Abstract
We describe the spermatogenic cycle of the African sideneck turtle (Pelusios castaneus) (Reptilia: Testudines). We further classify the pattern of spermatogenesis in the species, generating new comparative baseline data on the spermatogenic cycle of freshwater turtles in the tropics. Seventy-two adult male P. castaneus collected at different times from various river drainages in Ibadan, Nigeria, were used for the study. The average body weight of the turtles was 723 ± 23.36 g while the curved carapace and plastron lengths of the turtles were 24.4 ± 1.47 cm and 15.7 ± 1.23 cm, respectively. Histology of the testis and epididymis, gonadosomatic index, epididymal mass index, seminiferous tubule diameter and epididymal ductal diameter all suggest the postnuptial pattern of spermatogenesis. Sperm production in P. castaneus starts in May and peaks in September. It is thereafter released into the epididymis, and the degree of sperm packing of the epididymis increases from October, reaching its peak in January. Spermatozoa were found in the epididymis of the turtles all through the year. The study shows that the postnuptial pattern of turtle spermatogenesis is not unique to turtles in the temperate regions of the world and that the spermatogenic cycle of P. castaneus is similar to those of other chelonians with minor variations. This report, the first of its kind for a pelomedusid turtle, therefore serves as baseline information for the family in comparative studies of the spermatogenic cycle of turtles.
Introduction
The reproductive biology of tropical freshwater turtles has received little attention in comparison with that of temperate species. Reproduction of freshwater turtles in temperate regions is largely constrained by seasonal variation in temperature, with mating, egg development, nesting and hatching confined to warmer months, whereas freshwater turtles in tropical climates generally experience warm temperatures all year round (Kennett Citation1999). The African sideneck turtle Pelusios castaneus (Schweigger, 1812) (Reptilia: Testudines) is a freshwater turtle of the family Pelomedusidae, widely distributed in West Africa, occurring from Guinea and Senegal to northwestern Angola (Kirkpatrick Citation1995).
Spermatogenesis in turtles has been described by several authors as following one of three patterns: prenuptial, postnuptial (dissociated pattern) and acyclic (Georges Citation1983; Rao & Shaad Citation1985; Kennett Citation1999; Meylan et al. Citation2002). In the prenuptial pattern, spermatogenesis immediately precedes mating, while in postnuptial spermatogenesis, spring mating uses spermatozoa produced the previous summer and or fall that have been stored in the epididymides over winter during a sexually quiescent phase (Lofts Citation1977; Lofts & Tsui Citation1977; Meylan et al. Citation2002). However, in acyclic spermatogenesis, there is a continual production of spermatozoa without a defined peak or quiescence (Meylan et al. Citation2002). It has been reported that certain turtles that show a postnuptial pattern of spermatogenesis (spring mating in North American forms) also mate at the height of spermatogenesis (fall mating in North American forms) as in the prenuptial pattern (Gibbons & Greene Citation1990; Gist et al. Citation1990).
The spermatogenic cycles of various marine and freshwater turtles have been documented, including the Stinkpot, Sternotherus odoratus (McPherson & Marion Citation1981); the Australian freshwater turtle Emydura krefftii (Georges Citation1983); the freshwater turtle Trionyx gangeticus (Rao & Shaad Citation1985); the Loggerhead turtle, Caretta caretta (Wibbles et al. Citation1990); the Kemp’s Ridley sea turtle, Lepidochelys kempi (Rostal Citation1991); the Australian freshwater turtles Chelodina rugosa and Elseya dentata (Kennett Citation1999) and the Florida Softshell Turtle, Apalone ferox (Meylan et al. Citation2002). With the exception of the recent report of Olukole et al. (Citation2011), on the topographical anatomy of the male reproductive organs of the African sideneck turtle, little is known about the reproductive cycle in this family. The existing research reports on the genus Pelusios had mainly focused on its conservation, nutrition and history of migration (Broadley & Boycott Citation2009), morphometry of the external body anatomy (Olukole et al. Citation2010) and haematology (Omonona et al. Citation2011).
The spermatogenic cycle of pelomedusids has not been previously studied with respect to reproductive biology; most of what is known is based on anecdotal reports (Broadley & Boycott Citation2009). Also, there is a scarcity of research reports on the spermatogenic cycle of tropical turtles, especially those indigenous to the African continent. This study was therefore designed to investigate the spermatogenic cycle of Pelusios castaneus with the view of generating information that would be useful in the development of a hypothesis of the evolution of spermatogenic cycles in turtles, and hence serve as baseline data on comparative spermatogenic cycles of freshwater turtles of the tropical and temperate regions of the world.
Materials and methods
Experimental animals
Over a 12-month period, a total of 72 adult male Pelusios castaneus (six for each month of the year) were used for the study. They were bought from local farmers who picked up these turtles at different times in various river drainages in Ibadan, Nigeria. The animals were kept in artificial ponds and were stabilized for 72 hours prior to the investigations carried out. They were fed with commercial fish pellets ad libitum. Standard body parameters were all determined using a Draper® 115-mm vernier caliper and metric tape. The body weight of the animals was taken with the aid of a Microvar® weighing balance. The turtles were anaesthetized using ketamine hydrochloric acid (HCl) at 25 mg/kg body weight intramuscularly on the medial aspects of the thigh. Anatomical nomenclature used in the study follows the reports of Lapparent De Broin (Citation2001) and Wyneken (Citation2001).
The animals were sacrificed by cervical decapitation. In order to separate the plastron from the carapace, a straight line cut through the bridge of each animal was made craniocaudally. This was followed by the detachment of the thick connective tissue between the pectoral apparatus from the anterior part of the plastron and cutting through of the pelvic bones attached to the coelomic surface of the plastron. The superficial and deep structures of the coelomic cavities of the turtles were exposed. The testis and epididymis were removed and weighed using the digital Microvar® weighing balance.
Seasonal studies
In order to determine the seasonal changes in the sizes of the testis and epididymis, the combined mass of the left and right of each organ was used to calculate the gonadosomatic index (GSI) and epididymal mass index (EMI) respectively, according to the formula given by Pickford (Citation1953):
Samples of the testes and epididymis were fixed in Bouin’s fluid and embedded in paraffin blocks. Sections 2–4 mm thick were stained with haematoxylin and eosin (Rao & Shaad Citation1985). The slides of testis and epididymis were studied under a light microscope. In order to determine the testicular and epididymal seasonal variation in the turtle, the seminiferous tubular diameter and epididymal duct diameter were measured. For each parameter, 10 measurements were made per section using a calibrated eye-piece micrometer (Graticules Ltd., Tonbridge, Kent). The testis from each turtle was classified using a spermatogenic stage schedule modified from Licht (Citation1967) and Meylan et al. (Citation2002):
Stage 1. Seminiferous tubule with single layer of Sertoli cells lining epithelium with spermatogonia interspersed.
Stage 2. Seminiferous tubules with several layers of Sertoli cells and spermatogonia.
Stage 3. Seminiferous tubules with primary spermatocytes; several cell layers of spermatogonia, and Sertoli cells.
Stage 4. Seminiferous tubules with more primary than secondary spermatocytes; spermatozoa and spermatids.
Stage 5. Seminiferous tubules with secondary spermatocytes more abundant with fewer primary spermatocytes; spermatids and spermatozoa present.
Stage 6. Seminiferous tubules with primary spermatocytes, abundant secondary spermatocytes, spermatids, lumen packed with spermatozoa (sperm peak of spermatogenesis).
Stage 7. Seminiferous tubules with fewer spermatids, abundant spermatozoa; mixing of clumped debris in the center of lumen; appearance of clearing out of spermatozoa.
For each month, the degree of packing of sperm in the epididymis of each turtle was scored subjectively as follows: 1: empty; 2: almost empty; 3: few loosely packed clumps of spermatozoa; 4: lumen full of loosely packed clumps of spermatozoa; 5: some loose and some dense clumps of spermatozoa; 6: lumen full of dense clumps of spermatozoa (Meylan et al. Citation2002).
Statistical analysis
All data obtained on a monthly basis were expressed as means with the standard error of mean using the GraphPad Prism version 4.00 for Windows, GraphPad Software. Analysis of variance was performed using two-way analysis of variance (ANOVA) and significance reported at P < 0.05.
Results
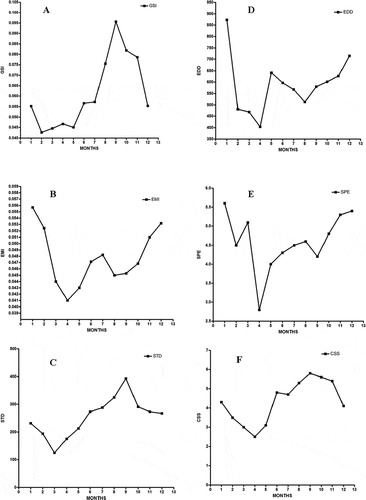
The average body weight of the turtles used for the study was 723 ± 23.36 g. The curved carapace and plastron lengths of the turtles were 24.4 ± 1.47 cm and 15.7 ± 1.23 cm, respectively. There was a significant difference (P < 0.0001) in the monthly body weight of the turtles. The average weights of the testis and epididymis were 0.51 ± 0.041 g and 0.36 ± 0.018 g, respectively. The weights of the testis and epididymis also significantly differed (P < 0.0001) among the months. Spermatozoa were present in the epididymis of all the turtles used for the study regardless of the variation in body weight; the smallest turtle (300 g) had mature spermatozoa in its testis and epididymis. The mean GSI and EMI for the 12-month period were 0.061% and 0.048%, respectively. The monthly GSI and EMI also differed significantly (P < 0.0001). The maximum value for GSI (0.096%) was observed in the month of September, with an average testicular weight of 0.45 g. This coincided with the period of maximal sperm production as spermatogenesis clearly begins in July, reaching its peak in September, with the lowest value being recorded in February (A). The GSI also showed a slow increase from February to May (period of low testicular activity in the animal) and a rapid increase between July and November, with the highest value being that of September (A).
Figure 1. Monthly variation in parameters of the testis and epididymis of the African sideneck turtle (Pelusios castaneus). A: Gonadosomatic index (GSI, %); B: Epididymis mass index (EMI, %); C: Seminiferous tubule diameter (STD, µm); D: Epididymal ductal diameter (EDD, µm); E: Degree of sperm packing of epididymis (SPE); F: Classification of spermatogenic stage (CSS). Notice that the months of the year are represented by their corresponding numerals in the figure above.
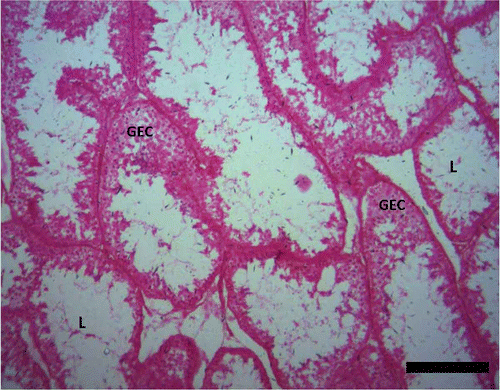
The spermatogenic stages in different months of the year showed slight variations, stage for stage. The seminiferous tubules were in a regressed condition and reduced germinal epithelium between the months of February and May ().
Figure 2. Photomicrograph of the testis of Pelusios castaneus. GEC: germinal epithelial cells; L: lumen (February). H&E: haematoxylin & eosin. Scale bar: 25 µm.
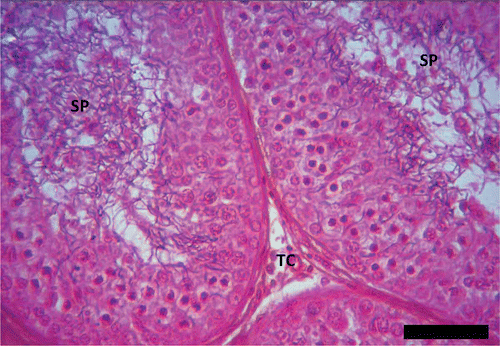
However, remnants of spermatozoa were found within the lumen of a few seminiferous tubules during the period. In the months of May and June, the seminiferous tubules were characterized by the presence of spermatogonia, spermatocytes and spermatids with few spermatozoa (). Between July and September, the histological analysis of the testis revealed the seminiferous tubules exhibiting increased spermatogenic activity (). This period was characterized by the presence of Sertoli cells, myoid cells (flattened contractile cells found outside the basement membrane of each seminiferous tubule), some spermatogonia, spermatocytes (more of secondary than primary spermatocytes), numerous spermatids (more of elongated than round spermatids) and the enlarged lumen of seminiferous tubules being fully packed with spermatozoa ().
Figure 3. Photomicrograph of the testis of Pelusios castaneus. GEC: germinal epithelial cells; SP: spermatozoa (May). H&E: haematoxylin & eosin. Scale bar: 25 µm.
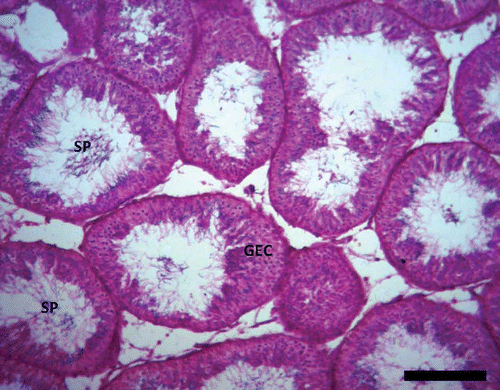
Figure 4. Peak of spermatogenesis in Pelusios castaneus. SP: spermatozoa; TC: interstitial cell (September). H&E: haematoxylin & eosin. Scale bar: 100 µm.
Some of the slides also revealed clumped debris in the centre of the lumen of the seminiferous tubules. The epididymis showed reduced epithelial height and an enlarged lumen filled with spermatozoa in the month of January ( and ). The histology of the epididymis showed that the epididymal duct is lined by a ciliated pseudostratified columnar epithelium and 5–8 ductuli efferentes that were lined by simple columnar epithelium ().
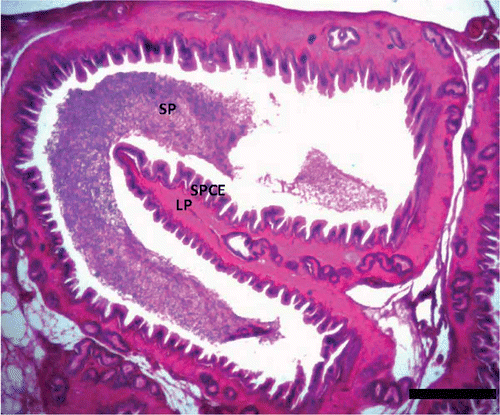
Figure 5. Photomicrograph of the epididymis of Pelusios castaneus showing spermatozoa within the lumen of the duct of the epididymis with efferent ducts (ED) within the lamina propria (LP); PSCE: pseudostratified columnar epithelium; SP: spermatozoa (January). H&E: haematoxylin & eosin. Scale bar: 25 µm.
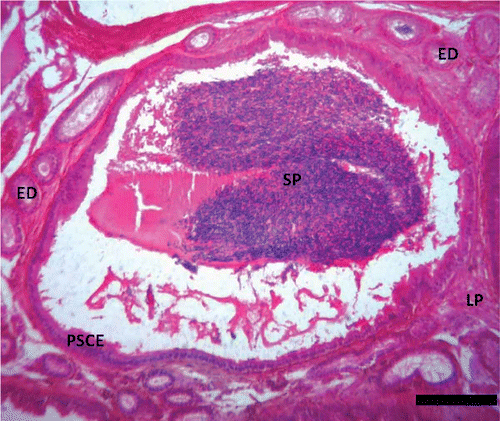
Figure 6. Photomicrograph of the epididymis of Pelusios castaneus showing spermatozoa within the lumen of the duct of the epididymis with efferent ducts within the lamina propria (LP); SPCE: pseudostratified columnar epithelium; SP: spermatozoa (April). H&E: haematoxylin & eosin. Scale bar: 25 µm.
The EMI starts increasing in November, peaks in January (0.056), declines by March and reaches its lowest value in April (0.041), (B). Data from the mean seminiferous tubule diameter (STD) also show that testicular activity is at its peak in September (393 µm) while the lowest value was recorded in March (125 µm) (C). The mean epididymal ductal diameter (EDD) was lowest in April (403 µm), while its highest value was recorded in January (873 µm) (D). The degree of sperm packing in the epididymis (SPE) was lowest in April and highest in January (E), while the classification of spermatogenic stage (CSS) was at its peak in September and its lowest value was recorded in April (F).
Discussion
The reproductive cycle of Pelusios castaneus is similar to those of other chelonians with minor variation. The fact that spermatozoa were found in the epididymis of all the turtles used for this study confirms that they were all sexually matured, including the smallest of these turtles, weighing a mere 300 g. This is similar to the findings of McPherson and Marion (Citation1981) on the stinkpot turtle (Sternotherus odoratus) in that spermatozoa were found in the epididymis thoughout the year. Spermatozoa had been reported to be generally present throughout the year in the epididymis of adult male turtles (McPherson & Marion Citation1981; Rao & Shaad Citation1985). In temperate regions, more spermatozoa had been reported to be present in the epididymis of turtles during the winter phase of the reproductive cycle (Gibbons Citation1968). The maximum value for the GSI obtained in this study is similar to that reported in the freshwater turtle Trionyx gangeticus (Rao & Shaad Citation1985).
The histology of the testis and epididymis of the turtle is similar to that of other chelonians (McPherson & Marion Citation1981; Rao & Shaad Citation1985; Meylan et al. Citation2002). The histological features of both the testis and epididymis of the African sideneck turtle are also similar to those reported in mammals (Oke Citation1982; Bacha & Bacha Citation2000; Massanyi et al. Citation2003; Olukole & Obayemi Citation2010). Histological data from both the testis and epididymis, the GSI, the EMI, the EDD and STD obtained in the study all suggest that spermatogenesis in P. castaneus follows a postnuptial pattern. Hence, mating at the onset of the wet season followed by spermatogenesis at the peak of the wet season appears to be the pattern in P. castaneus. The period of active spermiogenesis in this turtle is therefore between August and November. The patterns of seasonal changes of the testis and epididymis observed in this study have been reported by several authors (Christiansen & Dunham Citation1972; Lofts & Tsui Citation1977; McPherson & Marion Citation1981; Rao & Shaad Citation1985; Kuchling Citation1988; Meylan et al. Citation2002).
It could be inferred that once sperm production in P. castaneus peaks in September, it is immediately released into the epididymis and the degree of sperm packing of the epididymis increases from October reaching its peak in January. Hence, as the testis reaches its maximum size, the spermatic duct also enlarges and is ready to receive the shedding spermatozoa (Rao & Shaad Citation1985). This therefore suggests that the P. castaneus mates between March and May using spermatozoa produced between July and November that had been stored in the epididymis from the previous year. This is similar to earlier reports on spermatogenesis in tropical reptiles. In seasonally breeding mammals, regression of the epididymis following the mating period is accompanied by a loss of sperm (Millar & Glover Citation1970; Bidwai & Bawa Citation1981; Gist et al. Citation2001).
The postnuptial pattern of spermatogenesis observed in P. castaneus has also been reported in the freshwater turtle Trionyx gangeticus (Rao & Shaad Citation1985); the Florida Softshell Turtle, Apalone ferox (Meylan et al. Citation2002); the temperate softshelled turtle, Pelodiscus sinensis (Lofts & Tsui Citation1977) and the snapping turtle, Chelydra serpentina (Mahmoud & Cyrus Citation1992). Nevertheless, the prenuptial pattern of spermatogenesis has been reported in the Loggerhead turtle, Caretta caretta (Wibbles et al. Citation1990); the Kemp’s Ridley sea turtle, Lepidochelys kempii (Rostal Citation1991) and the Green turtle, Chelonia mydas (Owens Citation1980). However, in tropical environments a prenuptial pattern of gonadal recrudescence should also be expected because it has been described as being primitive for turtles, and a postnuptial pattern of spermatogenesis must have arisen in most cases as a result of movement of turtles into temperate and subtropical regions of the world (Crews Citation1987; Meylan et al. Citation2002). The acyclic pattern of turtle spermatogenesis has been reported in the Chelydridae, Macrochleys temminckii (Dobie Citation1971) and Kinosternidae, Claudius angustatus (Flores-Villela & Zug Citation1995). Turtles in the temperate zones have an unusual reproductive cycle whereby spermatogenesis and ovulation have been reported to be out of phase with each other, with the former occurring as an episodic event, commencing in early summer (June), while spermatozoa leave the testis and enter the epididymis in autumn, September to October (Gist et al. Citation2001).
The information made available in this study shows that the postnuptial pattern of turtle spermatogenesis is not unique to turtles in the temperate regions of the world and that the spermatogenic cycle of the P. castaneus is similar to those of other chelonians with minor variation. It therefore serves as baseline data in the comparative spermatogenic cycle of sea and freshwater turtles. However, more studies involving long-term monitoring of the male hormone profiles of the turtle, electron microscopic studies of the male reproductive organs at different seasons of the year as well as nesting activities in the female P. castaneus would be needed to further explain the full process of spermatogenesis.
Acknowledgements
This work was supported by University of Ibadan Senate Research Grants (SRG/FVM/2010/4A and SRG/FVM/2010/1B).
Conflict of interest: none.
References
- Bacha WJ, Bacha LM. 2000. Color atlas of veterinary histology. New York: Lippincott William and Wilikins. 219 pp.
- Bidwai PP, Bawa SR. 1981. Correlative study of the ultrastructure and the physiology of the seasonal regression of the epididymal epithelium in the hedgehog Paraechinus micropus. Andrologia 13:20–32.
- Broadley DG, Boycott RC. 2009. Pelusios sinuatus (Smith 1838) – serrated hinged terrapin. Conservation biology of freshwater turtles and tortoises. Chelonian Research Monographs 5:036.1–036.5.
- Christiansen JL, Dunham AE. 1972. Reproduction of the yellow mud turtle (Kinoster non flavescens flavescens) in New Mexico. Herpetologica 28:130–137.
- Crews D. 1987. Diversity and evolution of behavioral controlling mechanisms. In: Crews D, editor. Psychobiology of reproductive behavior: An evolutionary approach. Englewood Cliffs, NJ: Prentice-Hall. pp. 88–119.
- Dobie J. 1971. Reproduction and growth in the alligator snapping turtle Macroclemys temminckii. Copeia 4:645–658.
- Flores-Villela O, Zug GR. 1995. Reproductive biology of the chopontil, laudius angustatus (Testudines: Kinosternidae) in southern Veracruz, Mexico. Chelonian Conservation & Biology 1:181–186.
- Georges A. 1983. Reproduction of the Australian freshwater turtle Emydura krefftii (Chelonia: Chelidae). Journal of Zoology 201:331–350.
- Gibbons JW. 1968. Reproductive potential, activityand cycles in the painted turtle, Chrysemys picta. Ecology 49:399–409.
- Gibbons JW, Greene JL. 1990. Reproduction in the slider and other species of turtles. In: Gibbons JW, editor. Life history and ecology of the slider turtle. Washington, DC: Smithsonian Institution Press. pp. 124–134.
- Gist DH, Dawes SM, Terry W, Turner TW, Sheldon S, Congdon JD. 2001. Sperm storage in turtles: A male perspective. Experimental Zoology 292:180–186.
- Gist DH, Michaelson JA, Jones JM. 1990. Autumn mating in the painted turtle, Chrysemys picta. Herpetologica 46:331–336.
- Kennett RM. 1999. Reproduction of two species of freshwater turtle, Chelodina rugosa and Elseya dentata, from the wet-dry tropics of northern Australia. Journal of Zoology 247:457–473.
- Kirkpatrick DT. 1995. An essay on taxonomy and the genus pelusios. Available: http://www.unc.edu/~dtkirkpa/stuff/pel.html. Accessed Feb 2013 22.
- Kuchling G. 1988. Gonadal cycles of the Western Australian long-necked turtles Chelodina oblonga and Chelodina steindachneri (Chelonia: Chelidae). Records of the Australian Museum 14:189–198.
- Lapparent De Broin FDE. 2001. The European turtle fauna from the Triassic to the present. Dumerilia 4:155–217.
- Licht P. 1967. Reptiles. In: Lamning GE, editor. Marshall’s physiology of reproduction. 4th ed. Edinburgh: Churchill Livingstone. pp. 206–282.
- Lofts B. 1977. Patterns of spermiogenesis and steroidogenesis in male reptiles. In: Tyndale-Discoe CH, editor. Reproduction and evolution. Proceedings of the 4th symposium on comparative biology of reproduction. Canberra, Australia Capital Territory, Australia. pp. 127–136.
- Lofts B, Tsui HW. 1977. Histological and histochemical changes in the gonads and epididymides of the male soft-shelled turtle, Trionyx sinensis. Journal of Zoology 181:57–68.
- Mahmoud IY, Cyrus RV. 1992. The testicular cycle of the common snapping turtle, Chelydra serpentina, in Wisconsin. Herpetologica 48:193–291.
- Massanyi P, Jancova A, Uhrin V. 2003. Morphometric study of male reproductive organs in the rodent species Apodemus Sylvaticus and Apodemus flavicollis. Bulletin of the Veterinary Institute in Pulway 47:133–138.
- Mcpherson RJ, Marion KR. 1981. Seasonal testicular cycle of the stinkpot turtle (Sternotherus odoratus) in central Alabama. Herpetologica 37:33–40.
- Meylan PA, Sculer R, Moler P. 2002. Spermatogenic cycle of the Florida softshell turtle, Apalone ferox. Copeia 3:779–786.
- Millar RP, Glover TD. 1970. Seasonal changes in the reproductive tract of the male rock hyrax, Procavia capensis. Journal of Reproduction and Fertility 23:497–499.
- Oke BO. 1982. Some studies on the reproductive organs of the African giant rat (Cricetomys gambianus, Waterhouse) during the climatic seasons at Ibadan. M.Sc dissertation, Nigeria: Department of Veterinary Anatomy, University of Ibadan.
- Olukole SG, Aina OO, Okusanya BO. 2010. Morphometric analysis of the external body anatomy of the African sideneck turtle Pelusios sinuatus. Conference proceedings of the 30th Annual Symposium on Sea Turtle biology and conservation held in Goa, India, 313.
- Olukole SG, Obayemi TE. 2010. Histomorphometry of the testis and epididymis in the domesticated adult African great cane rat (Thryonomys swinderianus). International Journal of Morphology 28:1251–1254.
- Olukole SG, Oyeyemi MO, Oke BO. 2011. Gross anatomy of the male reproductive organs of the African sideneck turtle (Pelusius sinuatus). Book of Abstracts, 48th Annual Conference of the Nigerian Veterinary Medical Association, held in Ilorin, Kwara State, Nigeria, 58.
- Omonona AO, Olukole SG, Kushe FA. 2011. Haematology and serum biochemical parameters in freeranging African sideneck turtle (Pelusios sinuatus) in Ibadan, Nigeria. Acta Herpetologica 6:267–274.
- Owens DW. 1980. The comparative reproductive physiology of sea turtles. American Zoologist 20:549–563.
- Pickford GE. 1953. A study of the hypophysectomised male kill-fish Fundulus heteroclitus (Linn). Bulletin of the Bingham Oceanographic Collection 14:5–41.
- Rao RJ, Shaad FU. 1985. Sexual cycle of the male freshwater turtle, Trionyx gangeticus (Cuvier). Herpetologica 41:433–437.
- Rostal DC. 1991. The reproductive behavior and physiology of the Kemp’s Ridley sea turtle, Lepidochelys kempi (Garman, 1880). Ph.D. dissertation. Texas A&M Univ.: College Station.
- Wibbles TD, Owens W, Limpus CJ, Reed PC, Amoss MS. 1990. Seasonal changes in serum gonadal steroids associated with migration, mating, and nesting in the loggerhead sea turtle (Caretta caretta). General and Comparative Endocrinology 79:154–164.
- Wyneken J. 2001. The Anatomy of Sea Turtles. U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFSC-470. pp. 1–172.
