Abstract
The red deer of the Bosco Mesola Nature Reserve (northern Italy) constitute a population with relevance for zoogeography, genetics and conservation. We have analysed morphometrics of Mesola red deer (body weight, craniometry, stature, antler conformation and size) over a c. 30 year period (1980–2012), to (i) describe in detail their physical traits, (ii) compare them with those of other European populations and (iii) assess the effects of conservation actions on biometric measures of individuals. Mesola red deer were on average 15–50% lighter and at least 8–15% smaller than other European red deer. The sexual size dimorphism was low and significant only for adults. Body growth rate was also slower than that of the other European populations. While the average relative production of antler bone tissue appeared not to be different from that of other European red deer, antlers of Mesola stags were small and scarcely branched, with short trez tines. Bez tine and crown were rare and present almost exclusively in fully mature stags (2.2 and 3.6% of antler beams in adult stags, respectively). Environmental improvements led to significant favourable effects on antler size and complexity. All observed individuals had a distinct but slight spotting of the summer coat. These morphological characteristics, coupled with genetic peculiarities, make the Mesola red deer unique, deserving special protection.
Introduction
Morphometrics can give a detailed description of the physical characteristics of a population, which can be influenced by genetic and/or environmental factors (Anderson et al. Citation1974; Feldhamer et al. Citation1984; Terada et al. Citation2012). The long-term analysis of morphometric measures of a population can also help to assess changes in traits, which could be determined by e.g. variation of autoecological/sinecological or climatic factors (see Moyes et al. Citation2011 for birth weight and antler weight changes over time in Scottish red deer). In populations with conservation relevance, the monitoring of morphometrics could help to assess how individuals react to conservation measures (e.g. increase in body size).
The Western red deer Cervus elaphus Linnaeus, 1758 is among the most investigated mammal species, with a very large distribution range, encompassing Europe, North Africa and western and central Asia (Mattioli Citation2011). However, studies on its morphometry are rare (cf. Ingebrigsten Citation1924; Beninde Citation1937a; Mystkowska Citation1966; Lowe & Gardiner Citation1974 for craniometric investigations). If we exclude research derived from the routine monitoring of body weights and antler size on harvested individuals of hunted populations (Isakovic Citation1969; Drechsler Citation1980; Radler & Hattemer Citation1982; Bečejac et al. Citation1984; Mysterud et al. Citation2001), very few investigations have reconstructed the morphological and biometric characteristics of a red deer population (cf. Langvatn Citation1986). In particular, while some studies have been carried out on populations from central-northern Europe, information is scarce for red deer living in southern Europe (Mattioli Citation1993; Azorit et al. Citation2002; Mattioli et al. Citation2003; Martı́nez et al. Citation2005; Torres-Porras et al. Citation2009). Thus, the variability of this species in morphological and biometric features still remains poorly known.
The red deer Cervus elaphus italicus (Zachos et al. Citation2014) inhabiting the Bosco Mesola Nature Reserve (northern Italy) constitute a population with relevance for zoogeography, genetics and conservation (Lovari & Nobili Citation2010; Zachos & Hartl Citation2011). They are the only native red deer of peninsular Italy (Castelli Citation1941; Mattioli Citation1990; Mattioli et al. Citation2001) and show a mitochondrial DNA genotype with a sequence significantly different from those of all other populations of red deer (Lorenzini et al. Citation2005; Hmwe et al. Citation2006). Previous studies on morphometry of antlers, and some biometric measures, have suggested that these deer show some morphological peculiarities (small body size, reduced sexual dimorphism, persistent spotting in the summer coat, small antlers of simplified design, low reproductive performance; Mattioli Citation1990, Citation1993; Mattioli et al. Citation2003). Mesola red deer are threatened, because of (i) their very small distribution range (c. 1000 ha) and numbers (less than 200 individuals, in 2010; Ferretti & Mattioli Citation2012), (ii) their very low genetic variability and (iii) the impact of a dense population of sympatric fallow deer Dama dama on their natural food resources (Lorenzini et al. Citation1998; Mattioli et al. Citation2003; Zachos et al. Citation2009; Lovari & Nobili Citation2010; Zachos & Hartl Citation2011). In the last 15 years, conservation measures have been implemented (e.g. culling of fallow deer, recurrent mowing in the pastures, reseeding of pastures and winter supplementary feeding; Mattioli et al. Citation2003), which should be expected to have led to environmental improvements for red deer. In turn, physical conditions of red deer should improve (Mattioli et al. Citation2003), resulting in an increase of e.g. body weight, antler size and complexity.
Here we have collected and analysed all the available data on morphometrics of Mesola red deer, over a c. 30 year period (1980–2012), to (i) describe in detail the physical traits of Mesola red deer, (ii) compare them with those of other European populations and (iii) assess the effects of conservation actions on biometric measures of individuals.
Methods
Study area
The Bosco della Mesola Nature Reserve (1058 ha) is an enclosed area located in the Po river delta (Ferrara Province, northern Italy; ). This reserve is composed of woodland (93%, mainly holm oak Quercus ilex with manna ash Fraxinus ornus, hornbeam Carpinus spp., Caucasian ash Fraxinus oxycarpa, pedunculate oak Quercus robur, aspen Populus spp. and elm Ulmus spp.), wetland (4%) and grassland (3%: dry and wet meadows, glades and artificial pastures; Mattioli et al. Citation2003). Fallow deer were introduced first in the 16th century and again between 1957 and 1965, after having been exterminated during World War II (Mattioli et al. Citation2003).
Morphometric analyses
To assess the morphometric characteristics of Mesola red deer, we used several datasets. Body weights were gathered during the capture sessions (1980–1991 and 1995–1999). Ratios of height at withers:head-trunk length and hind foot:head-trunk length (taken following Langvatn Citation1977, Citation1986; Mattioli & De Marinis Citation2009) were collected in 1995–1999 (see Mattioli et al. Citation2003 for descriptive statistics of the main somatic measures). Antler conformation (number and kind of tines per antler pair and antler beam) of the total stag population was recorded from 1982 to 2012. Every year (1980–2012), cast antlers were collected in late winter and spring and measured according to the CIC (Conseil International de la Chasse) rules (Trense et al. Citation1981), except for the bez tine (not included among the traits of the CIC formula), which was measured as the trez tine. Antler measurements were performed also for captured stags and animals found dead. Skulls were measured with a digital caliper to 0.01-mm precision according to von den Driesch (Citation1976). Red deer age was determined exactly for individuals captured and marked when calves or yearlings. For other captured individuals, the age was estimated on the basis of tooth eruption, replacement and wear patterns (Wagenknecht Citation1984). For the other stags, age was estimated through visual assessment of body shape (body size and proportions, neck size and posture, head traits, length of pedicles; cf. Drechsler Citation1988). We recognized five biologically meaningful age classes of stags: calves, yearlings, subadults (2–4 years old), young adults (5–9 years old) and fully mature adults (> 9 years old) (Drechsler Citation1988), and four of hinds: calves, yearlings, young adults (2–4 years old) and fully mature adults (> 4 years old). Besides ear tags of captured animals, individual recognition was based on skin scars and coat colour patterns.
In 1996, environmental improvements (culling of fallow deer, recurrent mowing in the pastures, reseeding of pastures and winter supplementary feeding) were introduced, leading to favourable effects on physical conditions and population dynamics of the deer (Mattioli et al. Citation2003; Ferretti & Mattioli Citation2012). Thus, for the morphometric characterization of antlers we considered two sub-periods (Period 1: 1982–1996, before the conservation measures; Period 2: 1997–2012, i.e. after the beginning of conservation measures). We could not test the differences of body weights and somatic linear dimensions between periods, as our sample of captured deer was small for Period 2.
Body weights of adults, yearlings and calves of both sexes were normally distributed (Kolmogorov-Smirnov test: Z = 0.390–0.652; P = 0.790–0.982). For adults, yearlings and calves, sexual differences in body weights were tested through the t-test (Sokal & Rohlf Citation1995). For each age class and period, all biometric measures were normally distributed (Kolmogorov-Smirnov test: Z = 0.391–1.107; P = 0.172–0.998), except for the number of tines/antler beam (Z = 1.735–3.726; P < 0.006). We used linear models (Crawley Citation2007) to assess the effects of (i) age class, period and interaction age class × period on antler beam length, brow tine length, circumference at the burr, lower and upper circumference and antler beam weight, in adult stags; (ii) period on antler length, brow tine length and circumference at the burr, in subadult stags; (iii) period on antler length, in yearling stags. Simple and interactive effects of age class and period on the number of tines/antler beam of adult stags, as well as effects of period on number of tines/antler beam of subadult and yearling stags, were assessed through generalized linear models with Poisson errors (Crawley Citation2007).
Relationships between antler characteristics (antler beam length, antler beam weight, circumference at the burr and log-transformed number of tines per beam) were analysed through linear regressions (cf. McCullough Citation1982; Sokal & Rohlf Citation1995). Somatic ratios were compared between Mesola and those derived from a dataset on an Apennine red deer population of Alpine origin (S. Mattioli unpublished) by means of Mann-Witney U-tests (Sokal & Rohlf Citation1995).
Results
Body size
We collected 136 live body weights of Mesola red deer (1980–1999; ). Yearling stags reached 46% and yearling hinds achieved 63% of the adult weight, and the maximum mass was attained only after the age of 4 years for hinds and 9 years for stags. Adult stags were on average 43% heavier than adult hinds (t = –10.115, df = 72, P < 0.001). The heaviest recorded stag was 132 kg; the heaviest hind was 100 kg. Sexual size dimorphism was not apparent for yearlings (t = –0.336, df = 18, P > 0.05) and calves (t-test: t = –1.891, df = 27, P > 0.05).
Table I. Body weights of Mesola red deer, divided by sex and age classes (data collected from 1981 to 1999) (enlarged sample compared with that in Mattioli et al. Citation2003).
Craniometry
The scanty material could only give a rough idea of skull size variability of Mesola red deer (). Scandinavian and central European red deer (mean = 388.6 mm for stags and 339.0 mm for hinds; n = 6 populations) have skulls on average about 10% larger than the Mesola ones (), the same order of magnitude found for the external somatic measures (Mattioli et al. Citation2003). Eastern European populations (mean = 417.8 mm for stags; n = 6) have on average skulls c. 20% larger than those of Mesola red deer. Mesola skulls are of about the same size as the Iberian and the Scottish ones and only slightly larger than those of the Tyrrhenian red deer Cervus elaphus corsicanus (). The mean length of the upper tooth row (106.5 mm in stags and 100.3 mm in hinds) is similar to that of other European populations (cf. Beninde Citation1937a).
Table II. Craniometry of adult Mesola red deer.
Table III. Condylobasal length (mean in mm) of different populations of red deer, in Europe.
Stature
All the measures considered were significantly greater in Apennine red deer (AD) than in Mesola red deer (MD; medians, torso length, MD stags and hinds: 186.9–167.3 cm, AD stags and hinds: 198.4–178.0 cm; height, MD: 108.6–94.4 cm, AD: 128.0–113.0; hind foot-length, MD: 48.9–45.1 cm, AD: 57.0–53.0 cm; height:torso length ratio, MD: 0.58–0.57, AD: 0.64; hind foot-length:torso length ratio, MD: 0.26–0.27, AD: 0.29–0.30; Mann-Whitney U test, females: Z = –7.333/-4.524; P < 0.001; n = 122; males: –4.112/-3.223, P ≤ 0.001, n = 106).
Antler conformation
We observed 642 sets of cranial appendages. The mean number of tines/antler pair of adult stags was 6.2 ± 1.7 (s.d., n = 392). The number of tines/antler pair was significantly greater in fully mature stags (7.0 ± 1.6, n = 183) than in young adults (5.6 ± 1.4, n = 209; B = 0.239, s.e. = 0.041, P < 0.001) and was significantly greater in Period 2 (all adults: 6.7 ± 1.6, n = 248, young adults: 6.0 ± 1.4, n = 136, mature adults: 7.6 ± 1.4, n = 112) than in Period 1 (all adults: 5.4 ± 1.5, n = 144, young adults: 4.9 ± 1.2, n = 73, mature adults: 6.1 ± 1.4, n = 71; B = 0.218, s.e. = 0.043, P < 0.001; ). The mean number of tines/antler pair was much lower in Mesola stags than in stags of other European populations of red deer ().
Figure 2. Number of tines/antler pairs in Mesola red deer stags of different age classes, in Period 1 and Period 2.
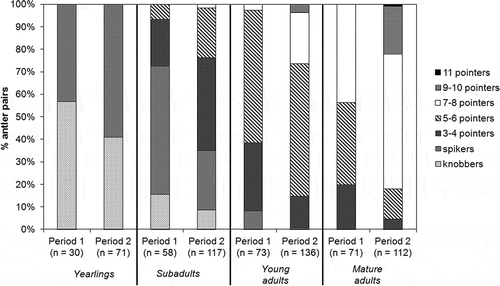
Table IV. Mean number of tines per antler pair in adult red deer of different European populations. I: Italy; UK: United Kingdom; CH: Switzerland; D: Germany; PL: Poland. Other abbreviations: L. Saxony: Lower Saxony; N. Apennines: Northern Apennines.
Antler beams with three tines prevailed (42.1% out of n = 783 antler beams; one tine: 2.2%, two tines: 22.0%, four tines: 28.9%, five tines: 4.7%, six tines: 0.1%, only one case). Only 2.2 and 3.6% of adult antler beams had a bez or a crown tine, respectively, and only 3.3% of young adults had a bez tine or a crown. Frequencies of occurrence of crown and bez tine were much lower than those observed in other European populations of red deer except for those from Corsica ().
Table V. Percentage of occurrence of crown and bez tine in adult stag antlers from some European populations. F: France; I: Italy; CH: Switzerland; D: Germany; SLO: Slovenia; PL: Poland; SK: Slovakia; RO: Romania; HR: Croatia.
Among subadults, 10.9% were antlerless, mostly with bony buttons (). Antlered subadults were mainly spikers and three- to four-pointers (). Among yearlings 45.5% were antlerless and the antlered ones were all spikers ().
Antler biometry
We measured 353 antler beams ( and ). Linear regressions among antler characteristics of adults were highly significant (r2 = 0.281–0.794, P < 0.001, n = 190–247). For adult stags, all the measures were significantly greater in fully mature adults than in young ones (B = 0.180–392.910, s.e. = 0.074–26.680, P < 0.05) and in Period 2 than in Period 1 (B = 0.264–109.370, s.e. = 0.080–29.880, P < 0.01), except the upper circumference, which did not differ significantly between periods (P > 0.05). The interaction age class × period was significant only for antler length (B = –5.181, s.e. = 2.233, P = 0.021), which increased significantly in Period 2 only for young adults (t-test: t = –3.692; df = 40.599; P < 0.001) but not for mature ones (t = –1.574; df = 60.539; P = 0.121). In adults, brow tine length was on average c. 32% of the main beam while the trez tine length was c. 18% of the beam. The bez tine was relatively short, on average c. 15% of the main beam length. The longest antler beam measured 92 cm, the heaviest 1366 g.
Table VI. Morphometric characteristics (linear measures: cm; weight: g; means ± s.d.) of antler beams of Mesola red deer, in 1981–2012.
Table VII. Antler beam length (mean) of different populations of red deer in Europe. I: Italy; E: Spain; D: Germany; PL: Poland; SLO: Slovenia; HR: Croatia.
For subadult stags, antler length and brow tine length increased significantly in Period 2 (B = 4.154–12.139, s.e. = 1.427–3.174, P < 0.006). There was a not-significant increase of circumference at the burr and of the number of tines/antler beam in Period 2 (B = 0.899–0.356, s.e. = 0.200–0.468, P = 0.060–0.075). Trez tine length did not differ significantly between periods (B = 1.333; s.e. = 1.464; P = 0.377).
For yearling stags, antler beam length was significantly greater in Period 2 than in Period 1 (B = 8.790; s.e. = 2.070; P = 0.001). The number of tines/antler beam did not differ between periods (B = 0.182; s.e. = 0.548; P = 0.739).
Antler investment
For Mesola adult stags, the average antler investment was 11.8 g of net weight/kg of body weight. Adult stags invested 73.9 g of gross antler weight (antlers plus 1.2 kg of skull)/kg of metabolic weight or 4.4 gr/kg BW1.35 (Geist Citation1987, Citation1998). The best stags, with the highest body weight and the highest antler mass, have a production of 5.5 g of gross antler mass per kg BW1.35 (2.7 kg of antlers plus skull for stags of 130 kg). By comparison with other European populations, the mean relative antler mass of Mesola red deer falls within the general pattern ().
Table VIII. Antler mass production per unit of body weight in average adult stags (cf. Geist Citation1987, Citation1998). I: Italy; UK: United Kingdom; D: Germany; PL: Poland.
Coat spotting
The totality of adult Mesola red deer had a distinct slight yellowish spotting of the summer coat, especially on the haunches. The colour patterns of the rump patch and tail are the same as those of the central European populations (cf. Dolan Citation1988; Geist Citation1998).
Discussion
Morphometrics is mostly unsuitable for differentiating genetic, epistatic, environmental and true statistical variation (cf. Geist Citation1991), but certainly can be used to give a detailed description of the physical characteristics of a population. Our data show that Mesola red deer are among the smallest red deer in Europe. Mean adult body weight is 30–35% (stags) and 15–30% (hinds) lighter than that of central European populations (Dauster Citation1940; Radler & Hattemer Citation1982; Bützler Citation1986; Wagenknecht Citation1986; von Raesfeld & Reulecke Citation1988) and 45–50% (stags) and 35–40% (hinds) lighter than that of eastern European ones (Szunyoghy Citation1963; Paller & Csányi Citation1999). Mean external linear dimensions were reduced by 8–15% compared with Swiss, German, Norwegian or Croatian populations (Buchli Citation1979; Drechsler Citation1985; Langvatn Citation1986; von Raesfeld & Reulecke Citation1988; Tucak Citation1997; Tucak et al. Citation1999). The sexual size dimorphism was low and apparent only for adults: stags have about 10% longer linear dimensions and are on average 43% heavier than females. Usually, size dimorphism is already apparent in newborn calves (Albon, Guinnes & Clutton-Brock Citation1983), and European red deer adult stags are on average 12–15% larger and 70–90% heavier than hinds (Radler & Hattemer Citation1982; Bützler Citation1986; Geist & Bayer Citation1988; von Raesfeld & Reulecke Citation1988). Dimorphism in size decreases as a consequence of the greater sensitivity of males to food limitation (Clutton-Brock et al. Citation1982; Leberg & Smith Citation1993; Ashley et al. Citation1998; LeBlanc et al. Citation2001).
Body growth rate of the Mesola red deer was slower than that of the other European populations. The ratio of yearling to adult mass was 26–29% lower than that observed in other populations (Radler & Hattemer Citation1982; von Raesfeld & Reulecke Citation1988). Young adult (i.e. 2–4 years old) hinds were on average 15 kg lighter than fully mature ones, which may contribute to explain the low birth rate (Mattioli et al. Citation2003; Ferretti & Mattioli Citation2012), since in hinds ovulation and pregnancy are related to the attainment of threshold body mass (Albon et al. Citation1983; Langvatn et al. Citation1996). The retarded body growth is also responsible for the delay in antler development of juvenile stags. Pedicles, which usually begin to grow at 8–10 months of age, in Mesola Wood did not start before 11–13 months, and sometimes later. When pedicles grew at 11–13 months, Mesola yearlings produced short spikes. When pedicles grew at 14–16 months, yearlings failed to produce true antlers and were true knobbers, with skin-covered pedicles, or “buttons” with small, bony outgrowths. When the pedicle fails to develop until 16–20 months of age, the first vestigial antlers, consisting of just two bony buttons, appear only in 2-year-old stags, whereas spikes arise in 3-year-old animals. Pedicle initiation is associated with the attainment of a threshold body weight and the growth of the first antler set is possible only when the pedicle reaches a critical length (Suttie & Kay Citation1983; Fennessy & Suttie Citation1985; Gaspar-López et al. Citation2008).
Mesola red deer fall within small-bodied red deer populations, like the Tyrrhenian Cervus e. corsicanus, the Barbary stag C. e. barbarus, the Iberian C. e. hispanicus and the Scottish populations C. e. scoticus of the Highlands. They are all clear adaptations to poorly productive habitats (i.e. Mediterranean woods and scrubs or Scottish open moorlands). Reduced sexual size dimorphism, slow body growth and delay in antler initiation of stags are typical traits of resource-restricted deer populations. Mesola red deer, together with Tyrrhenian, Iberian, North African and Scottish populations are often defined as “maintenance phenotypes” (cf. Geist Citation1987, Citation1998). It remains to be determined just to what extent in the Mesola red deer body size is genetically fixed and what instead is related to phenotypic plasticity. Habitat improvements in the Nature Reserve of Mesola Wood have caused a statistically significant slight increase in size (Mattioli et al. Citation2003; this study), which reflects a certain plasticity.
Western Red Deer are characterized by great size flexibility, as demonstrated by Scottish red deer transferred into English parks or New Zealand (Mitchell et al. Citation1977), or by the low-performance Silesian red deer subjected to a high nutrition plane in the experiments by F. Vogt in the 1920s and 1930s of the 20th century (Vogt Citation1937, Citation1947; Geist Citation1986). When reared with a high-quality diet, Iberian red deer attain the body weights of central European populations. Iberian stags, which normally weigh about 125 kg (Martı́nez et al. Citation2005), in farm can reach an average body mass of 150–200 kg (Gaspar-López et al. Citation2010).
The low stature of Mesola red deer, already observed by Perco (Citation1984) and caused by a slight leg shortening, is particularly noteworthy. The adult height at the withers is about 57–58% of the head-trunk length, against a standard of 62–64%, and the hind foot length is 26–27% of the head-trunk length against a standard of about 30% (cf. Langvatn Citation1986; Raesfeld & Reulecke Citation1988). It is not clear if this shrinking of limbs is simply a by-product of body size reduction, a consequence of genetic drift, an effect of malnutrition or an adaptive change (related for example to the locomotion needs in sandy and swampy terrain). Metapodial length tends to vary in several deer species with habitat and body condition. Populations in high-quality environments have relatively longer legs than those in poor habitats. Changes in leg proportions have been observed in a single population after only three decades of nutritional stress (Klein Citation1964; Terada et al. Citation2012; Putman & Flueck Citation2013). An evident shortening of limbs has been documented for the Tyrrhenian red deer C. e. corsicanus (Vigne & Marinval-Vigne Citation1988; Banwell Citation1998; Geist Citation1998).
The length of the upper tooth row is similar to that of other European red deer (cf. Mystkowska Citation1966), regardless of the smaller body size, as expected with a high-priority tissue like teeth, which tends to develop irrespective of environment (Geist Citation1998).
Antlers were relatively small, with a narrow spread and typically with a short trez tine. For a full array of the types of cranial appendages in Mesola Wood see Cortesi (Citation2012). Mean antler length was 2.0 times the condylo-basal length of the skull, in substantial accord with other European populations (Geist Citation1998). The average relative antler mass was not very different from that of other European red deer (), while the antler investment of the largest Mesola stags (5.5 g/kg BW1.35) was rather lower than that of the best trophy stags in free conditions (6.5–9 g/kg BW1.35) (cf. Geist Citation1998). If the sizes of antlers of Mesola red deer are simply in line with the small body size, their antler conformation is quite peculiar. The structure is oversimplified, with a low and scarcely variable number of branches, a very low frequency of bez tine and crown and a short trez tine ( and ). While the brow tine length is comparable to that observed in other red deer populations (c. 30–35% of the beam), the trez tine on average is short (18% of the main beam) with respect to other red deer populations (30–35% of the beam) (Bečejac et al. Citation1984; Mattioli Citation1996; Hafner Citation2011). The infrequent bez tine is short too (on average 15% of the main beam against a standard of 18–20%) (Mattioli Citation1996; Hafner Citation2011). The rare crown is rudimentary, three-pointed and short-tined. It can be cup-like, the classical shape in Europe (Beninde Citation1937b), or fan-like, a peculiar configuration of Mesola, with the three tines (a short dagger and a terminal fork) arising transversally and medially from different points of the main beam. This last crown type resembles the upper parts of wapitoid antlers (Geist Citation1998).
Scottish adult stags may reach a maximum of 14–16 tines per antler pair (Mitchell et al. Citation1986; V.P.W. Lowe 1986, pers. comm. to S.M.) and Sardinian stags 12 tines (Beccu Citation1989; Mattioli pers. obs.). The highest number of antler tines recorded in Mesola Wood over 30 years was 11 (only once). It is remarkable that from 1957 to 1996, bez tine and crown, typical traits of the European-type Cervus elaphus taxa, were totally absent and that for all the first part of the 20th century they both were only very rarely recorded (Mattioli Citation1993). Habitat enhancement measures carried out in the Reserve led to a significant improvement of antlers: the mean number of tines increased by 30%, the frequency of eight-point antlers grew from 9.0 to 24.6%, (), and bez tine and crown began to appear, mostly in fully mature stags. Antlers of young adults were 14.0% longer and 13.7% heavier in Period 2 than in Period 1, whereas those of mature stags showed a 20.8% increase in weight and only a not-significant 3.0% increase in main beam length. These results suggest that, when environmental conditions improved, the investment of mature and young adults favoured different antler characteristics. While the latter allocated energy to increase the main beam length, the weight and the branching of antlers, the former particularly favoured the rearrangement of the antler bone structure and the complexity of their cranial appendages. Thus, in antler growth, the main beam length seems to have priority, followed by the weight (through the increase of the more compact cortical bone at expense of spongiosa) and then by the development of tines. Also, Tyrrhenian red deer have small antlers, with a relatively simple antler design and a peculiar tendency to slight palmation in fully mature stags (Caboni et al. Citation2006).
The slight spotting of the summer coat is a common trait of all Mesola red deer. A speckled coat is very frequent in the Caucasian and Iranian C. e. maral and in yearlings and subadults of the North African C. e. barbarus (Meyer Citation1972; Dolan Citation1988) but is generally absent in C. e. corsicanus (Mattioli pers. obs.). Some spotting was observed in some Tyrrhenian red deer by Banwell (Citation1998). Spotted individuals can sometimes be present in European red deer populations but their frequency is normally low. In Hohenbucko, a research area in eastern Germany, spotting occurred in about 10% of animals (Wagenknecht Citation1986).
Our morphometric study, together with a genetic one, could also help to understand the taxonomic status of this population (Zachos et al. Citation2014), already considered a potential independent conservation unit (Lovari & Nobili Citation2010). Their morphological characteristics, coupled with genetic peculiarities (Lorenzini et al. Citation2005; Hmwe et al. Citation2006; Zachos et al. in press) make the Mesola red deer unique, deserving special protection (Lovari & Nobili Citation2010; Zachos & Hartl Citation2011; Ferretti & Mattioli Citation2012).
Acknowledgements
We wish to thank G. Nobili of the Corpo Forestale dello Stato, Chief of the Ufficio Territoriale per la Biodiversità of Punta Marina, Ravenna, who made our study easier in every way. The personnel of Posto Fisso of Bosco Mesola provided us assistance, logistical support and information on red deer in Bosco della Mesola Nature Reserve. A. Benassi helped us by collecting in the last few years several cast antlers. We thank S. Lovari, who stimulated us to carry out this work. We would like to acknowledge F. Perco who directed the first capture session in 1980–1982, and R. Fico who supervised the veterinarian aspects of the second session in 1993–1999. R. Dziedzic, M. Hafner and D. Zalewski kindly made available unpublished data, and E. Roskaft and G. Markov their precious theses. We are grateful to M. Festa-Bianchet and T. Landete-Castillejos for their opinions and suggestions on some parts of our work. V. Geist, R. Gill and F. Zachos critically read a previous draft of the manuscript. We are grateful to two anonymous referees, for their comments and suggestions to an earlier version of our manuscript.
References
- Ahlén I. 1965. Studies on the red deer Cervus elaphus L., in Scandinavia. II Taxonomy and osteology of prehistoric and recent populations. Viltrevy 3:89–176.
- Albon SD, Guinness FE, Clutton-Brock TH. 1983. The influence of climatic variation on the birth weights of red deer (Cervus elaphus). Journal of Zoology 200:295–298. doi:10.1111/j.1469-7998.1983.tb05793.x.
- Albon SD, Mitchell B, Staines BW. 1983. Fertility and body weight in female red deer: a density dependent relationship. The Journal of Animal Ecology 52:969–980. doi:10.2307/4467.
- Anderson AE, Medin DE, Bowden DC. 1974. Growth and morphometry of the carcass, selected bones, organs, and glands of mule deer. Wildlife Monographs 39:1–122.
- Ashley EP, McCullough GB, Robinson JT. 1998. Morphological responses of white-tailed deer to a severe population reduction. Canadian Journal of Zoology 76:1–5. doi:10.1139/z97-159.
- Azorit C, Analla M, Carrasco RE, Monoz-Cobo J. 2002. Influence of age and environment on antler traits in Spanish red deer (Cervus elaphus hispanicus). Zeitschrift für Jagdwissenschaft 48:137–144.
- Bališ M. 1959. Predbežná správa o štúdiu kraniologického materiálu jeleňa hôrneho (Cervus elaphus L.) z Tatranského národného parku. Sborník prác o Tatranskom národnom parku 3:164–173.
- Banwell B. 1998. Description of Cervus elaphus corsicanus. Deer Specialist Group Newsletter 14:9.
- Beccu E. 1989. Il cervo sardo. Sassari: Delfino Editore.
- Bečejac B, Brna J, Mikuška J, Valter J. 1984. Veličina rogovlja jelena obinog (Cervus elaphus L., 1758) na području Baranje i sjeverozapadne Bačke s obzirom na starosnu dob [Size of red deer antlers in the area of Baranja and northwestern Bačka according to age]. RAD Jugoslavenske akademije znanosti i umjetnosti. Razred za prirodne znanosti 20:121–149.
- Beninde J. 1937a. Zur Naturgeschichte des Rothirsches. Monographie der Wildsäugetiere. Vol. 4. Leipizg, Germany: P. Schoeps. 223 pp.
- Beninde J. 1937b. Zur Vererblichkeit der Kronenform beim Rothirsch. Zeitschrift für Forst- und Jagdwirtschaft 69:201–213.
- Buchli C. 1979. Zur Populationsdynamik, Kondition und Konstitution des Rothirsches im und um den Schweizerischen Nationalpark. Dissertation. Zürich, Switzerland: University of Zürich.
- Buchli C. 1992. Auswertung der Hirschstrecken 1991: Grundlagen zur Jagdplanung Vorschläge zu Eingriffmöglichkeiten. Zernez, Switzerland: Mimeo, Zernez.
- Bützler W. 1986. Cervus elaphus – Rothirsch. In: Niethammer J, Krapp F, editors. Handbuch der Säugetiere Europas. Band 2/II Paarhufer. Wiesbaden, Germany: AULA. pp. 107–139.
- Caboni A, Murgia C, Mattioli S. 2006. Antler characteristics of the Sardinian red deer (Cervus elaphus corsicanus): a preliminary analysis. In: Bartoš L, Dušek A, Kotrba R, Bartošová-Víchová J, editors. Advances in deer biology. Deer in a changing world. Proceeding of the 6th Deer Biology Congress, 7–11 August 2006, Prague: 266.
- Castelli G. 1941. Il cervo europeo. Firenze, Italy: Olimpia.
- Clutton-Brock TH, Guinness FE, Albon SA. 1982. Red deer: behaviour and ecology of two sexes. Chicago, USA: The University of Chicago Press.
- Cortesi P. 2012. I cervi delle nebbie. [Deer of the mist]. Argelato (Bologna): Minerva Edizioni.
- Crawley M. 2007. The R Book. Chichester, UK: J. Wiley and Sons.
- Danilkin AA. 1999. Oleny (Cervidae). Moskva, Russia: GEOS.
- Dauster KH. 1940. Das Wildbretgewicht nordwestdeutscher Rotwildstämme. Zeitschrift für Jagdkunde 2:91–111.
- Dolan JM. 1988. A deer of many lands - a guide to the subspecies of the red deer Cervus elaphus. Zoonooz 62:4–34.
- Drechsler H. 1980. Ueber di Geweihbildung bei Rothirschen im ‘Rotwildring Harz’ in den Jahren 1959–1978. Zeitschrift zur Jagdwissenschaft 26:207–219.
- Drechsler H. 1985. Ueber das körperliche Wachstum beim Rotwild. Niedersächsischer Jäger 6:305–306.
- Drechsler H. 1988. Altersentwicklung und Altersansprache beim Rotwild. Hamburg and Berlin, Germany: P. Parey.
- Dzięciołowski R, Babinska-Werka J, Wasilewski M, Goszczynski J. 1996. Physical condition of red deer in a high density population. Acta Theriologica 41:93–105.
- Dziedzic R, Flis M, Beeger S, Wojcik M. 1999. Occurrence and types of crowns in red deer males in middle-eastern Poland. Unpublished report, Lublin:1–10.
- Feldhamer GA, Stauffer JR, Chapman JA. 1984. Body morphology and weight relationships of sika deer in Maryland. Zeitschrift für Säugetierkunde 50:88–106.
- Fennessy PF, Suttie JM. 1985. Antler growth: nutritional and endocrine factors. In: Drew PF, Drew KR, editors. Biology of Deer Production. Wellington, New Zealand: Royal Society of New Zealand. pp. 239–250.
- Ferretti F, Mattioli S. 2012. The Mesola red deer: present numbers and conservation perspectives. Hystrix 23:35–43.
- Gaspar-López E, García AJ, Landete-Castillejos T, Carrión D, Estevez JA, Gallego L. 2008. Growth of the first antler in Iberian red deer (Cervus elaphus hispanicus). European Journal of Wildlife Research 54:1–5. doi:10.1007/s10344-007-0096-0.
- Gaspar-López E, Landete-Castillejos T, Estevez JA, Ceacero F, Gallego L, García AJ. 2010. Biometrics, testosterone, cortisol and antler growth cycle in Iberian red deer stags (Cervus elaphus hispanicus). Reproduction in Domestic Animals 45:243–249. doi:10.1111/j.1439-0531.2008.01271.x.
- Geist V. 1986. Super antler and Pre-World War II European research. Wildlife Society Bulletin 14:91–94.
- Geist V. 1987. On the evolution of optical signals in deer. A preliminary analysis. In: Wemmer CM, editor. Biology and management of Cervidae. Washington, USA: Smithsonian Institution Press. pp. 235–255.
- Geist V. 1991. Phantom subspecies: the wood bison Bison bison “athabascae” Rhoads 1897 is not a valid taxon, but an ecotype. Arctic 44:283–300.
- Geist V. 1998. Deer of the world: their evolution, behavior, and ecology. Mechanicsburg, USA: Stackpole Books.
- Geist V, Bayer M. 1988. Sexual dimorphism in the Cervidae and its relation to habitat. Journal of Zoology 214:45–53. doi:10.1111/j.1469-7998.1988.tb04985.x.
- Hafner M. 2008. Jelejad. Ljubljana, Slovenia: Lovska zveza Slovenije.
- Hafner M. 2011. Morfološke značilnosti rogovja navadnega jelena v vzhodnih Karavankah in Kamniško-Savinjskih Alpah [Morphological characteristics of antlers in the red deer population of eastern Karavanke and Kamnik Savinja Alps). Lovec 94:71–74.
- Hartl G, Lang G, Klein F, Willing R. 1991. Relationships between allozymes, heterozygosity and morphological characters in red deer (Cervus elaphus), and the influence of selective hunting on allele frequency distributions. Heredity 66:343–350. doi:10.1038/hdy.1991.43.
- Hell P, Herz S. 1972. Príspevok ku kraniometrickému štúdiu jeleňa obyčajného (Cervus elaphus L.) zo západnej polovice Slovenska [Contribution to the craniometric study of red deer in western Slowakia). Lynx 13:15–25.
- Hmwe SS, Zachos FE, Eckert I, Lorenzini R, Fico R, Hartl GB. 2006. Conservation genetics of the endangered red deer from Sardinia and Mesola with further remarks on the phylogeography of Cervus elaphus corsicanus. Biological Journal of the Linnean Society 88:691–701. doi:10.1111/j.1095-8312.2006.00653.x.
- Ingebrigsten O. 1924. Das norwegische Rotwild (Cervus elaphus L.): eine kraniometrische Untersuchung. Bergens Mus. Arbook 1922–23. Naturvidensk 7:1–258.
- Isakovic I. 1969. Morphologija jelenjih parogova Belja [Morphology of red deer antlers in Belje). Jelen 8:5–59.
- Klein DR. 1964. Range-related differences in growth of deer reflected in skeletal ratios. Journal of Mammalogy 45:226–235. doi:10.2307/1376985.
- Klein F. 1987. La gestion du cerf dans le secteur de La Petite Pierre (Vosges du Nord). Ciconia 11:97–108.
- Langvatn R. 1977. Criteria of physical condition, growth and development in Cervidae, suitable for routine studies. Stockholm: Nordic Council for Wildlife Research. pp. 1–27.
- Langvatn R. 1986. Size and weight relationships in Norwegian red deer. In: Linn S, editor. Das Rotwild. Austria: Proc. C.I.C. Symp. Graz. pp. 244–266.
- Langvatn R, Albon SD, Burkey T, Clutton-Brock TH. 1996. Climate, plant phenology and variation in age of first reproduction in a temperate herbivore. The Journal of Animal Ecology 65:653–670. doi:10.2307/5744.
- Leberg PL, Smith MH. 1993. Influence of density on growth of white-tailed deer. Journal of Mammalogy 74:723–371. doi:10.2307/1382294.
- LeBlanc M, Festa-Bianchet M, Jorgenson JT. 2001. Sexual size dimorphism in bighorn sheep (Ovis canadensis): effects of population density. Canadian Journal of Zoology 79:1661–1670. doi:10.1139/cjz-79-9-1661.
- Lorenzini R, Fico R, Mattioli S. 2005. Mitochondrial DNA evidence for a genetic distinction of the native red deer of Mesola, northern Italy, from the Alpine populations and the Sardinian subspecies. Mammalian Biology - Zeitschrift für Säugetierkunde 70:187–198. doi:10.1016/j.mambio.2004.11.018.
- Lorenzini R, Mattioli S, Fico R. 1998. Allozyme variation in native red deer Cervus elaphus of Mesola Wood northern Italy: implications for conservation. In: Hartl GB, Markowski J, editors. Ecological Genetics in Mammals. Acta Theriologica Suppl. 5:63–74.
- Lovari S, Nobili G. 2010. Programma nazionale di conservazione del cervo della Mesola. Quad. Cons. Natura, n. 36. Roma: Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Ministero delle Politiche Agricole Alimentari e Forestali - Corpo Forestale dello Stato, I.S.P.R.A. [in Italian with an English summary].
- Lowe VPW, Gardiner AS. 1974. A re-examination of the subspecies of Red deer (Cervus elaphus) with particular reference to the stocks in Britain. Journal of Zoology 174:185–201. doi:10.1111/j.1469-7998.1974.tb03151.x.
- Markov G. 1998. The population variability, morphological and genetic differentiation and systematics of representative species of family Cervidae in Palearctic. PhD thesis. Sofia, Bulgaria: Institute of Zoology, Bulgarian Academy of Sciences.
- Martı́nez M, Rodrı́guez-Vigal C, Jones OR, Coulson T, San Miguel A. 2005. Different hunting strategies select for different weights in red deer. Biology Letters 1:353–356. doi:10.1098/rsbl.2005.0330.
- Mattioli S. 1990. Red deer in the Italian peninsula, with particular reference to the Po delta population. Deer 8:95–98.
- Mattioli S. 1993. Antler conformation in red deer of the Mesola Wood, northern Italy. Acta Theriologica 38:443–450.
- Mattioli S. 1996. Morfometria dei palchi. In: Mazzarone V, Mattioli S, editors. Indagine sulla popolazione di cervo dell’Acquerino: relazione finale 1993–1995. Firenze, Italy: Regione Toscana. pp. 13–20.
- Mattioli S. 2011. Family Cervidae (Deer). In: Wilson D, Mittermeier RA, editors. Handbook of the Mammals of the World. 2nd volume. Hoofed Mammals. Barcelona: Lynx Edicions. pp. 350–443.
- Mattioli S, De Marinis AM. 2009. Guida al rilevamento biometrico degli Ungulati. Documenti Tecnici ISPRA 28:1–216.
- Mattioli S, Fico R, Lorenzini R, Nobili G. 2003. Mesola red deer: physical characteristics, population dynamics and conservation perspectives. Hystrix 14:87–94.
- Mattioli S, Meneguz PG, Brugnoli A, Nicoloso S. 2001. Red deer in Italy: recent changes in distribution and numbers. Hystrix 12:27–35.
- McCullough DR. 1982. Antler characteristics of George Reserve white-tailed deer. The Journal of Wildlife Management 46:821–826. doi:10.2307/3808583.
- Meyer P. 1972. Zur Biologie und Ökologie des Atlashirsches Cervus elaphus barbarus, 1833. Zeitschrift für Säugetierkunde 37:101–116.
- Mitchell B, McCowan D, Parish T. 1986. Performance and population dynamics in relation to management of red deer Cervus elaphus at Glenfeshie, inverness-shire, Scotland. Biological Conservation 37:237–267. doi:10.1016/0006-3207(86)90084-4.
- Mitchell B, Staines BW, Welch D. 1977. Ecology of red deer: a research review relevant to their management in Scotland. Banchory, Scotland: Institute of Terrestrial Ecology. pp. 1–74.
- Moyes K, Nussey DH, Clements MN, Guinness FE, Morris A, Morris S, Pemberton J, Kruuk LEB, Clutton-Brock TH. 2011. Advancing breeding phenology in response to environmental change in a wild red deer population. Global Change Biology 17:2455–2469. doi:10.1111/j.1365-2486.2010.02382.x.
- Mysterud A, Meisingset E, Langvatn R, Yoccoz NG, Stenseth NC. 2005. Climate-dependent allocation of resources to secondary sexual traits in red deer. Oikos 111:245–252. doi:10.1111/j.0030-1299.2005.14197.x.
- Mysterud A, Yoccoz NG, Stenseth NC, Langvatn R. 2001. Effects of age, sex and density on body weight of Norwegian red deer: evidence of density-dependent senescence. Proceedings of the Royal Society B: Biological Sciences 268:911–919. doi:10.1098/rspb.2001.1585.
- Mystkowska ET. 1966. Morphological variability of the skull and body weight of the red deer. Acta theriologica 11:129–194.
- Neumann A. 1968. Rotwildpopulation Hohenbucko. Beiträge zur Jagd- und Wildforschung 6:93–101.
- Paller A, Csányi S. 1999. A Lábodi gímszarvasbikák kondícióiváltozása a bőgési időszakban 1996–1997 [Condition changes of red deer stags during the rut at Lábod, 1996–1997). Vadbiológia 6:73–80.
- Perco F. 1984. Ricerca sui Cervidi. In: Minerbi B, editor. Riserva Naturale Gran Bosco della Mesola: piano di gestione faunistica per il decennio 1980–1989. Roma: Ministero dell’Agricoltura e Foreste. pp. 107–165.
- Putman R, Flueck WT. 2013. Intraspecific variation in biology and ecology of deer: magnitude and causation. Animal Production Science 51:277–291. doi:10.1071/AN10168.
- Radler K, Hattemer HH. 1982. Unterschiede im Körpergewicht des Rotwildes aus verschiedenen Gebieten der Bundesrepublik Deutschland. Zeitschrift für Jagdwissenschaft 28:79–88.
- Raesfeld Fvon, Reulecke K. 1988. Das Rotwild. Hamburg and Berlin, Germany: P. Parey.
- Røskaft E. 1978. Kraniometriske målinger hos hjort Cervus elaphus L. – en vurdering av vekst- og størreiesrelasjoner. [Craniometry of red deer: an assessment of growth and size relationships]. Thesis. Trondheim, Norway: University of Trondheim.
- Salvioni M. 1999. Analisi della morfologia di tre specie di Ungulati in Ticino. Bellinzona, Switzerland: Ufficio Caccia e Pesca Cantonale.
- Sokal RR, Rohlf FJ. 1995. Biometry. New York, USA: Freeman and Company.
- Suttie JM, Kay RNB. 1983. The influence of nutrition and photoperiod on the growth of antlers of young red deer. In: Brown RD, editor. Antler development in Cervidae. Kingsville, USA: Caesar Kleberg Wildlife Research Institute. pp. 61–71.
- Szunyoghy J. 1963. A magiarországi szarvas. Das ungarische Rotwild. Budapest: Hungarian Natural History Museum. pp. 1–193.
- Terada C, Tatsuzawa S, Saitoh T. 2012. Ecological correlates and determinants in the geographical variation of deer morphology. Oecologia 169:981–994. doi:10.1007/s00442-012-2270-7.
- Torres-Porras J, Carranza J, Pérez-González J. 2009. Combined effects of drought and density on body and antler size of male iberian red deer Cervus elaphus hispanicus: Climate change implications. Wildlife Biology 15:213–221. doi:10.2981/08-059.
- Toschi A. 1965. Mammalia: Lagomorpha, Rodentia, Carnivora, Ungulata, Cetacea. Fauna d’Italia VII. Bologna, Italy: Calderini.
- Trense W, de Boislambert AJH, Whitehead GK. 1981. Die Jagdtrophaen der Welt; Les trophées de chasse du monde; The game-trophies of the world. Germany: Hamburg and Berlin: P. Parey.
- Tucak Z. 1997. Morphometrische Eigenschaften der Rothirsche (Cervus elaphus L.) aus dem Donaugebiet in Baranja. Zeitschrift für Jagdwissenschaft 43:141–153.
- Tucak Z, Banaj B, Šubaric D. 1999. Beitrag zur Morphometrie des Rotwildes (Cervus elaphus Linné 1758) aus dem Donaugebiet Bačka. Zeitschrift für Jagdwissenschaft 45:127–133.
- Vigne JD, Marinval-Vigne MC. 1988. Contribution a la connaisance du cerf de Corse (Cervus elaphus, Artiodactyla, Mammalia) et de son histoire. Bulletin d’Ecologie 19:177–187.
- Vogt F. 1937. Neue Wege der Hege. Berlin, Germany: Neumann-Neudamm.
- Vogt F. 1947. Das Rotwild. Wien, Austria: Oesterreiches Jagd- Fischereiverlag.
- von den Driesch A. 1976. A guide to the measurement of animal bones from archeological sites. Peabody Museum Bulletin 1:1–137.
- Wagenknecht E, editor. 1984. Altersbestimmung des erlegten Wildes. Melsungen, Germany: Neumann-Neudamm.
- Wagenknecht E. 1986. Rotwild. Melsungen, Germany: Neumann-Neudamm.
- Wierzbowska I. 1999. Zmienność wymiarów czaszek populacji jelenia szlachetnego (Cervus elaphus L) w Karpatach [Size variation in skulls of red deer populations of Polish Carpathians]. Ph. D. Thesis. Kraków, Poland: University of Kraków.
- Zachos FE, Hajji GM, Hmwe SS, Hartl GB, Lorenzini R, Mattioli S. 2009. Population viability analysis and genetic diversity of the endangered red deer Cervus elaphus population from Mesola, Italy. Wildlife Biology 15:175–186. doi:10.2981/07-075.
- Zachos FE, Hartl GB. 2011. Phylogeography, population genetics and conservation of the European red deer Cervus elaphus. Mammal Review 41:138–150. doi:10.1111/j.1365-2907.2010.00177.x.
- Zachos FE, Mattioli S, Ferretti F, Lorenzini R. 2014. The unique Mesola red deer of Italy: taxonomic recognition (Cervus elaphus italicus nova ssp., Cervidae) would endorse conservation. Italian Journal of Zoology 81:136–143.