Abstract
Sabella pavonina Savigny, 1920 is a species common to sheltered areas of northwest Europe and reported also in several Mediterranean sites. However, most Mediterranean records refer to faunistic lists with only few papers reporting morphological descriptions supported by drawings or photographs, most of which are short and out-dated. Moreover, no analysis of intraspecific variations among Mediterranean populations is available. In the present paper we provide the descriptions of individuals identified as S. pavonina belonging to four different Mediterranean populations and deposited at the Laboratory of Zoology of the Salento University. We analyzed the species morphological variation within the Mediterranean area compared to the deposited neotype and some other specimens collected from the type locality (Plymouth) and from Portsmouth (English Channel). The examined specimens from the four Mediterranean localities all differ from the English Channel Plymouth specimens with respect to a higher crown/body length ratio and smaller body size. Among them, individuals from Taranto and Gela resemble the morphology of the neotype specimens from Plymouth more closely than the others, with only a few differences, mostly involving the branchial crown features. Statistical analysis shows the occurrence of two different groups, one formed by specimens collected in sites located at deeper depths (100–130 m), separated by another containing specimens collected at shallower depths (5–10 m). Based on these considerations, it could be hypothesized that the specimens collected from greater depths do not actually belong to the S. pavonina species. However, the poor condition of the material does not yet allow the establishment of a new taxon. Only the examination of additional material coupled with further data including genetic analysis, will clarify the status of the taxon S. pavonina within the Mediterranean area.
Introduction
In a large revision of the genus Sabella Linnaeus, 1767, Knight-Jones and Perkins (Citation1998) considered S. spallanzanii (Gmelin, 1791), S. pavonina Savigny, 1920, and S. discifera Grube, 1874 as the only valid species. The remaining 55 taxa listed in Hartman (Citation1959) and previously ascribed to the Sabella genus were either synonymized with one of these three species or placed in the genera Bispira Krøyer, 1856 and Stylomma Knight-Jones, 1997.
The genus Sabella is characterized by:
base of the crown unflanged and forming two semicircles with ventral edges involuted or with involution only on one side; radioles with shallow basal web (one third of the length); composite eyes, ocelli or flanges absent; dorsal lips with dorsal radiolar appendage sometime longer than web; collar margin separate dorsally by distinct gap; abdominal fascicles arising from conical lobe and forming pencil-like tufts of slightly geniculate chaetae arranged in spiral pattern of one or more whorls; interramal eyespot present (Knigth-Jones & Perkins Citation1998).
Some of these features are shared with other genera which form a quite homogeneous clade containing Sabella, Bispira, Branchiomma Kølliker, 1858, Pseudobranchiomma Jones, 1962, Sabellastarte Krøyer, 1856 and Stylomma, whose monophyly is strongly demonstrated even though the relationships within these genera are still unresolved (Fitzhugh Citation1989; Rouse & Fitzhugh Citation1994; Fitzhugh & Rouse Citation1999; Fitzhugh Citation2003; Nogueira et al. Citation2004). This clade has been defined by the presence of interramal eyespots in the thorax and abdomen, inferior thoracic chaetae arranged in longitudinal bundles, abdominal neuropodia as conical lobes,“spine-like” chaetae in the thorax and abdomen, and abdominal neurochaetae arranged in “C”-shaped fascicles or in spiral (Fitzhugh Citation1989; Rouse & Fitzhugh Citation1994; Fitzhugh & Rouse Citation1999; Capa Citation2008). In addition to these synapomorphies, a radiolar skeleton is also present in the dorsal radiolar appendage, a feature reported for some species of Laonome Malmgren, 1866 (Fitzhugh Citation2003). Among these genera, Sabella is the most closely related to Bispira from which it differs in the spiral arrangement of abdominal chaetae. Moreover, most of the Bispira species show eyes on the radioles. After phylogenetical analyses performed by several authors, the genus Bispira has been proposed either as the sister group of Sabella, because of the presence of companion chaetae with an asymmetrical distal membrane (Fitzhugh Citation1989; Rouse & Fitzhugh Citation1994), or the sister group of the clade including Sabella, Sabellastarte, Pseudobranchiomma and Branchiomma (Fitzhugh Citation2003) or an unresolved polytomy (Fitzhugh & Rouse Citation1999).
From the above considerations, it turns out that the only apomorphy for the genus Sabella is the spiral arrangement of abdominal chaetae.
Among the three Sabella species, S. spallanzanii described for the Mediterranean Sea is the most common taxon easily recognizable for the characteristic spiralization of the crown. Sabella discifera, reported for the Mediterranean as Branchiomma linaresi (Rioja, 1917), is a small-sized sabellid easily recognizable by the swollen tip of the radioles (Knight-Jones & Perkins Citation1998). Lastly, S. pavonina is a species common in sheltered areas of northwest Europe and reported also in several Mediterranean sites (Knight-Jones & Perkins Citation1998). However, most of the Mediterranean records refer to faunistic lists (e.g. Bellan Citation1958; Pozar-Domac Citation1978; Albertelli et al. Citation1981; Gambi et al. Citation1982; Giangrande Citation1985); the only description of Mediterranean material supported by drawings or photographs is that provided by Giangrande et al. (Citation2005) for specimens collected along the Italian coast (Ionian Sea, Taranto). Other morphological descriptions of Mediterranean specimens of S. pavonina are short and out-of-date (Lo Bianco Citation1893; Iroso Citation1921) while an analysis of intraspecific variations among Mediterranean populations has never been provided.
Knight-Jones and Perkins (Citation1998) re-described the species and erected the neotype from type locality material (Plymouth). Moreover, these authors underlined the high degree of intraspecific dimensional variation. Some individuals may grow to a large size in sheltered areas under particular environmental conditions, whilst in other sites, including the Mediterranean, specimens can be smaller. However, Knight-Jones and Perkins (Citation1998) examined only a few Mediterranean specimens, mainly focusing attention on the extramediterranean material.
In the present paper we provide the descriptions of individuals identified as S. pavonina belonging to four different Mediterranean populations and deposited at the Laboratory of Zoology of the Salento University (PCZL). We analyze the species’ morphological variation within the Mediterranean area compared to the deposited neotype (NHM, ZB 1988.10) and other material collected from English Channel.
Material and methods
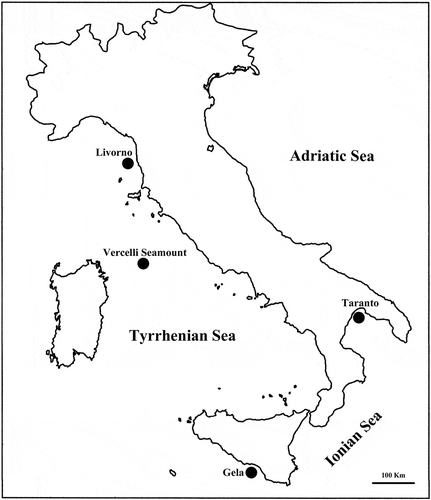
The following material of S. pavonina was examined: Plymouth. Neotype, deposited in the National History Museum, London (NHM), plus two specimens from Phyllis Knight-Jones’ private collection; Portsmouth, four specimens kindly provided by Dr Gordon Watson (Institute of Marine Sciences, School of Biological Sciences, University of Portsmouth, Portsmouth, UK); Mediterranean material (): Ionian Sea (Taranto, three specimens; Gela, eight specimens); Tyrrhenian Sea (Livorno, six specimens; Vercelli Seamount, four specimens). All the Mediterranean material is deposited in the Polychaete Collection of the Laboratory of Zoology of the University of Salento (Lecce), Italy (PCZL).
For each specimen the following features have been measured: body length, crown length, length of radiolar bare tip, length of radiolar appendages and height of the basal web, as well as number of radioles, and thoracic and abdominal chaetigers. Moreover, the morphology of chaetae and uncini was checked.
Drawings were made with the aid of a camera lucida attached to stereo- and compound microscopes. Photographs were taken using a stereomicroscope equipped with a Nikon Coolpix 990 camera. Multivariate analyses were performed using morphological measures of each specimen as variables; when necessary, size-dependant variables were standardized on body length or crown length (i.e. body length/crown length, radiolar length/crown length, number of right radioles/number of left radioles, number of radioles/body length, number of radioles/crown length, number of abdominal chaetigers/body length, number of thoracic chaetigers/body length); a 0–1 binary code was used to formalize variables such as shape of thoracic chaetae, presence/absence of eye spots, and features of the radiolar tip (0, short; 1, long). Differences among specimens were represented in a similarity matrix based on Gower’s similarity coefficient to take the different data types (numerical, binary) into account, and then analysed by cluster analysis. Analyses were performed using the computer program PRIMER (Plymouth, UK).
Results
Taxonomic accounts
Material from English Channel. A–F; A–C
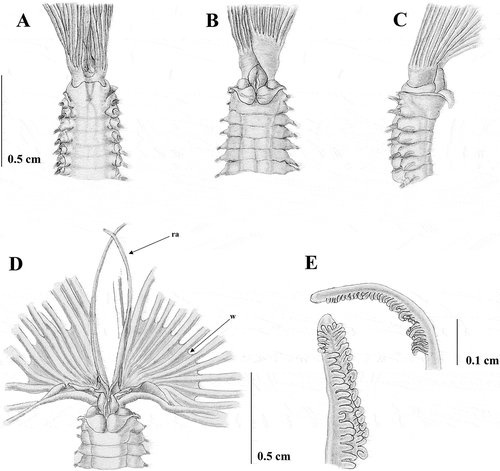
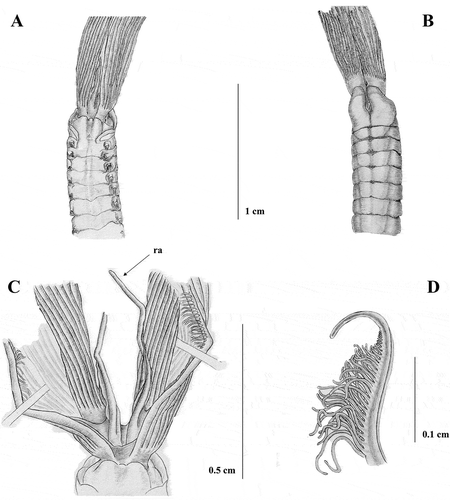
Figure 2. Sabella pavonina Neotype. A, anterior end dorsal view; B, anterior end ventral view; C, anterior end lateral view; D, dorsal lips with dorsal radiolar appendages (ra); E, variability of the tip of the radioles; F, particular of the basal web of the branchial crown (w).
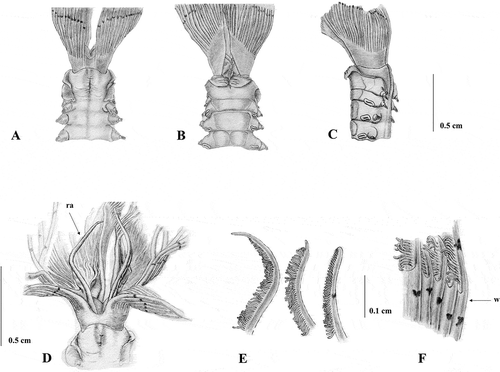
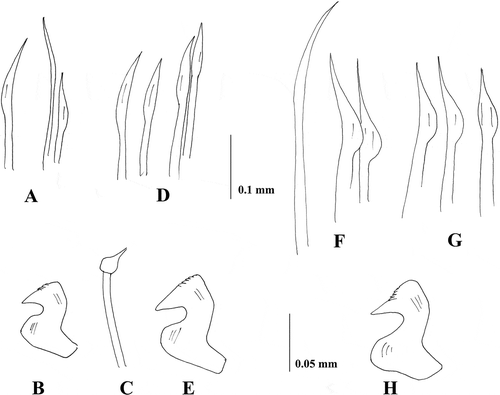
Figure 3. Shape of thoracic chaetae and uncini of Sabella pavonina specimens from neotype (A–C),Taranto (D, E), Vercelli Seamount (F–H). A, superior (left) and inferior (right) thoracic chaetae; B, thoracic uncinus; C, companion chaetae; D, inferior thoracic chaetae; E, thoracic uncinus; F, superior thoracic chaeta; G, inferior thoracic chaetae; H, thoracic uncinus.
Plymouth: neotype (NHM, ZB 1988.10)
Plymouth UK (Tamar Estuary, Plymouth Sound) Knight-Jones personal collection: two specimens, 50°22'34'' N–4°8'37''W, 4–5 m, 1988, on muddy sand
Portsmouth UK (Swartd Sands, Langstone Harbour). PCZL 2C: four specimens 50°49′N–1°05′W, 3 m, 2010, on muddy sand
The following description is based on the neotype. Body length without crown 20 cm. Crown length 4 cm. About 200 chaetigers of which eight chaetigers belong to thorax; first chaetiger about twice as long as the following ones (A). Collar widely separated dorsally with lateral margins scarcely projecting toward the midline beyond the first fascicles, and not reaching junction between crown and thorax (A–C); ventral margins forming rounded lappets protruding below large ventral sacs (B). First ventral shield wider than the following ones appearing more than twice as broad as long (B); dorsal radiolar appendages, 0.6 cm long (D). Crown with 40 pairs of radioles on each side, webbed basally for 0.4 cm (F). Radioles with short crowded pinnules, blunt bare tips and a rounded outer surface (E). Up to 10 dark spots on the radioles (F). Cross section of the radiolar skeleton near the web composed of four compact cells. Thoracic tori not reaching the ventral shields with gaps of similar width between thoracic shields and the adjacent tori (C).
Collar chaetae and superior thoracic notochaetae long and slender; inferior thoracic notochaetae more geniculate and wider limbate (A); thoracic uncini with finely toothed crest (B); companion chaetae geniculate with broad asymmetrical blades (C). Chaetae of abdominal fascicles resembling the morphology of the thoracic notochaetae.
The only difference between Plymouth and Portsmouth material is that the crown of the specimens from Portsmouth is more brown in colour with more evident dark spots on the radioles; moreover, one specimen also has dark pigments on the two dorsal-most radioles and on the ventral sacs and collar lappets. This coloration pattern is not present in the type material. Specimens from Portsmouth showed fewer dark spots on the radioles, numbered 7–8.
Material from Taranto. C–D; A–E; A–B
Taranto (Mar Grande, Ionian Sea) PCZL2A: three specimens, 40°29′07′′ N–17°16′39′′ E, 5 m, 2002, on muddy sand
The morphology of the collar (A–C) corresponds to that of the type material. Specimens are smaller with a longer crown, (body measuring up to 8 cm and crown 3.5 cm) and have about 120 abdominal and eight thoracic chaetigers. They also have a longer radiolar appendage measuring 1.2 cm (D). The crown bears about 20 pairs of radioles webbed basally for about 0.4 cm (D) having a rounded outer surface, short crowded pinnules and a blunt bare tip (E).
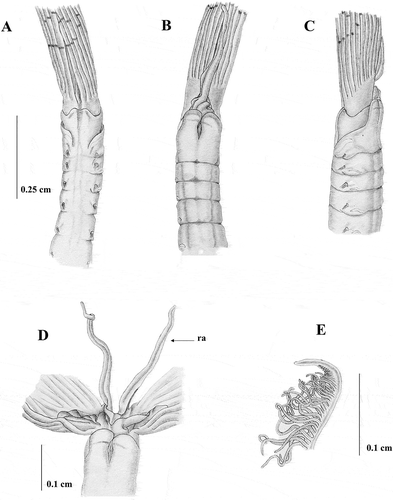
Figure 4. Sabella pavonia from Taranto. A, anterior end, dorsal view; B, anterior end, ventral view; C, anterior end, lateral view; D, Crown showing the dorsal lips with dorsal radiolar appendages (ra) and the basal web (w); E, tip of the radioles.
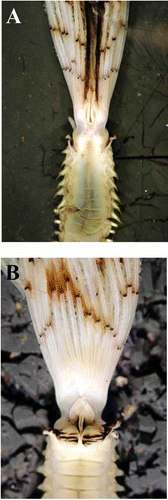
Similar to the type material, materials from Taranto have up to 10 dark spots on each radiole. However, dark pigments are also present on the two dorsal-most radioles and on the ventral sacs and collar lappets, even if they are evident only in freshly collected specimens (A and B). Up to 18 thoracic notochaetae, whose morphology is similar to that of the neotype, as well as the morphology of uncini (D and E).
Figure 5. Sabella pavonina from Taranto showing the coloration pattern of collar, crown and dorsalmost radioles. A, dorsal view; B, ventral view.
Material from Gela. A–E
Figure 6. Sabella pavonina from Gela. A, anterior end, dorsal view; B, anterior end, ventral view; C, anterior end, lateral view; D dorsal lips with dorsal radiolar appendages (ra); E, tip of the radiole.
Gela Harbour (Sicily Channel) PCZL2B: eight specimens, 37° 3′54.86′′N–14°15′4.94′′E, 10 m, 1987, on muddy sand
The morphology of the collar (A–C) is similar to that of the type material and the material from Taranto. Specimens are smaller with body length 3 cm, but with a long crown (1.25 cm) similarly to Taranto material, and have about 70 abdominal and eight thoracic chaetigers. Radiolar appendages are shorter than found in Taranto material, measuring 0.23 cm (D). The crown bears about 14 pairs of radioles webbed basally for 0.13 cm. Radioles with a rounded outer surface and a longer bare tip compared to Taranto material, but similar to the neotype (E). No pigmentation on collar and dorsal-most radioles are detected, even though the presence of pale pigments on the radioles was detected. Morphology and number of chaetae and uncini are similar to those of the Taranto material and neotype.
Material from Vercelli Seamount. F, H; A–E
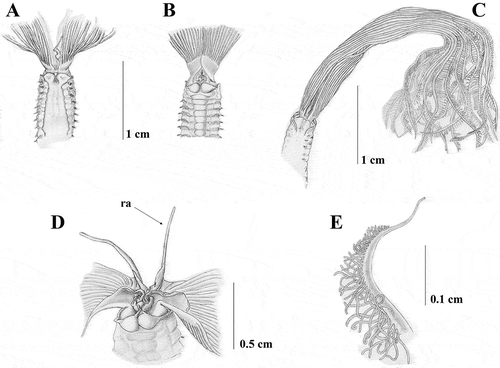
Figure 7. Sabella pavonina from Vercelli Seamount. A, anterior end, dorsal view; B, anterior end, ventral view; C, anterior end, dorsal view showing the branchial crown; D, dorsal lips with dorsal radiolar appendages (ra); E, tip of the radiole.
Vercelli Seamount (Tyrrhenian Sea) PCZL 3A: four specimens, 41°06.45′N–10°54.43′E, 130 m, 2009, on bioconcretions (coralligenous).
The morphology of the collar (A and B) is similar to that of the type material and that of the previously described Mediterranean material. Specimens are larger than the Taranto ones (9 cm in body length), with a longer crown (6.18 cm crown length) (C), bearing a larger number of radioles (30 on each side), and webbed basally for 0.45 cm. Specimens have about 130 abdominal and eight thoracic chaetigers. Radiolar appendages shorter than those found in Taranto material, measuring 0.60 cm (D). Radioles too damaged to ascertain the morphology, but with a longer bare tip than Taranto material (E). Neither pigmentation on collar and dorsal-most radioles nor the presence of pigments on the radioles is detected. The number of thoracic chaetae is greater than that recorded for the previous described material, numbering up to 30. Inferior thoracic notochaetae are larger and more broadly hooded than those described in previous material (F), whilst uncini are similar to all the previously described material (H).
Material from Livorno
G; A–D
Figure 8. Sabella pavonina from Livorno A, anterior end, dorsal view; B, anterior end, ventral view; C, dorsal lips with dorsal radiolar appendages (ra): D, tip of the radiole.
Livorno (Tyrrhenian Sea) PCZL3A: seven specimens, 43°33′44′′N–10°17′42′′E, 100 m, 1983, on mud
The morphology of the collar (A and B) is similar to that of the type material and that of the previously described Mediterranean material. Specimens have a number of thoracic and abdominal chetigers (130 abdominal and eight thoracic) similar to those of the Vercelli Seamont and are similar in size to the Taranto material with a similar crown length (8 cm in body length and 3.5 cm in crown length), but have a larger number of radioles (28 each side) webbed basally for 0.4 cm. Radiolar appendages slightly shorter than that those found in the Taranto material, measuring 0.9 cm (C). Radioles with a rounded outer surface and a longer bare tip than the Taranto material (D). Neither pigmentation on collar and dorsal-most radioles nor the presence of pigments on the radioles is observed, similarly to Vercelli material. Morphology and number of thoracic chaetae similar to those reported for Vercelli material (G).
All the reported features for the examined specimens are summarized in .
Table I. Morphological features examined in Sabella pavonina specimens from the six sampling localities. Length measures are given in cm (mean value ± standard deviation).
Statistical analysis
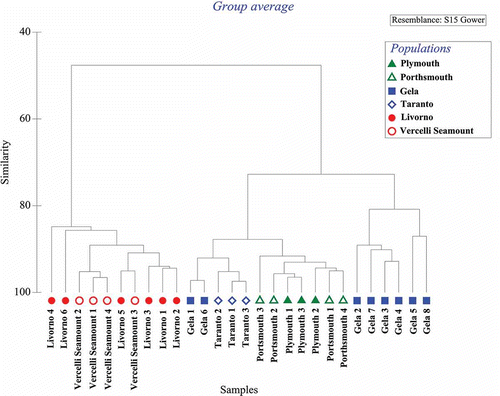
Most of the features considered in are related to the size of the specimens: for example, the number of abdominal chaetigers is highly correlated to the total body size (r = 0.88), or to the number or radioles in the crown (0.86). However, when the whole data set was considered, although total length was highly correlated to crown length (r = 0.87), total length is not correlated to the ratio between crown length and body length (r = 0.33). As already pointed out, crown length is instead correlated to the ratio between the length of radiolar appendage (0.61) to the height of the basal web (r = 0.90) and to the crown length. In the cluster analysis shown in , two main groups of specimens could be recognized: English Channel specimens, including the neotype, clustering together sharing high similarity (Glower similarity > 90%) within a larger group also including the specimens from both Gela and Taranto. This large cluster shares more than 70% of similarity and is clearly different from the second main group formed by specimens from Livorno and Vercelli Seamount that appear very similar to each other (Glower similarity > 80%) without differences between locations.
Concluding remarks
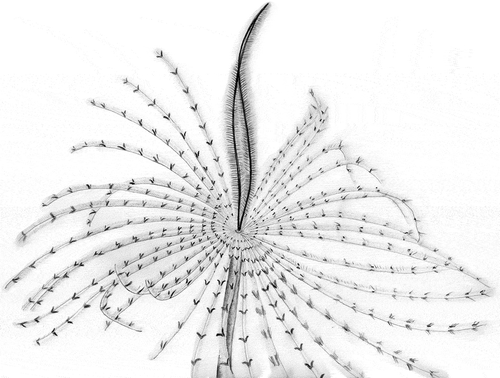
In their description of S. pavonina from Plymouth, Knight-Jones and Perkins (Citation1998) reported the presence of dark spots on the radioles and of pigmentate ventral sacs and collar, without mentioning the existence of dark colouration in the two dorsal-most radioles. In particular, the crown has been described as pale cream yellow-coloured, with up to 10 encircling bands of pigment and a series of red-brown blotches along the outer surface of each radiole. In comparison with the neotype, only the Mediterranean specimens from Taranto and Gela showed dark brown spots on radioles. In addition, Taranto specimens also showed a dark pigmentation not only on the ventral sacs and the collar lappets, but also on the two dorsal-most radioles. In live specimens, these features are so evident that the crown actually resembles a peacock’s tail, thus justifying the etymology of the species name “pavonina”: “pavonina” [“pavone” = “peacock”] ().
Figure 10. Drawing of Sabella pavonina showing the radiole position from a live specimen in its natural environment.
In this regard, however, it must be stressed that in the Taranto preserved material the dark pigmentation on the two dorsal-most radioles disappeared, while the spots on the radioles became pale but were still detectable. The two dorsal-most coloured radioles were evident not only in fresh material from Taranto, but also in one preserved specimen from Portsmouth. In addition, the crown of specimens from Portsmouth appeared darker, with particularly evident bands of pigment on the radioles and pigment on the ventral sacs and collar. The possible disappearance of coloration of preserved material may explain why a dark coloration of the two dorsal-most radioles was never mentioned by Knight-Jones and Perkins (Citation1998) for Plymouth specimens, as well as by Lo Bianco (Citation1893) for Mediterranean individuals from the Gulf of Naples. By contrast, in the description of some specimens collected from the Gulf of Naples, Iroso (Citation1921) reports the presence of these two “tentacles” intensely coloured in purple and clearly distinguishable from the other radioles showing a yellow coloration with purple spots. However, in the Mediterranean material from Vercelli Seamount and Livorno, pigmentation was completely absent and the crown appeared pale ().
Figure 11. Different development (length) of the branchial crown in specimens from Vercelli Seamount (A), Livorno (B) and Portsmouth (C).

As a whole, the examined specimens from the four Mediterranean localities all differ from the English Channel material for the higher crown/body length ratio (); moreover, Mediterranean material, especially that from shallower sites, is characterized by smaller body size. Among them, individuals from Taranto and Gela recall the morphology of the specimens from English Channel material more than do individuals collected in deeper sites, with only a few differences mostly involving the branchial crown features.
The examined specimens from Mediterranean localities also differ from each other as regards several features, most concerning the branchial crown. The individuals from Vercelli Seamount show the largest body size and the longest crown length, whilst the lowest values for both these features are reported for specimens from Gela. Among the examined Mediterranean material, the specimens from Vercelli Seamount showed the lowest value of radiolar appendages/crown length ratio as well as the lowest web/crown length ratio. By contrast, the other Mediterranean individuals showed similar higher values of web/crown length ratio, while the specimens from Taranto were characterized by the longest radiolar appendages and the highest value of radiolar appendages/crown length ratio. Moreover, differently from Vercelli Seamount and Livorno specimens, the branchial crown of S. pavonina specimens from Taranto and Gela show a lower number of radioles and also differ in the presence of dark spots and a shorter bare tip. Lastly, the inferior thoracic chaetae in the specimens from Vercelli Seamount and Livorno are larger and more broadly hooded than that reported for the individuals from Taranto and Gela, whose thoracic chaetae morphology is similar to that reported for the specimens from the English Channel.
Based on the foregoing statements, the absence of dark spots on the radioles, long basal webs and radiolar tips, and broadly hooded inferior thoracic chaetae all characterize the specimens collected from greater depths, and make it possible to differentiate them from the specimens collected from the shallower depths and also from English Channel material. This is also confirmed by the statistical analysis performed on our results that clearly showed the occurrence of two clusters, one representing the Vercelli Seamount and Livorno specimens and the other one representing Plymouth and Portsmouth material, together with the Mediterranean specimens from the shallowest sites, Taranto and Gela. However, some dissimilarity is also present within this cluster and, in particular, the population from Gela appears highly variable.
In addition to the already mentioned morphological differences, the Mediterranean populations from greater and shallower depths also colonize different habitats. The specimens from Taranto and Gela were collected from sheltered areas (Ports) on soft bottom (muddy sand) at a depth from 5 to 10 m, whilst those from Vercelli Seamount and Livorno were collected in open sea on hard bottom, represented by biogenic formation (coralligenous) or in mud at a depth from 100 to 130 m (Bo et al. 2011). Based on these considerations, it could be hypothesized that the specimens collected from greater depths do not actually belong to the S. pavonina species; therefore we refer to the specimens from Vercelli Seamount and Livorno as Sabella sp., whilst the specimens collected from Taranto may be considered as representative of S. pavonina in the Mediterranean Sea. However, the poor condition of the material collected at greater depths, especially concerning the crown, still makes it impossible at present to describe a new species, establishing the holotype. Moreover, the difference existing also between the Mediterranean material here ascribed to S. pavonina and the material from the type locality prompted further investigation concerning the variability existing within this taxon. Only the accurate examination of additional material, from both deep and shallower areas, coupled with further data including genetic analysis, will clarify the status of S. pavonina within the Mediterranean area.
References
- Albertelli G, Cattaneo M, Della Croce N, Drago N. 1981. Benthos della piattaforma continentale Ligure. Alassio, Savona, Chiavari, Corniglia (1977–1981). Università degli Studi di Genova, Istituto di Scienze Ambientali Marine, Cattedra di Idrobiologia e Pescicoltura, Rapporto Tecnico n. 18:1–28.
- Bellan G. 1958. Contribution à l’étude des Annélides Polychètes du Golfe de Genes (1). Doriana, Supplemento agli Annali del Museo Civico di Storia Naturale “G. Doria“ Genova 2:1–7.
- Bo M, Bertolino M, Borghini M, Castellano M, Covazzi Harriague A, Di Camillo CG, Gasparini GP, Misic C, Povero P, Pusceddu A, Schroeder K, Bavestrello G. 2011. Characteristics of the mesophotic megabenthic assemblages of the Vercelli Seamount (North Tyrrhenian Sea). PlosOne 6(2):e16357.
- Capa M. 2008. The genera Bispira Krøyer, 1856 and Stylomma Knight-Jones, 1997 (Polychaeta: Sabellidae): Systematic revision, relationships with close related taxa and new species from Australia. Hydrobiologia 596:301–327.
- Fitzhugh K. 1989. A sytematic revision of the Sabellidae-Caobangiidae-Sabellongidae complex (Annelida: Polychaeta). Bulletin of the American Museum of Natural History 192:1–104.
- Fitzhugh K. 2003. A new species of Megalomma Johansson, 1927 (Polychaeta: Sabellidae: Sabellinae) from Taiwan, with comments on sabellid dorsal lip classification. Zoological Studies 42:106–134.
- Fitzhugh K, Rouse GW. 1999. A remarkable new genus and species of fan worm (Polychaeta: Sabellidae: Sabellinae) associated with marine gastropods. Invertebrate Biology 118:357–390.
- Gambi MC, Giangrande A, Colognola R. 1982. Policheti e Archianellidi del Museo della Stazione Zoologica di Napoli. Bollettino dei Musei Istituti Biologici Università di Genova 50(Suppl):385.
- Gambi MC, Giangrande A, Fresi E.1983–84. Policheti di fondo mobile del Golfo di Salerno ipotesi di un modello di distribuzione generale. Nova Thalassia 6(suppl):575–583.
- Giangrande A. 1985. Policheti dei rizomi di Posidonia oceanica L. Delile (Helobie, Potamogetonacee) di una prateria dell’isola di Ischia (Napoli). Atti Società Toscana di Scienze Naturali Memorie, Serie B 92:195–206.
- Giangrande A, Pierri C, Trianni L. 2005. Su di una popolazione di Sabella pavonina (Annelida Polychaeta) nel Mar Grande di Taranto. Thalassia Salentina 28:9–16.
- Hartman O. 1959. Catalogue of the polychaetous annelids of the world. Allan Hancock Foundation Publications, Occasional Paper 23:355–628.
- Iroso I. 1921. Revisione dei Serpulidi e Sabellidi del Golfo di Napoli. Pubblicazioni della Stazione Zoologica di Napoli 3:47–91.
- Knight-Jones P, Perkins TH. 1998. A revision of Sabella, Bispira and Stylomma (Polychaeta: Sabellidae). Zoological Journal of the Linnean Society 123:385–467.
- Lo Bianco S. 1893. Gli anellidi tubicoli del Golfo di Napoli. Atti Accademia di Scienze Fisiche e Matematiche Napoli 5:65–81.
- Nogueira JM, López E, Rossi MCS. 2004. Kirkia heterobranchiata, a new genus and species of extratubular brooding sabellid (Polychaeta: Sabellidae) from São Paulo, Brazil. Journal of the Marine Biological Association of the UK 84:701–710.
- Pozar-Domac A. 1978. Katalog Monogocetinasa (Polychaeta) Jandrana. 1. Sjeverni i srednij Jadran. Acta Adriatica 19:1–59.
- Rouse GW, Fitzhugh K. 1994. Broadcasting fables: Is external fertilization really primitive? Sex, size, and larvae in sabellid polychaetes. Zoologica Scripta 23:271–312.