Abstract
Lake Faro (Sicily, Italy) is a natural confined brackish environment particularly subject to anthropogenic impact, resulting in a mixture of xenobiotic substances, i.e. heavy metals and polycyclic aromatic hydrocarbons (PAHs), and characterised by low hydrodynamics. In order to assess the water quality status of this pond, a multi-biomarker approach was applied on mussel Mytilus galloprovincialis (Lamarck, 1819) both inhabiting the lake and from a control site (Goro). Different biomarkers were investigated on mussel digestive glands and gills, including biomarkers of exposure (cytochrome P450 4, CYP4), neurotoxicity (acetylcholinesterase, AChE; choline acetyltransferase, ChAT), general stress (lysosomal membrane stability, LMS), and genotoxicity (micronucleus and comet assays). The results suggest significant responses related to the selected area. A statistically significant inhibition (P < 0.0001) of CYP4 in the digestive gland, and of AChE and ChAT in the gills, was found in specimens collected from Faro compared with the control. The lysosomal membrane stability of mussels from Lake Faro was lower than the reference site, while the DNA damages were significantly higher in mussels from the brackish area compared to the control. This study represents the first time the quality status of Lake Faro is assessed using a multi-biomarker approach on the mussel M. galloprovincialis, which proved to be suitable to identify the effects of environmental pollutants at molecular and cellular levels.
Introduction
In recent years, the rapid increase of anthropogenic activities has caused an incessant influx of organic and inorganic xenobiotics into aquatic environments, including polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), organophosphorus (OP) compounds and heavy metals. Bivalves, and in particular marine mussels, i.e. Mytilus galloprovincialis (Lamarck, 1819), have been widely used as bioindicators in pollution monitoring programs, due to their ability to accumulate in their tissues and tolerate high concentrations of organic and inorganic pollutants (Widdows & Donkin Citation1992; Fasulo et al. Citation2008; Ciacci et al. Citation2012; Cappello et al. Citation2013a, Citation2013b).
In this study, specimens of M. galloprovincialis were collected from a brackish environment, Lake Faro (Sicily, Italy), characterised by the presence of heavy metals (Giacobbe et al. Citation1996; Munaò et al. Citation2000; Mauceri et al. Citation2005; Fasulo et al. Citation2008) and PAHs (Giacalone et al. Citation2004; Arpa Sicilia Citation2007). This site is a typical example of a natural confined environment, where eutrophication can trigger stress with negative repercussions on the aquatic ecosystem. The lake is characterised by euryhaline waters, as there is an injection of fresh water from aquifers, which mixes with salt water through the channels communicating with the sea. It represents an example of a meromictic basin and, therefore, the sediments are not involved in a mixing process. The pond is also subject to the negative effects of anthropogenic impact, associated with a massive construction industry, sewage and fertilisers used in the surrounding areas, that may affect the ecological balance (Sorgi et al. Citation2006).
A battery of multiple biomarkers was applied on mussel specimens in two key organs, the digestive gland and gills, including cytochrome P450 (Peters et al. Citation1999; Solé & Livingstone Citation2005; Cappello et al. Citation2013a), esterases (Moreira & Guilhermino Citation2005), lysosomal membrane stability (Da Ros et al. Citation2002; Castro et al. Citation2004; Pisoni et al. Citation2004), micronucleus test and comet assay (Rank et al. Citation2007; Banni et al. Citation2010).
The digestive gland of mollusks is the main organ for metabolic regulation, participating in the processes of detoxification and elimination of xenobiotics (Marigómez et al. Citation2002; Moore & Allen Citation2002; Cappello et al. Citation2013a; D’Agata et al. Citation2013). The cytochrome P450 (CYP) enzyme family plays a key role in the mechanism of detoxification, being involved in metabolising a wide range of lipophilic organic endogenous and exogenous compounds. A few studies have been performed on invertebrates and, to date, only CYP4 was sequenced in M. galloprovincialis. A specific CYP gene, CYP4Y1, was identified in the digestive gland of M. galloprovincialis, and its expression was found to be inhibited after exposure to beta-naphthoflavone (Snyder Citation1998), and down-regulated in M. galloprovincialis caged in a heavily polluted petrochemical area (Cappello et al. Citation2013a).
The gills of the bivalves present several mechanisms controlling the functions involved in the maintenance of homeostasis (Gomez-Mendikute et al. Citation2005; Scarfì et al. Citation2006; Fasulo et al. Citation2008; Cappello et al. Citation2013b). The gills are constantly in contact with the surrounding aquatic environment, and thus highly exposed to environmental factors due to their large surface and their involvement in gas exchange and feeding (de Oliveira David et al. Citation2008). Therefore, the structural pattern of the gill tissues and their biochemical profiles reflect the adverse environmental impact on the animal (Gregory et al. Citation2002; Ciacci et al. Citation2012; Cappello et al. Citation2013b). Acetylcholine (ACh) is a neurotransmitter used in efferent systems and also in some central circuits (Woolf Citation1991). It is synthesized in the cytoplasm of cholinergic neurons by the enzyme choline acetyltransferase (ChAT), and degraded at the synaptic cleft by the enzyme acetylcholinesterase (AChE). AChE is involved in the transmission of nerve impulses and has been widely used as an indicator of potential neurotoxicity, both in invertebrates and lower vertebrates (Peakall Citation1992; Mora et al. Citation1999a, 1999b; Ciacci et al. Citation2012; De Domenico et al. Citation2013).
Furthermore, it is well known that the accumulation of xenobiotics and their related metabolic products inside the lysosomes weakens their membrane stability and may induce diffusion of hydrolytic lysosome enzymes into the cytosol. The assessment of lysosomal membrane stability has been proposed as a rational biomarker of general stress (Petrovic et al. Citation2001; Castro et al. Citation2004), and its use has been evaluated in marine organisms, both in field and laboratory studies (Da Ros et al. Citation2002; Castro et al. Citation2004; Maisano et al. Citation2013).
Inefficient detoxification or increased activation of xenobiotic compounds is believed to modulate the biologically active dose available for the molecular end target (DNA), and thus influence the levels of biomarkers reflecting exposure to and damage by genotoxic agents (Brescia et al. Citation1999). The impact of genotoxic chemicals on the integrity of cellular DNA is the first event after the exposure. Chromosomal damage expressed after cell replication represents an accumulated effect associated with long-term exposure (Dixon et al. Citation2002; Jha et al. Citation2005; Jha Citation2008). The alkaline elution test has been applied in marine invertebrates (Vukmirovic et al. Citation1994; Bolognesi et al. Citation1996) and allows the evaluation of a large range of DNA damages in terms of DNA single-strand breaks. The haemocytes have been used in such investigations for the assessment of DNA damage using single cell gel electrophoresis or comet assay (Dixon et al. Citation2002; Jha et al. Citation2005; Jha Citation2008; D’Agata et al. Citation2013). In addition, the micronucleus (MN) test, one of the most commonly applied techniques for identifying genomic alterations in animals (Jha et al. Citation2005; Fasulo et al. Citation2010), has also been performed on mussels as endpoint of genotoxicity testing.
The purpose of the present study was to evaluate the water quality status of a natural confined brackish environment, Lake Faro, by applying, for the first time in this site, a multi-biomarker approach on the mussel M. galloprovincialis, chosen as the sentinel organism. The battery of multiple biomarkers included biomarkers of exposure (CYP4), neurotoxicity (AChE; ChAT), general stress (LMS), and genotoxicity (MN and comet assays).
Materials and methods
Study area
Lake Faro (Sicily, Italy) was chosen as the natural confined brackish environment for this study. It is a meromictic basin, particularly subject to anthropogenic impact resulting in the presence of heavy metals and PAHs, and characterised by low hydrodynamics. In addition, increased population density and low rainfall occurs during the summer months. By contrast, the consortium of fishermen in Goro (Ferrara, Italy), a zone of type A according to the D.L. 530/92, the physico-chemical parameters of which have been previously reported (Fasulo et al. Citation2008), was chosen as the control site ().
Water sampling and analysis
At both sampling sites, surface water samples were collected in polythene bottles, and transferred to the laboratory under refrigeration. Temperature, pH, conductivity and dissolved oxygen (DO) were measured in the field by a portable instrument (Multi 340i/SET, WTW Wissenschaftlich, Weilheim, Germany). In the laboratory, the water samples were analysed for various physico-chemical parameters following standard methods (Grasshoff et al. Citation1983; APHA Citation1995). Nutrients (e.g. ammonia, phosphate, orto phosphate, nitrites) were estimated by colorimetric methods according to APHA (Citation1995) from samples filtered through a 0.45-μm Millipore membrane filter paper. All colorimetric estimations were performed using a spectrophotomer (Filterphotometer PF-11 MN, Macherey-Nagel GmbH and Co. KG – Düren, Germany).
Sampling of mussels
Specimens of M. galloprovincialis, 4–5 cm shell length, were collected in Lake Faro and in the control site during the summer season.
Gills and digestive glands of 30 individuals for each sampling site were fixed in a solution of 4% paraformaldehyde in phosphate buffered saline (PBS) 0.1 M, pH 7.4, dehydrated in increasing ethanol concentrations and embedded in Paraplast (Bio-Optica, Milano, Italy), or rapidly excised, frozen in liquid nitrogen, and stored at –80°C until processed for subsequent analyses.
Samples of haemolymph were withdrawn by syringe from the posterior adductor muscle and transferred to small plastic centrifuge tubes on ice, until use.
PAH analysis in tissues
PAH content was determined according to the method of Dafflon et al. (Citation1995), as previously described by Fasulo et al. (Citation2012b). For PAH analysis, the following solvents and reagents were used: acetonitrile ACN (Romil), water and cyclohexane (Chromanorm BDH), acetone (Pestinorm BDH), potassium hydroxide (KOH), ethanol and exane (Carlo Erba), all of high-performance liquid chromatography (HPLC) grade. The digestive glands dissected from 15 individuals were pooled in three samples (each with tissues of five specimens) per each sampling area. Approximately 3 g of each pooled sample were weighted with an analytical balance Mettler Toledo AT 104 and homogenized in a glass vial using an Ultra-TURRAX IKA T10 basic. The homogenized samples were saponified with 10 mL of 1 M KOH in an ethanol solution for 3 h at 80°C in a water bath. Then, 20 mL of cyclohexane was added and samples mixed by an orbital agitator for 10 min using dark glassware (Dafflon et al. Citation1995). The hexanic phase was recovered and the polar mixture washed once with cyclohexane, and then discharged. The extracts were filtered, concentrated under a nitrogen gas stream to about 1 mL, and the concentrated extract was removed with a pasteur pipette and loaded into a Varian Bond Elut C18 12-mL cartridge, previously conditioned. The eluates were dried under nitrogen flow and dissolved with 1 mL of acetonitrile before the analysis.
The concentrations of the following 16 PAHs identified by the Environmental Protection Agency (EPA) as priority pollutants, naphthalene (NA), acenaphthylene (ACY), acenaphthene (AC), fluorene (FL), phenanthrene (PHE), anthracene (AN), fluoranthene (FA), pyrene (PY), benzo(a)anthracene (BaA), chrysene (CH), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DahA), benzo(g,h,i)perylene (Bghi) and indeno(1,2,3-cd)pyrene (IP), were determined. Quantitative analysis of PAHs was carried out with an HPLC apparatus Pro-Star 363 (Varian, Palo Alto, CA) equipped with a 20-mL loop and a fluorescence detector (FLD Pro-Star 363). The software used was Star Chromatography Workstation version 5.2 (Varian, Palo Alto, CA).
Immunohistochemistry
Sections (4 μm thick) were prepared from paraffin-embedded digestive glands and gills. The sections were treated using the indirect immunofluorescence method (Mauceri et al. Citation1999). Non-specific binding sites for immunoglobulins were blocked by incubations for 1 h with normal goat serum (NGS) in PBS (1:5). The digestive glands sections were incubated overnight in a humid chamber at 4°C with the primary rabbit polyclonal antibody anti-CYP4 prediluted (the antibody CYP4 was kindly provided by Professor Anders Goksøyr of University of Bergen, Norway), while gill sections were incubated by co-localization with a rabbit polyclonal antibody anti-AChE (Chemicon International, Temecula CA) diluted 1:50, and a mouse polyclonal antibody anti-ChAT (Abcam, Cambridge UK), diluted 1:100. After a rinse in PBS for 10 min, the sections were incubated for 2 h at room temperature with fluorescein isothiocyanate (FITC) conjugated goat anti-rabbit IgG (Sigma) and tetramethylrhodamine isothiocyantate (TRITC) conjugated goat anti-mouse IgG (Sigma), diluted 1:100.
Negative controls for the immunohistochemical labeling were performed by substitution of non-immune sera for the primary antisera.
All observations were made on five randomly selected fields in each slide using a 40× oil-immersion objective with a motorized Zeiss Axio Imager Z1 microscope (Carl Zeiss AG, Werk Göttingen, Germany), equipped with an AxioCam digital camera (Zeiss, Jena, Germany) for the acquisition of images.
RNA extraction and polymerase chain reaction (PCR)
Total RNA was extracted from the digestive glands of specimens using TRIzol LS reagent (Invitrogen, Carlsbed, CA, USA) (Chomczynski & Sacchi Citation1987). The RNA content was quantified using a UV spectrophotometer (UV Mini 1240 Shimadzu, Milano, Italy).
The cDNA was synthesized using 4 μg of total RNA and oligo (dt)20 primer (150 pmol/μL) (Invitrogen), with Moloney Murine Leukemia Virus (MMLV) reverse transcriptase (Invitrogen) as prescribed by the manufacturer’s instructions. Two μl of the resulting cDNA were amplified in the PCR reaction.
The sequences of primers to CYP4Y1 were designed on the sequence no. AF072855 present in Genebank: sense primer 5′-AGGCTTTCACCAGTTCC-3′ and antisense primer 5′- TCCGGCAGAAATGGAGTAAA-3′, amplifying a 172-bp sequence.
The actin gene of each examined organism was used as positive control. The gene was amplified using sequence primers based on the actin cDNA sequence of M. galloprovincialis to obtain a fragment of 200 bp sequence.
PCR was prepared using 2.5 µL of 10× buffer, 0.13 µL of 5 U/µL Poly Taq polymerase (Invitrogen), 0.8 µL of 50 mM magnesium chloride (MgCl2), primers (50 µM each), 1 µL of cDNA template, 0.5 µL of 10 mM dNTPs (deoxynucleoside triphosphates), and Milli-Q water (Millipore, Vimodrone MI, Italy). The total reaction was performed in a 25-µL volume. The program used to amplify fragments of cytochrome P450 4Y1 was 95°C for 2 min and 35 cycles at 95°C for 30 s, 56°C for 30 s, 72°C for 30 s and a final extension at 72°C for 5 min.
To perform the reaction we used the Mastercycler Ep-Gradient (Eppendorf, Milano, Italy). RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) products were characterized by electrophoresis on SYBR Safe-stained agarose gel.
Enzyme activities
The activity of AChE was evaluated in the gills of the mussels. AChE was determined using the method of Ellman et al. (Citation1961) with slight modifications. Briefly, thiocholine derivatives are hydrolysed by acetylcholineterase to yield thiocholine. Subsequent combination with 5,5-dithiobis-2-dinitrobenzoic acid (DTNB) forms the yellow anion 5-thio-2-nitrobenzoic acid, which absorbs strongly at 412 nm. The activities were expressed as μmol/min/mg.
Lysosomal membrane stability
Haemolymph was analysed for lysosomal membrane stability as neutral red retention time (NRRT) (Regoli et al. Citation2004). Haemolymph collected from the adductor muscle of 30 specimens for each sampling site was incubated on a glass slide with a freshly prepared neutral red working solution (2 µL/mL saline from a stock solution of 20 mg neutral red dye dissolved in 1 mL dimethyl sulfoxide (DMSO)). The haemocytes were microscopically examined (63/100×, Zeiss Axio Imager Z1) at 15 min intervals (for up to 90 min) to determine at what point in time the dye, previously taken up by lysosomes, was lost into the cytosol in 50% of granular haemocytes.
Genotoxicity analysis
1. MN assay
Haemolymph of 30 organisms for each sampling site was spread on slides, transferred to a lightproof humidity chamber, and allowed to adhere. After, it was fixed in pure methanol for 10 min, hydrated and then stained with 5% Giemsa solution for 15 min. A total of 1000 haemocytes were examined for each specimen under the light microscope (Zeiss Axio Imager Z1) to determine the presence or absence of micronuclei or other nuclear abnormalities (NAs) such as nuclear buds, notched and lobed nuclei, bi-nucleated and fragmented-apoptotic cells, as well as micronucleus connected to the main nucleus, commonly known as “eight-shaped” cells. The NAs were identified using the criteria described by Fenech et al. (Citation2003) (Barsiene et al. Citation2012).
2. Comet assay
The comet assay was conducted at pH > 13, according to Steinert et al. (Citation1998), Jha et al. (Citation2005) and D’Agata et al. (Citation2013), with some modifications (Fasulo et al. Citation2010). Haemolymph samples were centrifuged at 2000 rpm for 5 min and pellets resuspended in Phosphate Buffered Saline (PBS). Ten microlitres of the diluted sample were mixed with 65 µL of 0.7% low-melting-point (LMP) agarose, then the 75 µL mixture was layered on the precoated slides on 1% normal melting point agarose. The slides were covered with a cover slip, and were left for 5 min in a refrigerator to solidify. The cover slip was gently removed and 75 µL of 0.7% low melting agarose were added and another cover slip was placed on top. The samples were left for another 5 min in the refrigerator. After removing the cover slip, the slides were placed in lysis buffer (2.5 M NaCl, 100 mM EDTA-Na, 10 mM Tris-HCl) for 2 h at 4°C. After lysis, the slides were placed in an electrophoresis box for 20 min in running buffer, to allow the unwinding to occur. Electrophoresis was performed using the same running buffer at 20 V and 240 mA for 20 min. The slides were then neutralized with Tris buffer 0.4 M pH 7.5, and stained with Sybr Safe (2.5 µL/mL).
The slides were examined with the Zeiss Axio Imager Z1 fluorescence microscope. To determine whether visual scoring correlated with computerized image analysis, the same cells were also scored for DNA damage using the Comet assay IV software (Perceptive instruments, Suffolk, UK). To quantify the induced DNA damage, two parameters were considered: the tail length (TL) and the tail moment (TM). TM is a measure of the migrated DNA in the tail, multiplied by the TL (Olive et al. Citation1990).
Statistical analysis
Immunoreactive cell quantification was performed by counting the positive cells using Axio Vision Release 4.5 software (Zeiss, Göttingen, Germany). The band intensities of CYP4Y1 were measured with Quantity One software (BioRad, Marnes-la-Coquette, France). Results were expressed as mean ± S.D. All the obtained data were statistically analysed with Graph Pad software (Instat, La Jolla, CA, USA) using one-way analysis of variance (ANOVA), and applying the Mann-Whitney U test.
Results
Water physico-chemical analysis
During the summer, in Lake Faro, the water temperature at the surface was 27°C, with a salinity of 37‰ PSU (Practical Salinity Units). In Goro, the water surface temperature was 25°C, while the salinity was 31‰ PSU. Additional physico-chemical parameters (i.e. oxygen, pH, ammonium, chlorine, phosphate, nitrites, etc.) measured in both sampling sites are reported in .
Table I. Physicochemical parameters of the Faro and Goro environments during the summer season. D.L. = Detection limit.
PAH analysis
PAH analysis in gills did not show significant results since the concentrations were lower than 0.006 µg/g in both sites. Conversely, in the digestive gland tissues of mussels from Lake Faro, the significant PAH concentrations were: naphthalene (0.302 ± 0.2 µg/g), dibenzo(a,h)anthracene (0.318 ± 0.2 µg/g), benzo(a)pyrene (0.022 ± 0.01 µg/g) and fluoranthene (0.026 ± 0.02 µg/g) ().
Table II. Polycyclic aromatic hydrocarbons (PAH) concentration (mean ± SD) in the digestive glands (μg/g). n.d. = not detectable.
Immunohistochemical analysis
The immunohistochemical investigation of the digestive glands of M. galloprovincialis sampled in Goro revealed a significant number of CYP4 immunopositive cellular cords (), while a drastic reduction in the number of immunopositive cells was observed in organisms from Lake Faro (B). The statistically processed results are shown in G.
Figure 2. Immunolocalization of CYP4 (A, B) on mussel (Mytilus galloprovincialis) digestive gland, and AChE (C, D) and ChAT (E, F) on gills. (A) Control specimens showing a significant number of CYP4 immunopositive cells (arrow). (B) Specimens from Lake Faro, displaying few CYP4 positive cells. (C) Control specimens showing AChE immunopositive fibers (arrow) and (E) ChAT immunopositivite fibers (arrowhead) along the gill epithelium. (D) Specimens from Lake Faro, displaying a drastic reduction of AChE immunopositivity, and (F) ChAT immunopositivite cells (arrowhead) along the gill epithelium. Mean and standard deviation (SD) of immunopositive cells (G, H). Asterisk indicates a significant difference at P < 0.0001. Scale bars: 10 μm. CYP4 = cytochrome P450 4; AChE = acetylcholinesterase; ChAT = choline acetyltransferase.
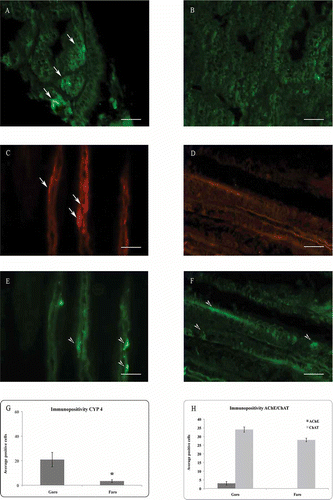
The presence of cholinergic neurotransmitters AChE and ChAT in the specimens collected from Goro is documented with AChE immunopositive fibers distributed along the filaments (C), and both ChAT immunopositivite cells and numerous fibers and varicosities along the gill epithelium (E). On the contrary, in the specimens collected from the brackish site, a drastic reduction of positive fibers to AChE was clearly noticed (D), while a number of epithelial cells showed positivity for ChAT (F).
Statistical analyses of the mean of immunoreactive cells are represented in H (P < 0.0001).
Amplification of CYP4Y1-specific complementary DNA
The molecular data, obtained by RT-PCR, have provided a discrete band of 172 bp for CYP4Y1. The results were normalized with the mRNA expression of cytoplasmic actin, chosen as the reference gene. The levels of CYP4Y1 expression were higher in the digestive gland cells of control mussels compared with the organisms from Lake Faro.
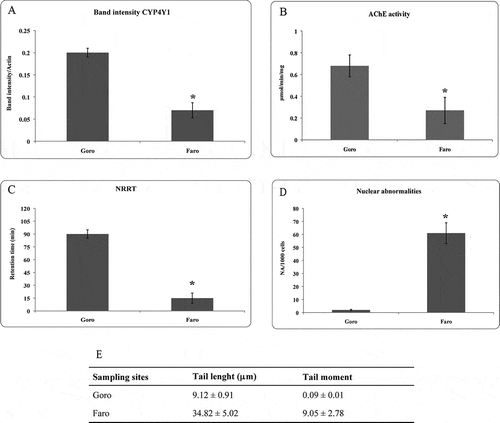
The results were analysed as a variation in band intensity, and showed statistically significant differences between Goro and Faro individuals (P = 0.0005) (A).
Figure 3. (A) Mean and standard deviation (SD) of the ratio between CYP4Y1 and actin band intensity. Asterisk indicates a significant difference at P = 0.0005. (B) AChE activity expressed in μmol/min/mg in Mytilus galloprovincialis collected from Goro and Lake Faro. Asterisk indicates a significant difference at P < 0.0001. (C) Neutral red retention time (NRRT) of haemocytes of M. galloprovincialis from Goro and Faro. In the specimens collected from Lake Faro, the loss of the neutral red was observed after 15 min in more than 50% of the haemocytes. Asterisk indicates a significant difference at P = 0.0005. (D) Mean frequency of nuclear abnormalities (NA), and (E) tail length and tail moment in the haemolymph of mussels collected from Goro and Faro. Asterisk indicates a significant difference at P < 0.0001. CYP4Y1 = cytochrome P450 4Y1; AChE = acetylcholinesterase.
Enzyme activities
AChE activity was inhibited in the gills of mussels collected from Faro with respect to the levels measured in the organisms of Goro. The mean value was 0.27 µmol/min/mg compared to 0.68 µmol/min/mg in the samples from Goro (B). The difference was statistically meaningful (P < 0.0001).
Lysosomal membrane stability
The results of the NR retention time measured in haemocytes of mussels are shown in C. In the mussels from the control group, there was almost no loss of dye from the lysosome to the cytosol for the duration of the assay, with a destabilization of the lysosomal membrane evidenced only after 90 min. Instead, in the specimens sampled from Lake Faro, the loss of the neutral red was observed after 15 min in more than 50% of the haemocytes. There was a statistically significant difference between control and mussels from Faro (P = 0.0005).
Genotoxicity analysis
1. MN assay
The MN test applied on the haemocytes of mussels did not reveal the presence of micronuclei, but the presence of several nuclear abnormalities in the specimens collected from Lake Faro. In detail, the main abnormalities detected were nuclear blebs and notched lobed. The statistical analysis performed on the average of cells with abnormal nuclei revealed a statistically significant difference (P < 0.0001) between the mussels of Faro and those of the control, with a frequency of nuclear abnormalities (FNA) of 61‰ in the specimens collected from Faro (D).
2. Comet assay
A statistically significant increase (P < 0.0001) of DNA damage was observed in haemocytes from mussels sampled at Faro by applying the comet assay. The % DNA in the comet tail was higher (a four-fold increase) compared to the control organisms. The ANOVA showed significant differences between the tail length (P < 0.0001) and the tail moment (P = 0.0001) values observed in all specimens collected from the two sites (E).
Discussion
The water quality status of Lake Faro, a natural confined brackish environment, was assessed in this study by applying, for the first time in this site, a multi-biomarker approach on the mussel M. galloprovincialis, chosen as the sentinel organism. Lake Faro is particularly subject to anthropogenic impact, resulting in a mixture of xenobiotic substances, including pesticides, organic pollutants, heavy metals and PAHs (Giacobbe et al. Citation1996; Munaò et al. Citation2000; Giacalone et al. Citation2004; Mauceri et al. Citation2005; Scarfì et al. Citation2006; Arpa Sicilia Citation2007; Fasulo et al. Citation2008), and characterised by low hydrodynamics.
In environmental risk assessment, the use of a battery of biomarkers is strongly recommended (Donnini et al. Citation2007; Monserrat et al. Citation2007; Fasulo et al. Citation2010, Citation2012a; Iacono et al. Citation2010) because a multi-biomarker approach provides a more comprehensive and integrated view of the biological responses of aquatic organisms, even where the levels of contaminants are not particularly high (Cravo et al. Citation2009). The biomarkers applied in this study were selectively chosen to investigate specific biological responses related to toxic compounds present in the environment.
In mussels collected from Lake Faro during the summer season, PAHs, and in particular naphthalene, dibenzo(a,h)anthracene, benzo(a)pyrene and fluoranthene, were found in the digestive gland tissue, confirming the presence of PAHs in the brackish water of the pond. An inhibition of CYP4 was observed in the digestive glands, according to the molecular analysis with CYP4Y1 mRNA levels significantly reduced. It is known that the major pathway for lipophilic xenobiotic elimination includes P450-mediated metabolism in the vertebrate liver (Fasulo et al. Citation2010), or analogous invertebrate tissues (Cappello et al. Citation2013a). Increases in total CYP content have been demonstrated in Mytilus sp. after exposure to organic contaminants (Michel et al. Citation1993), but one CYP4 fragment, named CYP4Y1 by the CYP nomenclature committee, has been described in M. galloprovincialis with decreasing gene expression as a result of exposure to naphtoflavone (Snyder Citation1998; Jonsson et al. Citation2006), and down-regulated both at the enzymatic and protein level in the digestive tissue of M. galloprovincialis caged in a petrochemical area (Cappello et al. Citation2013a).
The neurotoxic effects in mussels were measured through the study of an important neurotransmitter, ACh. Therefore, AChE and ChAT were investigated in this study, since both enzyme activities are strictly related to the levels of ACh, the main chemical mediator in the transmission of nerve impulses, with ChAT regulating ACh production and AChE responsible for its degradation (Walker & Thompson Citation1991; Parsons et al. Citation1993). The results presented in this paper show a strong inhibition of AChE in the gills of the specimens collected from Faro with respect to the control, suggesting the presence of xenobiotics in the brackish site. Although the AChE activity is inhibited by the presence of neurotoxic compounds, such as organophosphorus and carbamate (Day & Scott Citation1990; Bocquene & Galgani Citation1998), the responsiveness of AChE to many other chemical groups, e.g. heavy metals, hydrocarbons and detergents (Guilhermino et al. Citation1998; Fasulo et al. Citation2010; Ciacci et al. Citation2012), and algal toxins (Lehtonen et al. Citation2003) has also been acknowledged. Therefore, its inhibition is interpreted as a very reliable signal of exposure and effect.
As biomarker of biological stress, the stability of lysosomal membranes was also performed on mussel haemolymph. The measure of NRRT showed a decrease in the lysosomal membrane stability of the specimens collected from Lake Faro after only 15 minutes from the start of the observations, confirming a significant stress to which mussels are subjected in the brackish environment. Our results, in agreement with other studies in the field (Regoli et al. Citation2004; Mamaca et al. Citation2005; Moore et al. Citation2006, Citation2008; Nigro et al. Citation2006; Maisano et al. Citation2013), confirm the usefulness and sensitivity of the lysosomal membrane stability test as a biomarker of stress for detecting and monitoring sublethal changes in marine organisms.
Furthermore, the MN test and comet assay were used as genotoxicity tests because of heavy metals with genotoxic potential known to be present in Lake Faro (Fasulo et al. Citation2008). Both tests revealed the presence of mutagenic agents in the pond, through the presence of a large number of nuclear morphological changes and a substantial damage to DNA, as revealed by the FNA values, the tail length and tail moment. DNA damage evaluated by the method of alkaline comet assay may originate from single- or double-strand breaks, adduct formation, alkali-labile sites, or cross-linking DNA-DNA/DNA-protein (Tice et al. Citation2000).
Conclusion
This study represents the first time the quality status of Lake Faro has been assessed by using a battery of biomarkers on the mussel M. galloprovincialis, which proved to be suitable to identify the effects of environmental pollutants at both molecular and cellular levels. The advantage of using the mussel as the sentinel organism is that the changes observed in a sessile organism can be attributable to the local and seasonal conditions of the area examined. Indeed, the selected multiple biomarkers applied on M. galloprovincialis provided information on the presence of a mixture of pollutants in Lake Faro, resulting in metabolic disorders in mussels. Specifically, the observed inhibition of CYP4 and AChE could be linked to the presence of organic contaminants, and in particular of PAHs detected in the mussel digestive gland tissue. The change in the lysosomal stability is a general indicator of environmental stress, while the increased DNA damage is the result of exposure to mutagenic and genotoxic compounds. Moreover, the biological responses must also be related to the seasonal conditions. In fact, as is well known, summer represents the most stressful and critical situation due to higher temperature, and consequently environmental contamination worsens in a meromictic basin as Lake Faro.
Overall, the results from this work demonstrate the effectiveness of the use of a battery of biomarkers in studies of environmental risk assessment, since a multi-biomarker approach provides a comprehensive and integrated view of the biological responses of aquatic organisms.
References
- APHA. 1995. Standard methods for the examination of water and wastewater. 19th ed. Washington, DC: American Public Health Association.
- ARPA SICILIA. 2007. Piano di monitoraggio per la prima caratterizzazione dei corpi idrici superficiali della regione siciliana. Available: http://www.osservatorioacque.it/documenti/pta/allegati/02/volume%20i%20e%20allegati/allegato%20ii_transizioni.pdf. Accessed July 2013 10.
- Banni M, Negri A, Dagnino A, Jebali J, Ameur S, Boussetta H. 2010. Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicology and Environmental Safety 73:842–848.
- Barsiene J, Rybakovas A, Garnaga G, Andreikenaite L. 2012. Environmental genotoxicity and cytotoxicity studies in mussels before and after an oil spill at the marine oil terminal in the Baltic Sea. Environmental Monitoring and Assessment 184:2067–2078.
- Bocquene G, Galgani F. 1998. Biological effects of contaminants: Cholinesterase inhibition by organophosphate and carbamate compounds. In: Proceedings of the ICES Techniques in Marine Environmental Sciences 22:12. Copenhagen, Denmark: ICES.
- Bolognesi C, Rabboni R, Roggieri P. 1996. Genotoxicity biomarkers in M. galloprovincialis as indicators of marine pollutants. Comparative Biochemistry and Physiology 113:319–323.
- Brescia G, Celotti L, Clonfero E, Neumann HG, Forni A, Foà V, Pisoni M, Ferri GM, Assennato G. 1999. The influence of cytochrome P450 1A1 and glutathione S-transferase M1 genotypes on biomarker levels in coke-oven workers. Archives of Toxicology 73:431–439.
- Cappello T, Maisano M, D’Agata A, Natalotto A, Mauceri A, Fasulo S. 2013a. Effects of environmental pollution in caged mussels (Mytilus galloprovincialis). Marine Environmental Research 91:52–60.
- Cappello T, Mauceri A, Corsaro C, Maisano M, Parrino V, Lo Paro G, Messina G, Fasulo S. 2013b. Impact of environmental pollution on caged mussels Mytilus galloprovincialis using NMR-based metabolomics. Marine Pollution Bulletin in press. doi.org/10.1016/j.marpolbul.2013.10.019.
- Castro M, Santos MM, Monteiro NM, Vieira N. 2004. Measuring lysosomal stability as an effective tool for marine coastal environment monitoring. Marine Environmental Research 58:741–745.
- Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry 162:156–159.
- Ciacci C, Barmo C, Gallo G, Maisano M, Cappello T, D‘Agata A, Leonzio C, Mauceri A, Fasulo S, Canesi L. 2012. Effects of sub-lethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus galloprovincialis. Aquatic Toxicology 120–121:109–118.
- Cravo A, Lopes B, Serafim A, Company R, Barreira L, Gomes T, Bebianno MJ. 2009. A multibiomarker approach in Mytilus galloprovincialis to assess environmental quality. Journal of Environmental Monitoring 11:1673–1686.
- D’Agata A, Fasulo S, Dallas LJ, Fisher AS, Maisano M, Readman JW, Jha AN. 2013. Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology doi:10.3109/17435390.2013.807446.
- Da Ros L, Meneghetti F, Nasci C. 2002. Field application of lysosomal destabilization indices in the mussel Mytilus galloprovincialis: Biomonitoring and transplantation in the Lagoon of Venice (north-east Italy). Marine Environmental Research 54:817–822.
- Dafflon O, Gobet H, Koch H, Bosset JO. 1995. Le dosage des hydrocarbures aromatiques polycycliques dans le poisson, les produites carne’s et le fromage par chromatographie liquide a’haute performance. Travaux de chimie alimentaire et d‘hygiène 86:534–555.
- Day KE, Scott IM. 1990. Use of acetylcholinesterase activity to detect sublethal toxicity in stream invertebrates exposed to low concentrations of organophosphate insecticides. Aquatic Toxicology 18:101–114.
- De Domenico E, Mauceri A, Giordano D, Maisano M, Giannetto A, Parrino V, Natalotto A, D’Agata A, Cappello T, Fasulo S. 2013. Biological responses of juvenile European sea bass (Dicentrarchus labrax) exposed to contaminated sediments. Ecotoxicology and Environmental Safety 97:114–123.
- de Oliveira David JA, Salaroli RB, Fontanetti CS. 2008. Fine structure of Mytella falcate (Bivalvia) gill filaments. Micron 39:329–336.
- Dixon DR, Prusky AM, Dixon L, Jha AN. 2002. Marine invertebrate eco-genotoxicology: A methodological overview. Mutagenesis 17:495–507.
- Donnini F, Dinelli F, Sangiorgi F, Fabbri E. 2007. A biological and geochemical integrated approach to assess the environmental quality of a coastal lagoon (Ravenna, Italy). Environment International 33:919–928.
- Ellman GL, Courtney KD, Andres JV, Featherstone RM. 1961. A new and rapid colometric determination of acetylcholinesterase activity. Biochemical Pharmacology 7:88–95.
- Fasulo S, Brunelli E, Maisano M, Sperone E, Mauceri A, Bernabò I, Cappello T, D’Agata A, Tripepi S. 2012a. Toxicity of Foroozan crude oil to ornate wrasse (Thalassoma pavo): Ultrastructure and cellular biomarkers by using integrated approaches. Italian Journal of Zoology 79:182–199.
- Fasulo S, Iacono F, Cappello T, Corsaro C, Maisano M, D‘Agata A, Giannetto A, De Domenico E, Parrino V, Lo Paro G. 2012b. Metabolomic investigation of Mytilus galloprovincialis (Lamarck 1819) caged in aquatic environment. Ecotoxicology and Environmental Safety 84:139–146.
- Fasulo S, Marino S, Mauceri A, Maisano M, Giannetto A, D‘Agata A, Parrino V, Minutoli R, De Domenico E. 2010. A multibiomarker approach in Coris julis living in a natural environment. Ecotoxicology and Environmental Safety 73:1565–1573.
- Fasulo S, Mauceri A, Giannetto A, Maisano M, Bianchi N, Parrino V. 2008. Expression of metallothionein mRNAs by in situ hybridization in the gills of Mytilus gallorpovincialis from natural polluted environments. Aquatic Toxicology 88:62–68.
- Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. 2003. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutation Research 534:65–75.
- Giacalone A, Gianguzza A, Mannino MR, Orecchio S, Piazzese D. 2004. Polycyclic aromatic hydrocarbons in sediments of marine coastal lagoons in Messina, Italy: Extraction and GC/MS analysis, distribution and sources. Polycyclic Aromatic Compounds 24:135–149.
- Giacobbe MG, Oliva FD, Maimone G. 1996. Environmental factors and seasonal occurrence of the Dinoflagellate Alexandrium minutum, a PSP potential producer, in a Mediterranean lagoon. Estuarine, Coastal and Shelf Science 42:539–549.
- Gomez-Mendikute A, Elizondo M, Venier P, Cajaraville MP. 2005. Characterization of mussel gill cells in vivo and in vitro. Cell and Tissue Research 321:131–140.
- Grasshoff K, Ehrhardt M, Kremling K. 1983. Methods of sea water analysis. Weinheim: Verlag Chemie.
- Gregory MA, Marshall DJ, George RC, Anandraj A, McClurg TP. 2002. Correlations between metal uptake in the soft tissue of Perna perna and gill filament pathology after exposure to mercury. Marine Pollution Bulletin 45:114–125.
- Guilhermino L, Barros B, Silva MC, Soares A. 1998. Should the use of inhibition of cholinesterase as a specific biomarker for organophosphate and carbamate pesticides be questioned?. Biomarkers 3:157–163.
- Iacono F, Cappello T, Corsaro C, Branca C, Maisano M, Gioffre G, De Domenico E, Mauceri A, Fasulo S. 2010. Environmental metabolomics and multibiomarker approaches on biomonitoring of aquatic habitats. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 157:S50–S50.
- Jha AN. 2008. Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221.
- Jha AN, Dogra Y, Turner A, Millward GE. 2005. Impact of low doses of tritium on the marine mussel, Mytilus edulis: Genotoxic effects and tissue-specific bioconcentration. Mutation Research 586:47–57.
- Jonsson H, Schiedek D, Grøsvik BE, Goksøyr A. 2006. Protein responses in blue mussels (Mytilus edulis) exposed to organic pollutants: A combined CYP-antibody/proteomic approach. Aquatic Toxicology 78S:S49–S56.
- Lehtonen KK, Kankaanpää H, Leiniö S, Sipiä VO, Pflugmacher S, Sandberg-Kilpi E. 2003. Accumulation of nodularin-like compounds from the cyanobacterium Nodularia spumigena and changes in acetylcholinesterase activity in the clam Macoma balthica during short-term laboratory exposure. Aquatic Toxicology 64:461–476.
- Maisano M, Trapani MR, Parrino V, Parisi MG, Cappello T, D’Agata A, Benenati G, Natalotto A, Mauceri A, Cammarata M. 2013. Haemolytic activity and characterization of nematocyst venom from Pelagia noctiluca (Cnidaria, Scyphozoa). Italian Journal of Zoology 80:168–176.
- Mamaca E, Bechmann RK, Torgrimsen S, Aas E, Bjørnstad A, Baussant T, Le Floch S. 2005. The neutral red lysosomal retention assay and comet assay on haemolymph cells from mussels (Mytilus edulis) and fish (Symphodus melops) exposed to styrene. Aquatic Toxicology 75:191–201.
- Marigómez I, Soto M, Cajaraville MP, Angulo E, Giamberini L. 2002. Cellular and subcellular distribution of metals in molluscs. Microscopical Research and Technology 56:358–392.
- Mauceri A, Fasulo S, Ainis L, Licata A, Lauriano ER, Martinez A. 1999. Neuronal nitric oxide synthase (nNOS) expression in the epithelial neuroendocrine cell system and nerve fibers in the gill of the catfish, Heteropneustes fossilis. Acta Histochemica 101:437–448.
- Mauceri A, Fossi MC, Leonzio C, Ancora S, Minniti F, Maisano M, Lo Cascio P, Ferrando S, Fasulo S. 2005. Stress factors in the gills of Liza aurata (Mugilidae, Teleosts) living in polluted environments. Italian Journal of Zoology 72:285–293.
- Michel XR, Suteau P, Robertson LW, Narbonne JF. 1993. Effects of benzo(a)pyrene 3,3′,4,4′-tetrachlorobiphenyl and 2,2’,4,4′,5,5′-hexachlorobiphenyl on the xenobiotic-metabolizing enzymes in the mussel (Mytilus galloprovincialis). Aquatic Toxicology 27:335–344.
- Monserrat JM, Martìnez PE, Geracitano LA, Amado LL, Martins C, Lopes G, Pinho L, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A. 2007. Pollution biomarkers in estuarine animals: Critical review and new perspectives. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 146:221–234.
- Moore MN, Allen JI. 2002. A computational model of the digestive gland epithelial cells of marine mussels and its simulated responses to oil-derived aromatic hydrocarbons. Marine Environmental Research 54:579–584.
- Moore MN, Allen JI, McVeigh A. 2006. Environmental prognostics: An integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Marine Environmental Research 61:278–304.
- Moore MN, Koehler A, Lowe D, Viarengo A. 2008. Lysosomes and autophagy in aquatic animals. Methods in Enzymology 451:581–620.
- Mora P, Fournier D, Norbonne JF. 1999a. Cholinesterases from the marine mussels Mytilus galloprovincialis Lmk. and M. edulis L. and from the freshwater bivalve Corbicula fluminea Müller. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 122:353–361.
- Mora P, Michel X, Narbonne JF. 1999b. Cholinesterase activity as a potential biomarker in two bivalves. Environmental Toxicology and Pharmacology 7:253–260.
- Moreira SM, Guilhermino L. 2005. The use of Mytilus galloprovincialis acetylcholinesterase and glutathione S-transferases activities as biomarkers of environmental contamination along the northewest portuguese coast. Environmental Monitoring and Assessment 105:309–325.
- Munaò F, Di Pietro A, Scoglio ME, Piperno I. 2000. Elementi in traccia nel complesso lagunare Ganzirri-Faro. Rischio sanitario. Igiene Moderna 114:243–260.
- Nigro M, Falleni A, Del Barga I, Scarcelli V, Lucchesi P, Regoli F, Frenzilli G. 2006. Cellular biomarkers for monitoring estuarine environments: Transplanted versus native mussels. Aquatic Toxicology 77:339–347.
- Olive PL, Banath JP, Durand RE. 1990. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells using the ‘‘comet’’ assay. Radiation Research 122:86–94.
- Parsons SM, Prior C, Marshal IG. 1993. Acetylcholine transport, storage and release. Internationa Review of Neurology 35:280–390.
- Peakall D. 1992. Animal biomarkers as pollution indicators. London: Chapman & Hall.
- Peters LD, Shaw JP, Nott M, O‘Hara S, Livingstone DR. 1999. Development of cytochrome P450 as a biomarker of organic pollution in Mytilus sp.: Field studies in United Kingdom (‘Sea Empress’ oil spill) and the Mediterranean Sea. Biomarkers 4:425–441.
- Petrovic S, Ozretic B, Krajnovic-Ozretic M, Bobinac D. 2001. Lysosomal membrane stability and metallothioneins in digestive gland of mussels (Mytilus galloprovincialis Lam.) as biomarkers in a field study. Marine Pollution Bulletin 42:1373–1378.
- Pisoni M, Cogotzi L, Frigeri A, Corsi I, Bonacci S, Iacocca A, Lancini L, Mastrototaro F, Focardi S, Svelot M. 2004. DNA adducts, benzo(a)pyrene monooxygenase activity, and lysosomal membrane stability in Mytilus galloprovincialis from different areas in Taranto coasta waters (Italy). Environmental Research 96:163–175.
- Rank J, Lehtonen KK, Strand J, Laursen M. 2007. DNA damage, acetylcholinesterase activity and lysosomal stability in native and transplanted mussels (Mytilus edulis) in areas close to coastal chemical dumping sites in Denmark. Aquatic Toxicology 84:50–61.
- Regoli F, Frenzilli G, Bocchetti R, Annarumma F, Scarcelli V, Fattorini D, Nigro M. 2004. Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquatic Toxicology 68:167–178.
- Scarfì K, Mauceri A, Maisano M, Minniti F, Bruno R, Fasulo S. 2006. Morpho-functional alterations in branchial epithelium of Mytilus galloprovincialis (L.) living in natural and polluted environments. Biologia Marina Mediterranea 13:773–776.
- Snyder MJ. 1998. Cytochrome P450 enzymes belonging to the CYP4 family from marine invertebrates. Biochemical and Biophysical Research Communications 249:187–190.
- Solé M, Livingstone DR. 2005. Components of the cytochrome P450-dependent monooxygenase system and ‘NADPH-independent benzo[a]pyrene hydroxylase’ activity in a wide range of marine invertebrate species. Comparative Biochemistry and Physiology 141:20–31.
- Sorgi C, Cavallaro M, Brianti E, Ferlazzo M, Giannetto S. 2006. Cryptosporidium and Giardia in mussels (Mytilus galloprovincialis) from Faro salt-lake, Sicily. Parassitologia 48:296.
- Steinert SA, Streib-Montee R, Leather JM, Chadwick DB. 1998. DNA damage in mussels at sites in San Diego Bay. Mutation Research 399:65–85.
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. 2000. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environmental and Molecular Mutagenesis 35:206–221.
- Vukmirovic M, Bihari N, Zahn RK, Muller W, Batel R. 1994. DNA damage in marine mussel Mytilus galloprovincialis as a biomarker of environmental contamination. Marine Ecology Progress Series 109:165–171.
- Walker CH, Thompson HM. 1991. Phylogenetic distribution of cholinesterases and related esterases. In: Mineau P, editor. Cholinesterase inhibiting insecticides. Their impact on wildlife and the environment. Chemicals in agriculture. Vol. 2. Amsterdam: Elsevier. pp. 1–17.
- Widdows J, Donkin P. 1992. Mussels and environmental contaminants: Bioaccumulation and physiological aspects. In: Gosling E, editor. The Mussel Mytilus: ecology, physiology, genetics and culture, of developments in aquaculture and fisheries science. Vol. 25. Amsterdam: Elsevier Science Publishers. pp. 383–424.
- Woolf NJ. 1991. Cholinergic systems in mammalian brain and spinal cord. Progress in Neurobiology 37:475–524.