterior chaetigers, and thicker with up to 18 teeth in median and posterior chaetigers. Compound falcigers bidentate, in anterior chaetigers with proximal teeth slightly larger than distal teeth, both directed laterally (). Falcigers in posterior chaetigers with shorter blades, proximal teeth thicker and much larger than distal teeth, both directed laterally (). Anterior chaetigers with up to 9 falcigers; posterior ones with up to 3 falcigers. Acicula blunt, coloration changing from black to yellow: black from chaetiger 1 to 32 and yellow from 33 to the posterior end. Subacicular hooks from chaetiger 17, yellow, bidentate; proximal teeth larger than distal ones; distal teeth directed upward (); always single in each chaetiger.
Pygidium with two pairs of anal cirri without articulations; dorsal cirri longer than ventral ones.
Variations
L10 = 1.5–2.0 mm, W10 = 0.6–1.0 mm. The start of the subacicular hooks is size-dependant and varies from chaetiger 13 to 19.
Type locality
Carrie Bow Cay, Belize (Caribbean Sea).
Etymology
The species is named to honor the memory of Dr. Phyllis Knight-Jones, a great and esteemed colleague for her many contributions to the taxonomy of polychaetes.
Distribution
Belize. Some previous records of L. ninetta in the Grand Caribbean Region may belong to this species.
Remarks
Lysidice phyllisae n. sp. resembles L. caribensis n. sp., L. carriebowensis n. sp., L. collaris and L. ninetta in having yellow subacicular hooks. L. phyllisae n. sp. differs from all these species in having acicula changing in color from black to yellow. This feature is unique among all currently described Lysidice species. However, Iannotta et al. (Citation2009) described a morphotype, also with a distinct genotype, of ‘L. ninetta’ from the Mediterranean Sea, with both black and yellow acicula; unfortunately, no complete morphological descriptions of the Mediterranean specimens were given so complete comparison is not possible.
Lysidice thalassicola n. sp.
Figures 5A–H
Lysidice cf ninetta Gambi, van Tussenbroek & Brearley Citation2003:67.
Material examined
Type material
Holotype ECOSUR-0113, Mexican Caribbean, Quintana Roo, Puerto Morelos, C1+C2 as borer of the sea grass Thalassia testudinum, Caracol, 13 January 2007. Paratypes USNM, SZN-0019, same data as holotype. Additional materials: ECOSUR Mexican Caribbean, Quintana Roo, Puerto Morelos C3+C4 (3) as borer of the seagrass T. testudinum, 13 January 2007. ECOSUR C1+C2 same data as holotype. CARICOMP-UNAM(3) Mexican Caribbean, Quintana Roo, Puerto Morelos, as borer of the sea grass T. testudinum, 3 m, August 2008. Carrie Bow Cay, Belize; Sand Bore (35) as borer of the sea grass T. testudinum, 10 m, 13 October 2007.
Description
Holotype complete with 178 chaetigers; L10 = 2.0 mm, W10 = 0.7 mm. Prostomium rounded, median sulcus shallow. Lateral antennae to middle of first peristomial ring; median antenna to second peristomial ring, thicker than lateral antennae; no antennae outreaching prostomium when stretched forward. All ceratophores short, ceratostyles without articulations. Eyes rounded, lateral to the base of lateral antennae ().
Figure 5. Lysidice thalassicola n. sp. A, Anterior end, dorsal view. B, Chaetiger 3, frontal view. C, Chaetiger 16, frontal view. D, Chaetiger 169, frontal view (subacicular hook broken during mounting). E, Compound falcigers, chaetiger 3; F, Compound falcigers, chaetiger 89. G, Subacicular hook, chaetiger 89. H, Two sheaths of the seagrass Thalassia testudimun with specimens of Lysidice thalassicola n. sp. inside the sheath mesophyllum. Scale bars: A, 0.5 mm; B–D, 0.03 mm; E–G, 0.01 mm.
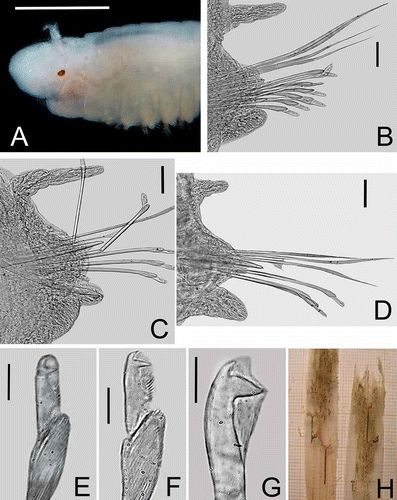
Peristomium slightly longer than prostomium, first peristomial ring slightly longer than second ring, separation between rings distinct on all sides. Maxillary apparatus with four paired and one single maxillae; MF = 1+1, 4+5, 6+0, 4+6, 1+1, MIII part of distal arc. Mandibles scoop-shaped.
All parapodia with inconspicuous prechaetal lobes; postchaetal lobes rounded, best developed in parapodia 1 to 13, inconspicuous in posterior parapodia. Notopodial cirri without articulations, tapering, best developed in parapodia 4 to 36, decreasing gradually in length in posterior parapodia. Ventral cirri in parapodia 1–3 thick, conical; from parapodia 4 to the posterior end with globular swollen bases and digitiform tips, tips relatively longer in posteriormost chaetigers. Notopodial cirri longer than ventral cirri from chaetiger 1 to 48, both of similar size from chaetiger 49 to 135; ventral cirri longer than dorsal cirri from chaetiger 136 to the posterior end (–D).
Supracicular chaetae limbate and pectinate. Pectinate chaetae heterodont with up to 22 teeth in anterior chaetigers, and with up to 25 teeth in median and posterior chaetigers. Compound falcigers bidentate, in anterior chaetigers with proximal teeth slightly larger than distal teeth, both directed laterally (). Falcigers in posterior chaetigers with proximal teeth thicker and larger than distal teeth, both directed laterally (). Anterior chaetigers with up to 11 falcigers; posterior ones with up to 3 falcigers.
Acicula tapering and yellow all along the body. Subacicular hooks present from chaetiger 22, yellow, bidentate, proximal teeth longer and thicker than distal ones, directed laterally; distal teeth directed slightly upward (); always single in each chaetiger.
Pygidium with two pairs of anal cirri without articulations, all of similar length; ventral cirri slender.
Variations
L10 = 1.0–2.0 mm, W10 = 0.6–0.8 mm. The start of subacicular hooks is size-dependent and varies from chaetiger 12 to 17.
Type locality
Puerto Morelos, Quintana Roo (Mexican Caribbean).
Etymology
The name of the species combines the generic name of the host plant (Thalassia testudinum) with the suffix indicating that this association is strong, possibly obligate.
Distribution
Caribbean Sea, Mexico and Belize.
Remarks
Lysidice thalassicola n. sp. resembles L. caribensis n. sp., L. carriebowensis n. sp., L. collaris, L. ninetta and L. phyllisae n. sp. in having yellow subacicular hooks. L. thalassicola n. sp. differs from L. carriebowensis n. sp., L. ninetta and L. phyllisae n. sp. in having a rounded prostomium, rounded eyes, and yellow acicula in all chaetigers instead of having black aciculae in at least one body region. In L. thalassicola n. sp. the region with basally inflated ventral cirri continues to the posterior end, whereas in all the other species it ends in late anterior or median chaetigers. Furthermore, L. thalassicola n. sp. is a borer of the sheaths of the sea grass T. testudinum (), while the other species are found preferentially associated with coral rocks or rubbles.
Reproductive features
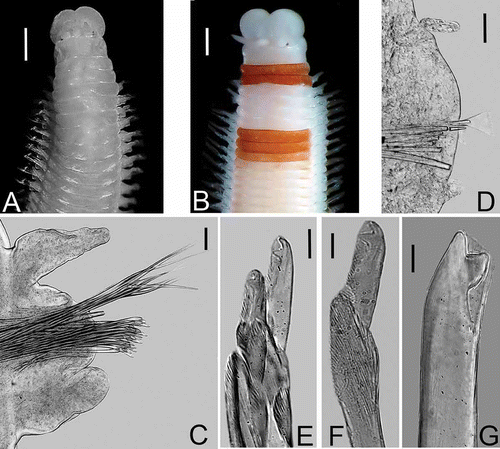
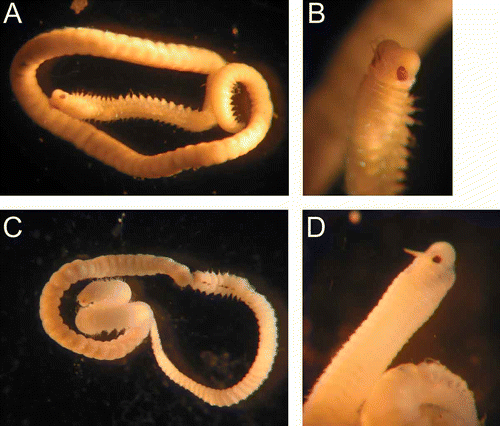
Mature males and females were present in material of L. thalassicola, L. adrianae and L. caribensis collected in November 2005 and October 2007 at Carrie Bow Cay (Belize), and in January 2007 at Puerto Morelos (Mexico). All species are gonochoric, and mature individuals have epitokous transformations with nearly two-thirds of the posterior body swollen and full of gametes, and with eyes extraordinarily enlarged (). Similar reproductive modes have been observed in two species of Lysidice, and in Nematonereis uniconis from the Mediterranean Sea (Gambi & Cigliano Citation2006).
Figure 6. A,B. Lysidice caribensis, epitokous specimen (male) and detail of its anterior part. C,D, Lysidice thalassicola, mature male specimen and detail of its anterior part. Note in both species the enlarged eyes.
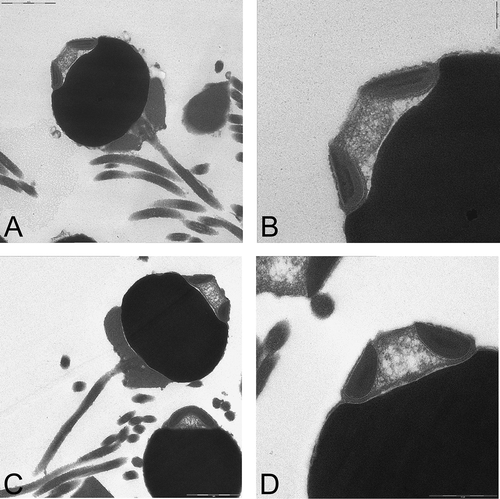
Egg size was measured in 3 females of L. thalassicola, ranging from 100 to 140 μm, and in a single female of L. caribensis (100–125 μm) and of L. adrianae (100–125 μm). The mature sperm morphology, examined by TEM, showed a very similar structure for the two species studied, L. caribensis and L. adrianae, with a rounded nucleus (,C) and a cap-like acrosome with a central sub-acrosomal space (,D).
Ecological features
A few observations on ecological features of the new species of Lysidice are here provided to highlight habitat preferences. Lysidice caribensis is the most common species in the material analyzed and in the whole studied geographic region. The species was mainly collected in dead coral rubble (down to 20 m depth), and with just one individual also found inside of a sponge (Tedania insignis).
Lysidice adrianae was observed exclusively in coral rubble and mainly in relatively shallow water (1–6 m depth).
Lysidice thalassicola shows the most interesting ecology, being exclusively found in association with Thalassia testudinum, as a borer of the seagrass sheaths (Gambi et al. Citation2003; Vasapollo et al. Citation2008) (). A few other species of Lysidice, as well as Nematonereis, have been observed with this unique and unusual life habit in the Mediterranean Sea (Gambi Citation2002). However, in the Mediterranean the boring species are also associated with other plant species and vegetated systems (Martin Citation1987), while L. thalassicola has been found only within the sheaths of Thalassia. This represents a very specialized habitat preference, and as far as we know L. thalassicola is the only species of polychaetes which seems so strictly associated to Thalassia. Lysidice carriebowensis and L. phyllisae, each represented by only a few individuals, have been found only at Carrie Bow Cay, in dead coral rubble. The number of specimens is still too limited to state anything more about their ecology and habitat preference, other than that these species seem to be associated with coral rocks and rubble.
Conclusive remarks
The present revision of the genus Lysidice in the Caribbean demonstrates the presence of five new species, all previously confused with Lysidice ninetta. Although we have considered various different habitats (coral rocks, rubbles and sands, Thalassia meadows), the geographic area investigated, as well as the depth range considered (0.5–20 m) are still relatively limited with respect to the extent and habitat diversity of the Great Caribbean region, therefore further investigations in different regions or depths may result in finding additional new species of Lysidice.
Among the new species recorded, we found some widely distributed taxa (L. thalassicola, L. caribensis, L. adrianae) and others with a more restricted geographic distribution (L. carriebowensis, L. phyllisae). Clearly, Lysidice displays a complex and diversified taxonomy in the Western Caribbean, showing clear habitat and geographic separation among the different species.
Reproductive habits appeared similar to congeneric species in temperate regions. The general spermatozoa structure, and the acrosome morphology in particular, is also very similar to that observed in other species of Lysidice from the Mediterranean Sea, as well as in N. unicornis (Gambi & Cigliano Citation2006), suggesting that this character is highly conservative in this group of closely related taxa. This ultrastructure is a typical ect-aquasperm morphology (Jamieson & Rouse Citation1989), and is generally related to free spawning and external fertilization. In the case of the Lysidice spp., this is consistent with epitokous reproduction.
Acknowledgements
We wish to thank Adriana Giangrande, Maria Alessandra Iannotta and Claudio Vasapollo for help in collecting material used for this study at Carrie Bow Cay and Puerto Morelos. Thanks are also due to Klaus Ruetzler, William Geoff Keel and Daniel Miller for support at Carrie Bow Cay, and to Brigit Van Tussenbroek for support at Puerto Morelos. The project was partially funded by the Caribbean Coral Reef Ecology Program of the Smithsonian Institution, and by a CNR (Italy)–CONACYT (Mexico) fellowship to MCG (2006–2008), and partially by the project ‘Taxonomía de poliquetos del Gran Caribe y evaluación molecular de especies anfiamericanas (Annelida: Polychaeta)’ by CONACYT (61609).
References
- Carrera-Parra LF, Fauchald K, Gambi MC. 2007. Revision of the taxonomic status of Lysidice Savigny (Polychaeta, Eunicidae) in the Western Caribbean Sea with observations on reproductive features and habitat preference of different species. Smithsonian Marine Science Symposium, 15–17 November 2007. (Abstract.) http://www.si.edu/ marinescience/symposium/speakers/fauchald_ab.htm (http://www.si.edu/ marinescience/symposium/speakers/fauchald_ab.htm)
- Carrera-Parra , LF and Salazar-Vallejo , SI. 1998 . Eunícidos (Polychaeta) del Caribe mexicano con claves para las especies del Gran Caribe: Eunice . Revista de Biología Tropical , 45 : 1481 – 1498 .
- Fauchald , K. 1970 . Polychaetous annelids of the family Eunicidae, Lumbrineridae, Iphitimidae and Dorvilleidae from the Western Mexico . Allan Hancock Monography, Marine Biology , 5 : 1 – 335 .
- Fauchald , K. 1992 . A review of the genus Eunice(Polychaeta: Eunicidae) based upon type material . Smithsonian Contributions to Zoology , 523 : 1 – 422 .
- Fauchald , K. 2010 . A revision of Nematonereis(Polychaeta, Eunicidae). 10th Polychaete Conference , Lecce 20–26 . June 2010 (Abstract).
- Gambi , MC. 2002 . Spatio-temporal distribution and ecological role of polychaete borers of Posidonia oceanica (L.) Delile scales . Bulletin of Marine Sciences , 71 : 1323 – 1331 .
- Gambi , MC and Cigliano , M. 2006 . Observations on reproductive features of three species of Eunicidae (Polychaeta) associated with Posidonia oceanica seagrass meadows in the Mediterranean Sea . Scientia Marina , 70S3 : 301 – 308 .
- Gambi , MC , Fauchald , K and Giangrande , A. 2006 . Taxonomic status and distribution of Lysidice and Nematonereis species (Polychaeta, Eunicidae) and other selected families (Sabellidae and Syllidae) in the Western Caribbean Sea , 12 – 14 . Do different habitat select for different species? CCRE Report 2005, Smithsonian Institution (January 2006) .
- Gambi , MC , van Tussenbroek , BI and Brearley , A. 2003 . Mesofaunal borers in seagrasses: World-wide occurrence and a new record of boring polychaetes in the Mexican Caribbean . Aquatic Botany , 76 : 65 – 77 .
- Iannotta , MA , Gambi , MC and Patti , FP. 2009 . Molecular evidence of intraspecific variability in Lysidice ninetta (Polychaeta: Eunicidae) in the Mediterranean Sea . Aquatic Biology , 6 : 121 – 132 .
- Iannotta , MA , Patti , FP , Ambrosino , M , Procaccini , G and Gambi , MC. 2007 . Phylogeography of two species of Lysidice (Polychaeta, Eunicidae) associated to the seagrass Posidonia oceanica in the Mediterranean Sea . Marine Biology , 150 : 1115 – 1125 .
- Jamieson , BGM and Rouse , GW. 1989 . The spermatozoa of the Polychaeta (Annelida): An ultrastructural review . Biological Review , 64 : 93 – 157 .
- Kurt Sahin , G and Cinar , ME. 2009 . Eunicidae (Polychaeta) species in and around Iskenderun Bay (Levantine Sea, Eastern Mediterranean) with a new alien species for the Mediterranean Sea and re-description of Lysidice collaris . Turkish Journal of Zoology , 33 : 331 – 347 .
- Martin , D. 1987 . Anélidos Poliquetos asociados a los concreciones de algas calcareas del litoral catalon. Miscellanea Zoologica 11 , : 61 – 75 .
- Salazar-Vallejo , SI. 1996 . Lista de especies y bibliografia de poliquetos (Polychaeta) del Gran Caribe . Anales Instituo Biologi Universidad Nacional Autonom Mexico, Serie Zoologia , 67 : 11 – 50 .
- Salazar-Vallejo , SI and Carrera-Parra , LF. 1998 . Eunicidos (Polychaeta) del Caribe mexicano con claves para las especies del Gran Caribe: Fauchaldius, Lysidice, Marphysa, Nematonereis y Palola . Revista de Biología Tropical , 45 : 1499 – 1521 .
- Vasapollo , C , Iannotta , MA , van Tussenbroek , BI and Gambi , MC. 2008 . First data on frequency and spatial distribution of borer polychaetes in Thalassia testudinum seagrass meadows off the Western Caribbean . Biologia Marina Mediterranea , 15 : 292 – 293 .