Abstract
Ophryotrocha diadema is an outcrossing, simultaneous hermaphroditic polychaete with external fertilization. In isolated pairs, mature worms take turn contributing eggs upon the condition that their partners reciprocate egg donation. In dense populations, these worms do not reciprocate. Instead, they strongly compete for mating in their preferred male role and produce few eggs. This plastic sex allocation may result in an overall different reproductive performance: mean individual reproductive output will be larger in sparse than in dense populations. We tested this hypothesis by measuring the individual reproductive output (paternal and maternal offspring) of worms in sparse and dense replicated populations. In dense populations, mean individual reproductive output was fourfold lower than that in sparse populations. We hypothesise that such dramatic demographic costs are potentially widespread in outcrossing simultaneous hermaphrodites with external fertilization and plastic sex allocation. The reproductive output of hermaphroditic organisms is a function of population density (i.e. the number of conspecifics) and studies on population growth and reproductive performance should take this effect into account.
Introduction
Simultaneous hermaphrodites have two sexual functions and partition their reproductive resources between them. Sex allocation theory predicts that they plastically adjust the proportion of resources allocated to each sex as a function of mating group size (Charnov Citation1982). Theory predicts that in sparse populations, where monogamous pairs may form, hermaphrodites increase their investment in eggs and limit the male function to the production of the few sperm needed to fertilize their partners’ eggs. In dense populations, hermaphrodites divert resources from egg production and invest more into the male function. Experiments or observations on different hermaphrodites have tested this theory and have often found overall support for it, although the patterns of resource allocation adjustments are highly species-specific (Raimondi & Martin Citation1991; Trouvè et al. et al. Citation1999; Hughes et al. Citation2002; Locher & Baur Citation2002; Tan et al. Citation2004; Brauer et al. Citation2007; Schärer & Janicke Citation2009). In some model systems, results show that sex allocation in hermaphrodites is plastic (i.e. it changes as a function of mating opportunities), as predicted by theory. For example, the hermaphroditic polychaete worms Ophryotrocha diadema CitationÅkesson (1976) (Annelida: Polychaeta: Dorvilleidae) have plastic female allocation that they adjust to mating opportunities, trading off with their investment in the male function. When mating opportunities are common (as in dense populations), worms reduce their egg production drastically and compete for mating in the male role; when mating opportunities are rare (as in sparse populations), they invest proportionally more resources in egg production and, in the absence of competitors, reduce their investment in the male function (Lorenzi et al. Citation2005, Citation2006). Sex allocation adjustments are the effect of sexual selection acting on both sexual functions in hermaphrodites (Lorenzi & Sella Citation2008; Anthes et al. Citation2010). These adjustments are typically hermaphroditic traits, and could explain why population growth rates (as measured in dense, lab populations) are higher in gonochoric than hermaphroditic species (Prevedelli et al. Citation2006).
If we assume a fixed budget for reproductive resources, we expect that hermaphrodites in large populations would use the same amount of resources for egg production than hermaphrodites in small populations, devalued of the resources diverted to increase the male function. Then we should find that hermaphrodites in large populations have a lower mean reproductive success than those in small populations. This reduced reproductive output should, in turn, affect population growth. We tested this hypothesis in the outcrossing simultaneously hermaphroditic polychaete worm O. diadema by measuring individual reproductive output of focal worms in sparse and dense populations.
Material and methods
The animal model
Ophryotrocha diadema (Annelida, Polychaeta, Dorvilleidae) is a polychaete worm originally found in the sediments of Californian harbors. Sampling from natural populations suggests that populations have low densities (Premoli & Sella Citation1995). For example, only few O. diadema individuals were isolated among hundreds of worms of a gonochoric Ophryotrocha species in the Pacific Coast (pers. comm. by B. Åkesson to G.S.) and 0.1–6.6 individuals kg−1 of mussel clusters were collected in the Mediterranean Sea (R. Simonini, pers. comm. to M.C.L.) (Schleicherová et al. Citation2013).
These worms are outcrossing simultaneous hermaphrodites with external fertilization. Before maturing as hermaphrodites, they have a protandrous phase during which they can fertilize the eggs laid by hermaphrodites (Sella & Lorenzi Citation2003). Then, they mature as hermaphrodites, and can both fertilize their partners’ eggs or lay eggs, but play one single role at each mating event. Eggs are laid in jelly cocoons and develop into larvae that leave their cocoons 8 days later and mature into simultaneous hermaphrodites in approx. 45 days. Mature hermaphrodites reproduce iteroparously for 7–10 weeks (Åkesson Citation1976, Citation1982).
In isolated pairs, worms take turns in laying cocoons of 20–25 eggs every third day (Sella Citation1985, Citation1988). When more than two worms are present, they adjust their sex allocation by investing proportionally more resources into the male function (Lorenzi et al. Citation2005, Citation2006), mate promiscuously (Sella & Lorenzi Citation2000) and can share the paternity of a single egg-cocoon with other hermaphrodites (Lorenzi et al. Citation2013). Sex allocation adjustments are not costly in the short term (Lorenzi et al. Citation2008) and polychaetes sense the number of conspecifics and/or potential mates through waterborne chemical cues (Schleicherová et al. Citation2006, Citation2010; Minetti et al. Citation2013).
Experimental procedure
Data were gathered from focal worms. The “focal” worms were identified through the colour of their eggs. In mature worms, eggs can be easily detected through the transparent body wall as either yellow or whitish eggs. In these worms, a dominant Y allele determines a yellow-egg phenotype, while the recessive y allele determines a white-egg phenotype (Sella & Marzona Citation1983). By means of this genetic marker, we can identify focal worms in a group and ascribe their progeny. The focal worms had yellow eggs and their mates had white eggs.
We carried out the experiment in glass bowls filled with 10 mL artificial sea water and kept in a thermostatic chamber at 20°C. Once a week water was replaced in the bowls and worms were fed with spinach ad libitum.
To obtain a sufficient number of worms for the experiment, 24 pairs of yellow-phenotype worms and 40 pairs of white ones were cultivated separately and allowed to reproduce. Their offspring supplied the virgin, newly mature, yellow- and white-phenotype worms of the same age to be used for the experiment. At sexual maturity, two worms from each yellow-phenotype offspring (n = 48 worms, hereafter, “focal worms”) were randomly assigned either to sparse populations (population size = 2; the population consisting of one focal, yellow-phenotype worm and one white-phenotype partner, n = 24 replicates) or to dense populations (population size = 12, consisting of one focal, yellow-phenotype worm and 11 white-phenotype potential partners, n = 24 replicates). With such a matched-sample design, each worm in the sparse population served as a control for its sibling in the dense population.
Experimental populations were checked daily for 12 days. At the first check, focal worms’ body size was measured as the number of chaetigerous segments. At each check, we recorded the number of yellow (laid by focal worms) and white (laid by focal partner/s) cocoons, and the number of eggs per cocoon. Adult worms were removed from the bowls on day 9.
Reproductive output of focal worms was quantified by rearing offspring until they were sexually mature. Indeed, in large populations, multiple potential “fathers” were present and paternity of the progeny could be assigned to either the focal worms or one of their rivals only after the progenies were sexually mature. When these worms matured and had eggs in their coeloms, they expressed their yellow or white phenotypes and we assessed their paternity (i.e. about 45 days after egg laying). Following Åkesson (Citation1976), this marker is neutral, since there is no difference in worm mortality rates before sexual maturity.
The ratio between the total number of cocoons produced in sparse populations and that produced in dense populations was approx 1:2, leading to more larvae per unit of volume in the dense population bowls. Therefore, to standardize rearing conditions, on day 9 the volume of sea water was doubled in the dense population bowls.
The total reproductive output of focal worms was estimated as the number of offspring (both maternal and paternal offspring) that on maturity had the yellow phenotype. Focal worms without offspring were included in the calculations.
In order to control for the potentially confounding effect of differential egg mortality in sparse and dense populations, we estimated egg mortality as the average proportion of eggs that disappeared from the cocoons in each bowl (with respect to the laid eggs).
Statistical analyses
Some replicates were excluded from calculations for various reasons (e.g. some worms died, altering population size). By using related worms in sparse and dense populations, we reduced the overall variability due to genetic differences (Howell Citation2010). We used a linear mixed model (LMM) to assess the significance of the differences in reproductive output between pairs of siblings in sparse and dense populations (dependent variable: reproductive output; within-subject factor: population size; random factor: family ID; covariate: body size).
Probabilities were two-tailed. Statistical analyses were performed using SPSS 20.0 statistical package (SPSS Inc, Chicago, IL).
Results
Reproductive output of focal worms
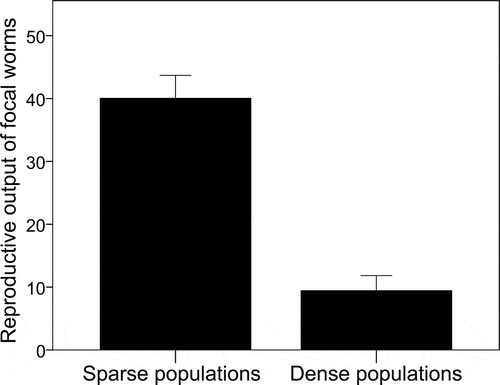
Focal worms had a dramatically lower reproductive rate in dense populations compared to that in sparse populations, with a fourfold reduction in their reproductive output (mature maternal + paternal offspring) (). The difference was highly significant, indicating that reproductive output in simultaneous hermaphrodites was strongly affected by population size (LMM, population size: F1,35.79 = 55.557, P < 0.0001; body size: F1,34.99 = 4.721, P = 0.037). The significant relationship between body size and reproductive output, which emerged in the LMM, occurred only in dense populations (Spearman’s rho, in dense populations: rho = 0.496, P = 0.022; in sparse populations: rho = 0.133, P = 0.545). Body size advantage in dense populations was not associated with the female function (correlation between body size and egg production in dense populations: rho = 0.180, P = 0.460; in sparse populations: rho = 0.288, P = 0.231). This suggests that larger hermaphrodites in dense populations might have a higher reproductive output because they were more successful in the competition for the male role.
Egg mortality was not significantly different between sparse and dense populations (Wilcoxon test, Z = 73.00, total n = 20, P = 0.376), suggesting that it did not affect the results (median proportion of eggs which disappeared in sparse populations: 5.56% vs. 4.86% in dense populations).
Discussion
In this study, we document that population size (i.e. the number of conspecifics) affects the reproductive output of simultaneous hermaphrodites, as they produce four times more offspring in sparse than in dense populations. We interpret these results as a consequence of the fact that hermaphrodites have a plastic sex allocation, which they adjust to mating opportunities. As population size increases, mating opportunities increase as well and hermaphrodites adjust their sex allocation in favour of the male function at the expense of the female function.
The reduced reproductive output of the worms in dense vs. sparse populations could be the result of uncontrolled density-dependent effects (e.g. mortality, oophagy, etc.) rather than a response to population size (e.g. the number of conspecifics). However, this hypothesis is not supported by evidence. First, egg mortality did not differ between sparse and dense populations. Second, a previous study documented that O. diadema worms had a higher egg production in sparse than in dense populations, irrespective of any density-dependent effects such as metabolite accumulation or encounter probability (Lorenzi et al. Citation2005). Furthermore, in other experiments, we simulated large population size, so that pairs of worms perceived cues as if population size were larger than two, and they reduced their egg output according to the perceived, and not the real, population size (Schleicherovà et al. Citation2006, Citation2010). All these observations support the hypothesis that worms reduce their egg output as population size increases.
It could be argued that, if worms decrease egg production in dense populations, the competition for mating as males should increase and worms with more female-biased allocation will gain higher reproductive success. Whilst this might be true in the short term, it might be disadvantageous in the long term, because fecundity often trades off with lifespan (Stearns & Hoekstra Citation2000). Indeed, hermaphrodites which skip the female role for long time periods live longer (Di Bona et al. Citation2010).
Mating in sparse populations is associated with small mating groups, i.e. low numbers of partners and few or no rivals over the male role. In small mating groups, hermaphrodites invest large proportions of their reproductive resources into eggs, trade eggs with their partners and take turns in the two sexual roles (Sella Citation1985; Sella & Ramella Citation1999). This is an evolutionary solution to the conflict over sex roles, since both partners prefer to play the cheaper male role than the expensive female role (Leonard Citation1993, Citation2005, Citation2006; Di Bona et al. Citation2010). In natural contexts, outcrossing hermaphrodites may be constrained to monogamous mating regimes when they live in very sparse populations, as O. diadema does (Sella & Ramella Citation1999; R. Simonini, pers. comm.). In other hermaphroditic species, the sizes of the populations are large but hermaphrodites are trapped in monogamous mating regimes by other life-history traits. For example, the serranid fish Hypoplectrus nigricans is an outcrossing hermaphrodite which mates monogamously (Fischer Citation1980). Here, monogamy is constrained by the short spawning period (a few hours per day), which reduces the chances that paired partners desert: reproductive gains from deserting the partner may be low, if most partners are paired.
In the present study, worms in dense populations reduced their reproductive output to less than 30% when compared to worms in sparse populations. Similarly, Plasmodium chabaudi adjust their sex allocation in response to the presence of unrelated conspecifics. Reece et al. (Citation2008) directly manipulated the mating-group sex ratio of these malaria parasites and measured the resulting reproductive output as the number of zygotes produced. As predicted by sex allocation theory, mating output was maximized at intermediate sex ratios, indicating that sex allocation in this malaria parasite is likely to be under stabilizing selection and reproductive output was maximized at female-biased sex ratios.
Overall, our study shows that the potential individual advantages in fitness due to opportunistic sex allocation are countered at the population level when populations are dense; opportunistic sex allocation is advantageous to the individual, but disadvantageous to the population, whose reproductive rate declines. Accordingly, Prevedelli et al. (Citation2006) found that dense populations of hermaphrodites had a demographic disadvantage compared to gonochorists. Here, we highlight that the demographic disadvantage of hermaphrodites is mainly due to their adaptive ability to adjust their sex allocation to mating group size and, ultimately, to population size. In this perspective, our study is an example of the tragedy of the commons (Hardin Citation1968), where traits that are advantageous at the individual level reduce population fitness. For example, strong cannibalism of larvae and pupae by adult flour beetles is adaptive at the individual level but impairs population growth (Wade Citation1977). Similarly, hyperaggressive water-strider males gain a slightly higher mating success than less aggressive males but reduce overall group mating in their pond (Chang & Sih Citation2013). More specifically, sexual selection can diminish population reproductive rates of Drosophila populations by imposing a “reproductive load” (Holland & Rice Citation1999). The reproductive load highlighted in Drosophila was caused by antagonist sexual selection and intersexual conflicts inherent to promiscuity. Similarly, sex allocation adjustments are promoted by sexual selection acting on the two sexes of simultaneous hermaphrodites (Lorenzi & Sella Citation2008; Anthes et al. Citation2010; Leonard Citation2013).
We highlight that the demographic advantage of hermaphroditism in sparse populations (relative to dense populations) is the bare outcome of sex allocation adjustments in hermaphrodites where the two sexual functions interfere with each other and resources are traded off between the male and female function (Lorenzi et al. Citation2006). Therefore, we expect that the results we obtained here could be obtained in other hermaphroditic systems as well, where the two sexual functions act in opposition and resources are partitioned between the male and the female function on the basis of population size. We hypothesise that such dramatic demographic costs of sex allocation are potentially widespread in outcrossing simultaneous hermaphrodites with external fertilization. If the reproductive output of hermaphroditic organisms is a function of population size, population growth studies (and their practical applications) should take the effect of sex allocation into account.
Acknowledgements
We thank Sergio Castellano and Janet Leonard for fruitful discussion on sexual selection, John Pannell for comments on a previous version of the manuscript and Silvia Natta for help in the laboratory. We also wish to thank two anonymous referees who made helpful comments on a previous version of the manuscript. This work was funded by MIUR (PRIN 2008 YS7AP3) and the University of Turin (ex 60% to M.C.L. and G.S.).
References
- Åkesson B. 1976. Morphology and life cycle of Ophryotrocha diadema, a new polychaete species from California. Ophelia 15:25–35.
- Åkesson B. 1982. A life table study on three genetic strains of Ophryotrocha diadema (Polychaeta, Dorvilleidae). International Journal of Invertebrate Reproduction 5:59–69.
- Anthes N, David P, Auld JR, Hoffer JNA, Jarne P, Koene JM, Kokko H, Lorenzi MC, Pélissié B, Sprenger D, Staikou A, Schärer L. 2010. Bateman gradients in hermaphrodites: An extended approach to quantify sexual selection. American Naturalist 176:249–263.
- Brauer VS, Schärer L, Michiels NK. 2007. Phenotypically flexible sex allocation in a simultaneous hermaphrodite. Evolution 61:216–222.
- Chang AT, Sih A. 2013. Multilevel selection and effects of keystone hyperaggressive males on mating success and behavior in stream water striders. Behavioral Ecology 24:1166–1176.
- Charnov EL. 1982. The theory of sex allocation. Princeton, N.J.: Princeton University Press.
- Di Bona V, Lorenzi MC, Sella G. 2010. Functional males in pair mating outcrossing populations of hermaphroditic animals. Biological Journal of the Linnean Society 100:451–456.
- Fischer EA. 1980. The relationship between mating system and simultaneous hermaphroditism in the coral reef fish, Hypoplectrus nigricans (Serranidae). Animal Behaviour 28:988–993.
- Hardin G. 1968. Tragedy of the commons. Science 162:1243–1248.
- Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proceedings of the National Academy of Sciences USA 96:5083–5088.
- Howell DC. 2010. Statistical methods for psychology. 7th ed. Belmont, CA: Cengage Wadsworth.
- Hughes RN, Manríquez PH, Bishop JDD. 2002. Female investment is retarded pending reception of allosperm in a hermaphroditic colonial invertebrate. Proceedings of the National Academy of Sciences USA 99:14884–14886.
- Leonard JL. 1993. Sexual conflict in simultaneous hermaphrodites: Evidence from serranid fishes. Environmental Biology of Fishes 36:135–148.
- Leonard JL. 2005. Bateman’s principle and simultaneous hermaphrodites: A paradox. Integrative and Comparative Biology 45:856–873.
- Leonard JL. 2006. Sexual selection: Lessons from hermaphrodite mating systems. Integrative and Comparative Biology 46:349–367.
- Leonard JL. 2013. Sexual selection and hermaphroditic organisms: Testing theory. Current Zoology 59:579–588.
- Locher R, Baur B. 2002. Nutritional stress changes sex specific reproductive allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Functional Ecology 16:623–632.
- Lorenzi MC, Schleicherová D, Sella G. 2006. Life history and sex allocation in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema: The role of sperm competition. Integrative and Comparative Biology 46:381–389.
- Lorenzi MC, Schleicherová D, Sella G. 2008. Sex adjustments are not functionally costly in simultaneous hermaphrodites. Marine Biology 153:599–604.
- Lorenzi MC, Schleicherová D, Sella G. 2013. Multiple paternity and mate competition in non-selfing, monogamous, egg-trading hermaphrodites. Acta Ethologica in press. doi:10.1007/s10211-013-0169-x
- Lorenzi MC, Sella G. 2008. A measure of sexual selection in hermaphroditic animals: Parentage skew and the opportunity for selection. Journal of Evolutionary Biology 21:827–833.
- Lorenzi MC, Sella G, Schleicherová D, Ramella L 2005. Outcrossing hermaphroditic polychaete worms adjust their sex allocation to social conditions. Journal of Evolutionary Biology 18:1341–1347.
- Minetti C, Sella G, Lorenzi MC. 2013. Population size, not density, serves as cue for sex ratio adjustments in polychaete worms. Italian Journal of Zoology. doi:10.1080/11250003.2013.841775
- Premoli MC, Sella G. 1995. Sex economy in benthic polychaetes. Ethology Ecology & Evolution 7:27–48.
- Prevedelli D, Massamba N’Siala G, Simonini R. 2006. Gonochorism vs. hermaphroditism: Relationship between life history and fitness in three species of Ophryotrocha (Polychaeta: Dorvilleidae) with different forms of sexuality. Journal of Animal Ecology 75:203–212.
- Raimondi PT, Martin JE. 1991. Evidence that mating group size affects allocation of reproductive resources in a simultaneous hermaphrodite. American Naturalist 138:1206–1217.
- Reece SE, Drew DR, Gardner A. 2008. Sex ratio adjustment and kin discrimination in malaria parasites. Nature 453:609–613.
- Schärer L, Janicke T. 2009. Sex allocation and sexual conflict in simultaneously hermaphroditic animals. Biology Letters 5:705–708.
- Schleicherová D, Lorenzi MC, Sella G. 2006. How outcrossing hermaphrodites sense the presence of conspecifics and suppress female allocation. Behavioual Ecology 17:1–5.
- Schleicherová D, Lorenzi MC, Sella G. 2013. Do stable environments select against phenotypic plasticity in hermaphroditic sex allocation? Italian Journal of Zoology 80:358–363.
- Schleicherová D, Lorenzi MC, Sella G, Michiels NK. 2010. Gender expression and group size: A test in a hermaphroditic and a gonochoric congeneric species of Ophryotrocha (Polychaeta). Journal of Experimental Biology 213:1586–1590.
- Sella G. 1985. Reciprocal egg trading and brood care in a hermaphroditic polychaete worm. Animal Behaviour 33:938–944.
- Sella G. 1988. Reciprocation, reproductive success and safeguards against cheating in the mating system of a hermaphroditic polychaete worm, Ophryotrocha diadema. Biological Bullettin 175:212–217.
- Sella G, Lorenzi MC. 2000. Partner fidelity and egg reciprocation in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema. Behavioural Ecology 11:260–264.
- Sella G, Lorenzi MC. 2003. Increased sperm allocation delays body growth in a protandrous simultaneous hermaphrodite. Biological Journal of the Linnean Society 78:149–154.
- Sella G, Marzona M. 1983. Inheritance, maternal influence and biochemical analysis of an egg color polymorphism in Ophryotrocha diadema. Experientia 39:97–98.
- Sella G, Ramella L. 1999. Sexual conflict and mating system in the dorvilleid genus Ophryotrocha and the dinophilid genus Dinophilus. Hydrobiologia 402:203–213.
- Stearns SC, Hoekstra RF. 2000. Evolution – an introduction. New York: Oxford University Press.
- Tan GN, Govedich FR, Burd M. 2004. Social group size, potential sperm competition and reproductive investment in a hermaphroditic leech, Helobdella papillornata (Euhirudinea: Glossiphoniidae). Journal of Evolutionary Biology 17:574–580.
- Trouvé S, Jourdane J, Renaud F, Durand P, Morand S. 1999. Adaptive sex allocation in a simultaneous hermaphrodite. Evolution 53:1599–1604.
- Wade MJ. 1977. An experimental study of group selection. Evolution 31:134–153.