Abstract
Social insects can live in densely populated colonies where mortality risks are increased by inter-individual transmission of pathogens. Thus, diverse strategies are employed against such infection risks, including the display of sophisticated behavioural traits. Considering that the waste of the leaf-cutting ant contains pathogens, worker ants that tend the fungus garden – here called fungus garden workers – should exhibit avoidance behaviour toward midden workers to minimize contamination of the fungus garden. We studied the behaviour of garden workers when confronted with midden and forager workers in colonies of Atta sexdens (Linnaeus, 1758). Eight colonies were used: in four colonies, the midden was inoculated with spores of the weed fungus Escovopsis weberi (Muchovej and Della Lucia, 1990), and in the other four colonies there was no artificial contamination. Grooming, self-grooming, inspection, immobilization and aggression behaviours were quantified. Additionally, we checked for fungal contaminants on the body surfaces of midden and garden workers from non-inoculated colonies. Garden workers displayed more intense behaviours (grooming, inspection and immobilization) toward midden workers than toward foragers; these behaviours did not differ between Escovopsis-inoculated and non-inoculated colonies. No antagonist behaviour was displayed by garden workers toward midden or forager workers independently of the inoculation treatment. Eight fungus species were isolated from the midden and garden workers, including Trichoderma sp., a probable antagonist of the fungus garden. Garden workers of A. sexdens discriminate against midden workers; however, a previous hypothesis that pathogens from the midden induce the aggressiveness of internal workers is not supported by our study.
Introduction
Most tasks performed by leaf-cutting ant workers are related to the hygiene of the colony. Midden workers deal with hazardous material including exhausted fungus garden, cadavers and dry leaves. This material is dangerous to the colony because it can shelter fungal contaminants such as Escovopsis spp. (Bot et al. Citation2001) and Trichoderma viride Pers ex Gray, 1794 (Lacerda et al. Citation2006), a parasite and an antagonist of the symbiotic fungus garden, respectively. A list of parasites and antagonists of the symbiotic fungus garden has been isolated from the midden of the leaf-cutting ants. Fisher et al. (Citation1996) isolated several fungi exclusively from refuse piles of Atta cephalotes (Linnaeus, 1758). These species include ubiquitous fungi, such as Aureobasidium pullulans (de Bari) Arnaud, 1918 that can be found in different environments, Trichoderma harzianum Rifai, 1969 which can parasitize other fungi, and Escovopsis weberi, a parasite of the symbiotic fungus of the leaf-cutting ants. Entomopathogenic fungi have been found in the colony midden, including Aspergillus flavus Link ex Gray, 1821 (Hughes et al. Citation2004; Rodrigues et al. Citation2005; Lacerda et al. Citation2006) and Metarhizium anisopliae (Metsch.) Sorokin, 1883 (Hughes et al. Citation2004; Rodrigues et al. Citation2005). It has been demonstrated that workers in contact with the refuse material of the colony die faster than those who are in a cleaner environment (Bot et al. Citation2001; Lacerda et al. Citation2010). Many of these species are hazardous to the leaf-cutting ants, but the role of most of them remains unknown.
Parasite avoidance can influence social behaviour (Moore Citation2002), and such behaviours have been observed in several social insects (Schmid-Hempel Citation1998). In the leaf-cutting ant Atta cephalotes, aggressive behaviour of garden workers toward midden workers has been reported (Hart & Ratnieks Citation2001). In this species, the workers apparently occupy two spatially separated environments in the colony: midden workers in the refuse pile and all other ants in the remainder of the nest. When midden workers tried to enter the fungus garden chamber, they were aggressively rejected by garden workers. In this case, aggressiveness would be an avoidance behaviour focused on the pathogens present on the midden workers (Hart & Ratnieks Citation2001). This finding contrasts with the observation that A. cephalotes workers did not detect the fungal agents M. anisopliae and T. viride contained in baits of wheat bran and orange juice. Furthermore, no detectable defensive behaviour was verified (Lopez & Orduz Citation2003), although the authors noted that the attractant (orange juice) might have prevented the ants from detecting the pathogens. Given these findings, more studies are needed to determine the conditions under which defensive behaviour occurs and against which pathogens it is directed in leaf-cutting ants.
Atta sexdens is a widespread species in Brazil. This species is economically important because it causes damage to agriculture and forestry resources. Its workers, like those of A. cephalotes, arrange their waste material in the interior of isolated underground chambers. Moreover, both species exhibit partitioned labour during waste transport (Hart & Ratnieks Citation2001; Lacerda et al. Citation2006), i.e., a waste item is frequently placed on a small deposit by the transporter workers, then taken to the main waste pile by the midden workers.
In the present work, we aimed to examine the occurrence of “grooming” behaviours (cleaning/licking), “self-grooming” (self-cleaning/self-licking), inspection and immobilization displayed by fungus garden workers toward midden and forager workers – here both are characterized as external task groups – when the latter are placed in the fungus garden. We also investigated the potential for aggressive behaviour of garden workers toward midden workers, as was observed in A. cephalotes (Hart & Ratnieks Citation2001) and Acromyrmex lobicornis (Emery, 1888) (Ballari et al. Citation2007). To test the relationship between the presence of pathogens and the level of aggressiveness, we isolated and identified fungal contaminant species on the body surfaces of midden and garden workers.
Materials and methods
Behaviour of fungus garden workers toward forager and midden workers in Escovopsis-inoculated and non-inoculated colonies
We used four colonies of A. sexdens (C1, C2, C3 and C4) kept at 25°C and 75% relative humidity with a 12-hour photoperiod. Each of these colonies had approximately 6 L of fungus garden which was maintained in a vial connected to a foraging arena.
For each test, five midden and five forager ants were alternately placed individually on the fungus garden. So, a forager was taken from the foraging arena and placed on the fungus garden, and its interactions with the fungus garden workers were observed for a period of 2 minutes. Subsequently, this forager worker was removed from the fungus garden and replaced by a midden worker that was observed in the same way. The following behaviours were observed: inspection by fungus garden workers, grooming, self-grooming, aggression and immobilization of the introduced worker. A total of 150 midden and 150 forager workers was observed in each colony. This methodology was modified from Hart and Ratnieks (Citation2001).
The number of workers displaying each social interaction (inspection, grooming and aggression) was quantified by observing the midden and forager workers in each colony. This value was divided by the total number of tests performed to obtain the mean occurrence rate of each interaction. Regarding self-grooming and immobilization behaviours, the sum of the number of times that each behaviour took place was divided by the total number of tests.
The above studies on the behaviour of garden workers in relation to midden and forager workers were also conducted in colonies in which the midden was inoculated with spores from the mycopathogen Escovopsis weberi isolated from A. sexdens colonies. In this experiment, four additional A. sexdens colonies, conditioned as the previous ones and with a similar fungus garden volume, were used. The waste of each colony was weighed and an average mass of 22.24 g was obtained. Then, each 22.24 g of waste was inoculated with 2.0 mL of a suspension in distilled water containing 107 spores/mL; the control colonies only received distilled water. We used a large number of spores to ensure the establishment of an infection and to increase the likelihood of Escovopsis detection and a subsequent response by the ants, as was done in another study conducted by Gerstner et al. (Citation2011). The inoculated colonies were named as follows: colonies C5, C6, C7 and C8. The tests as well as the types of behaviour evaluated were identical to those of the first experiment, utilizing 50 midden workers and 50 foragers in each colony.
This latter experiment was conducted 1 year after the testing of the non-inoculated colonies. In this way, the variable “year” was inserted into the statistical model to assess whether worker behaviour was influenced in some way. So, Escovopsis-inoculated and non-inoculated colonies were statistically analysed in the same model, using a variance analysis nested with fixed factors represented by the variables “year” (levels: Escovopsis-inoculated and non-inoculated) and “workers” (levels: forage and midden workers), and a random factor, being the variable “worker” when each level was nested inside the variable “colony”, this last one with eight levels, each level representing each one of the tested colonies.
Isolation and identification of the microorganisms present on the bodies of midden and fungus garden workers
Isolation procedures were performed to determine the fungal contaminants present on the cuticles of midden and fungus garden workers in colonies C1 to C4. Fungus isolation was conducted only on the non-inoculated colonies, as the aim of this procedure was to investigate whether the aggression level of the garden workers toward the midden workers could be associated with the absence/presence of contamination from refuse.
Thus, 0.1 g of midden and garden workers from each colony were separately placed into tubes containing 2.5 mL of 0.85% sterile saline solution. After vortex stirring, 0.1 mL of the suspension was spread with a Drigalski spatula in a Petri dish containing Potato Dextrose Agar (PDA) supplemented with Rose Bengal and streptomycin 10 mg/mL. We used four Petri dishes for each worker group (midden and garden) in each colony. Petri dishes were then kept in a chamber at 25°C until fungal colony growth zones formed. This procedure was adapted from that proposed by Alexander (Citation1977). These isolation procedures were repeated three times at intervals of 15 days.
After obtaining pure cultures, the cultures were subjected to microscopic analysis to identify the fungi with the aid of taxonomic keys (Alves Citation1998) and then classify them to the generic or species level.
Results
Behaviour of the fungus garden workers toward forager and midden workers
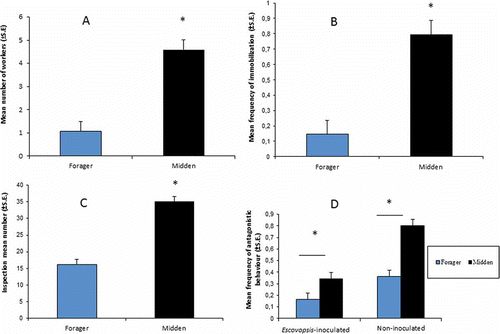
A large number of fungus garden workers performed more grooming of midden than of forager workers (F1,6 = 34.83, P < 0.01; A). However, midden inoculation with E. weberi did not alter grooming behaviour (F1,6 = 0.02, P = 0.91), i.e., this behaviour did not differ between Escovopsis-inoculated and non-inoculated colonies. Thus, this variable was not considered.
Figure 1. (A) Mean number of fungus garden workers that performed grooming on introduced forager and midden workers. (B) Frequency at which forager and midden workers were immobilized by fungus garden workers. (C) Mean number of garden workers that inspected foragers and midden workers. (D) Mean numbers of forager and midden workers antagonized by garden workers in colonies where the waste was Escovopsis-inoculated and where it was not. S.E., standard error.
There was no significant difference in the self-grooming behaviour of fungus garden workers when a midden vs. a forager worker (F1,6 = 0.16, P = 0.71) was placed on the fungus garden. Additionally, there was no significant difference in this behaviour between the year in which there was Escovopsis-inoculation of the refuse pile and the year in which the refuse was not inoculated (F1,6 = 2.93, P = 0.14).
Regarding worker immobilization, midden workers were significantly more immobilized by the fungus garden workers than foragers were (F1,6 = 24.82, P = 0.002; B). This behaviour did not differ based on whether the colony’s midden was inoculated with E. weberi (F1,6 = 0.18, P = 0.68). Fungus garden workers inspected the midden workers significantly more than the foragers (F1,6 = 66.52; P < 0.001; C). Again, this behaviour was not affected by midden inoculation (F1,6 = 0.0007, P = 0.98).
Midden workers of A. sexdens, in contrast with those of A. cephalotes (Hart & Ratnieks Citation2001), were not attacked or expelled by their nestmates in the fungus garden. Instead, we observed that the inserted worker sometimes received a bite from the garden worker or had one or more legs imprisoned in the garden worker’s mandibles for few seconds. However, after a few seconds, the midden worker was released and it could move about the fungus garden without obstruction. However, midden workers were more frequently attacked by garden workers than were the foragers (F1,6 = 14.91, P < 0.01; D). The effect of midden inoculation with E. weberi on this behaviour was the reverse of what had been expected, as midden and forager workers were less often attacked in inoculated than in non-inoculated colonies (F1,6 = 6.65, P = 0.04).
Isolation of microorganisms present on the bodies of midden and fungus garden workers
Fungus taxa isolated from the body surfaces of midden and fungus garden workers and their respective colonies are listed in . Eight fungus species were isolated including Trichoderma sp., which is recognized as antagonistic of the symbiotic fungus of leaf-cutting ants (Ortiz & Orduz Citation2000; Currie & Stuart Citation2001; Silva et al. Citation2006).
Table I. Fungus species found on the body surfaces of midden and forager workers from different colonies.
Discussion
In one way, our behavioural observations support the hypothesis that midden workers are recognized as a risk to the colony, as they received significantly more grooming from the fungus garden workers compared to the foragers. These results were expected, as the probability of contamination from the colony midden is greater. However, the fungus survey revealed that midden and fungus garden workers harboured the same contaminants. Some of these fungus species, like A. niger, A. melleus, F. solani and P. expansum, have been found parasitizing other insects, such as the beetle Melolontha melolontha (Linnaeus, 1758) the pine beetle Dendroctonus frontalis Zimmermann, 1868, Nomia bees or larvae of the beetle Tenebrio molitor Linnaeus, 1758 (Domsch et al. Citation1980). C. cladosporioides is a saprophyte that can associate with live plants such as the coffee tree (Pereira et al. Citation2005), and R. stolonifer and Pestalotiopsis sp. are phytopathogens (Cardoso et al. Citation2002; Hernández-Lauzardo et al. Citation2006). Trichoderma sp. is known for its antagonistic activity against the attine fungus garden (Ortiz & Orduz Citation2000), and it was found on the body surfaces of both midden and fungus garden workers from different colonies. Despite this, the authors verified that the presence of this fungus was not associated with an increase in grooming. Usually, grooming behaviour is performed in the fungus garden for the physical removal of contaminants such as spores of other fungi species that are present on leaves that are carried into the nest (Quinlan & Cherrett Citation1977; Bass & Cherrett Citation1994).
Self-grooming behaviour was performed constantly by the garden workers, and no significant difference was observed between introductions of midden and forager workers into the fungus garden, even across the two years – the first without and the second with Escovopsis inoculation of the midden. Such hygienic behaviour is important to prevent parasite transmission in ant societies. For example, Acromyrmex echinatior (Forel, 1899) workers exposed to M. anisopliae fungus exhibited an increase in survival when they were maintained together with their nestmates (Hughes et al. Citation2002). It was assumed that grooming and the antibiotic secretions of the metapleural gland were important to provide workers with immune resistance against this parasite. The addition of Escovopsis spores did not elicit the expected response, i.e., an increase in prophylactic behaviour, in our experiment. It is possible that the Escovopsis strain used was not very virulent. Currie (Citation2001) has observed that the negative impact of Escovopsis in colonies of Atta colombica Guérin-Méneville, 1844 was affected by the isolate of Escovopsis used in the study. Silva et al. (Citation2006) tested several strains of microfungi against the symbiotic fungus of Atta sexdens and they found variation among the strains. Two of these Escovopsis strains were more effective in inhibiting the symbiont than a third one.
Midden workers were significantly more immobilized than foragers by the fungus garden workers. Although we did not perform a pathogen inspection in foragers, the fact that the same fungus species were found on both midden and fungus garden workers suggests that the presence of fungal contaminants is not a reason per se to immobilize or to exclude nestmates from the nest. It is likely that there are other contributing factors that may not yet be well known. In social insects, nestmate recognition is generally mediated by the blend of chemical compounds (CC) present on the cuticles of individuals (D’Ettorre & Lenoir Citation2010). For instance, the role of the chemical substances present in the midden of the leaf-cutting ants is not known, and one untested hypothesis is that the individual odours of the midden workers change, at least temporarily, due to contact with the midden. If this is verified, chemicals should determine the task allocation of midden workers.
The expected avoidance behaviour against contaminated workers or materials is not a general rule in insect societies, including leaf-cutting ants. For example, leaf-cutting ants did not detect the fungal agents M. anisopliae and T. viride contained in baits that they introduced into their nests, and no detectable defensive behaviours were evident (Lopez & Orduz Citation2003). Walker and Hughes (Citation2009) exposed mini-nests of A. echinatior to the virulent fungal parasite M. anisopliae and observed that the workers from these nests received significantly more allogrooming than workers from naïve mini-nests. This behaviour increased the survival of the freshly treated ants. Therefore, allogrooming has been considered an adaptive social immune response.
Our study does not support the hypothesis that midden contamination induces the aggressive behaviour of fungus garden workers against midden workers that was observed in A. cephalotes (Hart & Ratnieks Citation2001) and in A. lobicornis (Ballari et al. Citation2007). In addition, it is interesting to note that none of the fungi found here are specific ant pathogens. Therefore, more investigation is necessary to determine, for example, whether differences in the virulence of Escovopsis and entomopathogens would induce differentiated behaviours in the workers.
Disclosure
The authors declare that they do not have any conflicts of interest.
Acknowledgements
We are thankful for the scholarships awarded by the National Council for Scientific and Technological Development and especially for grant 474819/2006-0.
References
- Alexander M. 1977. Introduction to soil microbiology. 2nd ed. NewYork: John Wiley and Sons. 467 pp.
- Alves SB. 1998. Controle Microbiano de Insetos. 2nd ed. FEALQ, Piracicaba. Alves, ed. Piracicaba, São Paulo, Brazil: FEALQ. pp. 289–381.
- Ballari S, Farji-Brener AG, Tadey M. 2007. Waste management in the leaf-cutting ant Acromyrmex lobicornis: Division of labour, aggressive behaviour, and location of external refuse dumps. Journal of Insect Behavior 20:87–98.
- Bass M, Cherrett JM. 1994. The role of leaf-cutting ant workers (Hymenoptera: Formicidae) in fungus garden maintenance. Ecological Entomolology 19:215–220.
- Bot ANM, Currie CR, Hart AG, Boomsma JJ. 2001. Waste management in leaf-cutting ants. Ethology Ecology and Evolution 13:225–237.
- Cardoso JE, Maia CB, Pessoa MNG. 2002. Ocorrência de Pestalotiopsis psidii e Lasiodiplodia theobromae causando podridão no caule da goiabeira no Ceará. Fitopatologia Brasileira 27:320–320.
- Currie CR. 2001. Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 128:99–106.
- Currie CR, Stuart AE. 2001. Weeding and grooming of pathogens in agriculture by ants. Proceedings of the Royal Society of London 268:1033–1039.
- D’Ettorre P, Lenoir A. 2010. Nestmate recognition. In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford: Oxford Universty Press. 432 pp.
- Domsch KH, Gams WP, Anderson T. 1980. Compendium of soil fungi (v. 1). New York: Academic Press. 1264 pp.
- Fisher PJ, Stradling DJ, Sutton BC, Petrini LE. 1996. Microfungi in the fungus gardens of the leaf-cutting ant Atta cephalotes: a preliminary study. Mycological Research 100:541–546.
- Gerstner AT, Poulsen M, Currie CR. 2011. Recruitment of minor workers for defense against a specialized parasite of Atta leaf-cutting ant fungus gardens. Ethology Ecology and Evolution 23:61–75.
- Hart AG, Ratnieks FLW. 2001. Task partitioning, division of labour and nest compartmentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behavioral Ecology and Sociobiology 49:387–392.
- Hernández-Lauzardo AN, Baustista-Bãnos S, Velázquez-del Valle MG, Trejo-Espino JL. 2006. Identification of Rhizopus stolonifer (Ehrenb.: Fr.) Vuill., causal agent of Rhizopus rot disease of fruits and vegetables. Revista Mexicana de Fitopatologia 24:65–69.
- Hughes WOH, Eilenberg J, Boomsma JJ. 2002. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proceedings of the Royal Society of London 269:1811–1819.
- Hughes WOH, Thomsen L, Eilenberg J, Boomsma JJ. 2004. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. Journal of Invertebrate Pathology 85:46–53.
- Lacerda FG, Della Lucia TMC, Lima ER, Campos LAO, Pereira OL. 2006. Waste management by workers of Atta sexdens rubropilosa (Hymenoptera: Formicidae) in colonies supplied with different substrates. Sociobiology 48:165–173.
- Lacerda FG, Della Lucia TMC, Pereira OL, Peternelli LA, Totola MR. 2010. Mortality of Atta sexdens rubropilosa (Hymenoptera: Formicidae) workers in contact with colony waste from different plant sources. Bulletin of Entomological Research 100:99–103.
- Lopez E, Orduz S. 2003. Metarhizium anisopliae and Trichoderma viride for control of nests of the fungus-growing ant, Atta cephalotes. Biological Control 27:194–200.
- Moore P. 2002. Parasites and the behaviour of animals. New York: Oxford University Press. 338 pp.
- Ortiz A, Orduz S. 2000. In vitro evaluation in Trichoderma and Gliocladium antagonism against the symbiotic fungus of fungus-growing ant Atta cephalotes. Mycopathologia 150:53–60.
- Pereira RTG, Pfenning LH, Castro HA. 2005. Caracterização e dinâmica de colonização de Cladosporium cladosporioides (Fresen.) de Vries em frutos do cafeeiro (Coffea arabica l. Ciência e Agrotecnologia 29:1112–1116.
- Quinlan RJ, Cherrett JM. 1977. The role of substrate preparation in the symbiosis between the leaf-cutting ant Acromyrmex octospinosus (Reich) and its food fungus. Ecological Entomology 2:161–170.
- Rodrigues A, Pagnocca FC, Bacci M Jr, Hebling MJA, Bueno OC, Pfenning LH. 2005. Variability of non-mutualistic filamentous fungi associated with Atta sexdens rubropilosa nests. Folia Microbiologica 50:421–425.
- Schmid-Hempel P. 1998. Parasites in social insects. Princeton: Princeton University Press. 392 pp.
- Silva A, Rodrigues A, Bacci M Jr, Pagnocca FC, Bueno OC. 2006. Susceptibility of ant-cultivated fungus Leucoagaricus gongylophorus (Agaricales: Basidiomycota) towards microfungi. Mycopathologia 132:115–119.
- Walker TN, Hughes WOH. 2009. Adaptive social immunity in leaf-cutting ants. Biology Letters 5:446–448.
