Abstract
The rapid rate of habitat loss in Madagascar urges a comprehensive assessment of the threats that its fauna faces by living in fragmented, secondary and/or exotic forests. The role of habitat disturbance in determining potential boosts of parasite infections in lemurs, for instance, has been only recently investigated. Here, we conducted a preliminary assessment of prevalence and intensity of gastrointestinal parasite infections in two populations of endangered red-collared brown lemurs, Eulemur collaris (Geoffroy, 1812) (Mammalia: Lemuridae), living in two littoral forest fragments of southeastern Madagascar. We collected faecal samples from 45 lemurs. The samples were stored in 70% alcohol, and we used a modified McMaster technique to analyze egg shedding. The analysis revealed only nematode infections. We found a higher prevalence of infection in the more disturbed fragment, while no differences were revealed in terms of infection intensity. Prevalence of infection was found to be sex-biased in the less degraded area, being higher in females. We hypothesize that this might have been caused by female immune suppression due to increased energetic costs during pregnancy and/or to avoid possible harm to the foetus. Since red-collared brown lemurs are the largest seed dispersers in the littoral forest and only small populations survive in a few fragments, the implications of our results should be considered in future conservation plans for this habitat.
Introduction
Anthropogenic habitat disturbance is known to influence the dynamics of parasite infections in wildlife populations (McCallum Citation2008). Among the different types of habitat disturbance, forest fragmentation affects the natural cycle of hosts and their parasites by the crowding effect, edge phenomena, stress due to decreased resource availability and a drop in genetic diversity (Gillespie et al. Citation2005; Schad et al. Citation2005; Chapman et al. Citation2006). In a fragmented situation, animals are forced to use corridors or edge areas where disease transmission by non-forest and/or non-native species, including humans, is more likely to happen (Nunn & Altizer Citation2006; Nunn & Dokey Citation2006). Non-human primates appear to be at particularly high risk of infection because of the rapid modification of their habitat (Cowlishaw & Dunbar Citation2000), their gregarious life and their close phylogenetic proximity to humans (Wallis & Lee Citation1999). In fact, parasite prevalence is high in areas where primates have more contacts with local people, visitors, researchers and/or with animals associated with human activities (Mul et al. Citation2007). But parasite richness and infection intensity in host organisms also depend on other factors such as age, sex, dominance rank, dispersal and seasons (Nunn & Altizer Citation2006).
Though a number of studies have documented gastrointestinal parasites of wild haplorhines, lemur endoparasites remain poorly investigated (Gillespie Citation2006; Junge & Sauther Citation2006; Junge & Louis Citation2007; Miller et al. Citation2007). Moreover, most reports are descriptive studies, which have not been related to ecological factors (for a review see Irwin & Raharison Citation2009). In southeastern Madagascar, mouse lemurs, Microcebus murinus (Miller, 1717) (Mammalia: Cheirogaleidae), in small forest fragments have higher prevalence and intensity of infection of nematodes, cestodes and protozoa (order Coccidia) compared to lemurs in larger fragments (Raharivololona & Ganzhorn Citation2009). A positive correlation between habitat degradation and parasite prevalence has also been found in Eulemur flavifrons (Gray, 1867) (Mammalia: Lemuridae) (Schwitzer et al. Citation2010). Seasons seem to also play a role in determining parasite species richness and infection intensity. Higher scores of infection have been recorded during hot-wet periods both in M. murinus and in Propithecus edwardsi (Grandidier, 1871) (Mammalia: Indridae) (Wright et al. Citation2009; Raharivololona & Ganzhorn Citation2010). Variations over different years and reproductive seasons have been reported also in E. rufifrons (Bennett, 1833) (Mammalia: Lemuridae) (Clough et al. Citation2010).
Understanding the risks the lemurs face by persisting in fragmented and/or in secondary forests is particularly important in habitats such as the southeastern littoral forests, which are today represented by archipelagos of forest patches (Bollen & Donati Citation2006; Vincelette et al. Citation2007). The largest lemur species occurring in this habitat, the endangered red-collared brown lemur (E. collaris), is restricted to the humid forests in the southeastern corner of the island (Mittermeier et al. Citation2008; Donati et al. Citation2011). Being the largest seed disperser in its habitat, the red-collared brown lemur has a crucial ecological role for the maintenance of these highly threatened forests (Bollen & Donati Citation2006; Donati et al. Citation2007). This lemur also represents an interesting model to investigate the link between anthropogenic forest disturbances and parasite infections, since it shows high ecological flexibility to cope with forest fragments at various levels of degradation (Donati et al. Citation2011).
The aim of this preliminary investigation was to assess (i) whether increased levels of anthropogenic habitat disturbance cause increased prevalence and/or intensity of parasite infections and (ii) whether the two sexes show a different susceptibility to endoparasites. For this, we chose two forest fragments differing in size, level of disturbance and distance from human settlements. We expected to record higher levels of infection prevalence and intensity with increasing forest disturbance and decreasing fragment size and distance from human settlements (Schwitzer et al. Citation2010). We also predicted males to be more vulnerable than females, since data collection was carried out during the red-collared brown lemur mating season (May–June), when high levels of testosterone are expected in males [as seen in congeneric species (Ostner et al. Citation2002) and other lemurids (Gould & Ziegler Citation2007)]. High levels of testosterone may act as an immune response depressor, thus increasing susceptibility to infections (Wingfield et al. Citation2001). However, during the second part of our sampling, females were at the beginning of their gestation (June), which could have exposed them to significant physiological stress (Cavigelli Citation1999; Tecot Citation2013). Thus, we also considered the alternative prediction that females may have been more vulnerable than males to infection.
Methods
Study sites
This study was conducted in two littoral forest fragments with differing disturbance levels in southeastern Madagascar. The fieldwork was carried out from May to June 2008 at two sites: Mandena (MAN) and Ste Luce (STL) (). The area is characterized by a tropical wet climate, with average monthly temperatures of 23°C, annual rainfall ranging from 1600 mm (MAN) to 2480 mm (STL) and no clear-cut dry season (Vincelette et al. Citation2007). Red-collared brown lemurs are arboreal strepsirhines living in multi-male, multi-female groups ranging from two to 17 individuals. Their mean body mass is 2.15 ± 0.25 kg and mean body length is 46.1 ± 2.6 cm (n = 11; Donati et al. Citation2007).
Figure 1. Location of sites. Study forest fragments are numbered (modified from Donati et al. Citation2007). North is up.
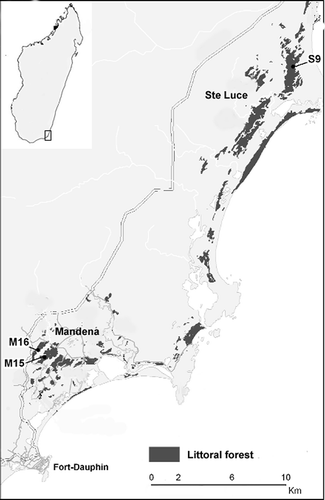
The conservation zone of MAN, 11 km northwest of Fort Dauphin (24°95’S 46°99’E) is on sandy soils at an altitude 0–20 m above sea level (Vincelette et al. Citation2007). The density of E. collaris in MAN is estimated to be around 0.16 animals per hectare (Donati et al. Citation2007). Since 2000, the area has been managed by the communes near the forest, Mandromondromotra and Ampasy, in collaboration with the Service de la Direction des Eaux et Forets and QIT (Quebec Iron and Titanium) Madagascar Minerals (QMM), a mining company which obtained a legal permit for ilmenite exploitation (Vincelette et al. Citation2007). The two largest forest fragments in MAN, M15 and M16, cover an area of 148 ha of degraded littoral forest. Approximately 82 ha of interspersed marsh and swamp connect the two fragments. M15/M16 are the only two forest fragments where red-collared brown lemurs still occur at this site.
The protected forests of STL, around 30 km north of Fort Dauphin (24°45’S 47°11’E), are among the most intact littoral ecosystems in Madagascar and possess a very high vegetation diversity (Bollen & Donati Citation2006). The density of E. collaris in S9 is estimated to be around 0.38 animals per hectare (Donati et al. Citation2007). Since 2005, the area has been managed by the communes nearby the forest, Ambandrika and Manafiafy, in collaboration with the Service de la Direction des Eaux et Forets and QMM (Vincelette et al. Citation2007). The 377-ha forest block S9, where this study was carried out, is one of the two fragments where collared lemurs still occur in the STL area. Vegetation analyses showed that floristically, MAN and STL littoral forests are very similar, suggesting that these two areas were once connected (Rabenantoandro et al. Citation2007). However, structural differences indicate that the forests present in MAN today are degraded forms of the vegetation type in STL (Rabenantoandro et al. Citation2007; Donati et al. Citation2011). These findings are further supported by a lack of certain tree families used for wood by local villagers in MAN but not in STL (Rabenantoandro et al. Citation2007). Analyses of forest degradation at S9 and M15/M16 resulted in two categories: “intact to slightly degraded” and “degraded to highly degraded” forest, respectively (Vincelette et al. Citation2007).
Faecal sampling and analysis
Duplicate faecal samples (72 hr apart) were collected from 45 collared brown lemurs immediately after defecation and divided into three equal parts totalling 270 samples (Gillespie Citation2006). The animals were individually identified via the help of the QMM local assistants who are trained to follow the lemur groups regularly. We sampled eight males and seven females belonging to four groups in MAN while 15 males and 15 females belonging to five groups were sampled in STL. No juveniles were included in the sample. Each sample was preserved in a 30-mL plastic tube which was then filled with 70% ethanol.
All samples were transported to the laboratory of Animal Ecology and Conservation in Hamburg, Germany, dried and examined by Anke Broll and Ole Theisinger for the presence of larvae or eggs of the following three classes of gastrointestinal parasites: Nematoda, Cestoda and Trematoda. An amount of 300 mg of dried faeces of each sample was weighed and rehydrated with distilled water. After 2 hr, the faeces were filtered out of the water. Drained faeces were then mixed with 4.5 mL of potassium iodide solution (density 1.5 g/mL) and filtered through a sieve (0.6 mm mesh size). Samples were analyzed with a McMaster counting chamber, using two counting areas per sample (80 × magnification) (Schad et al. Citation2005).
The analysis was based on two parameters: prevalence and intensity of infection (Rozsa et al. Citation2000). Prevalence (percentage of infected lemurs over the total number of individuals sampled) was based on the presence/absence of larvae or eggs. An individual was considered infected when larvae or eggs were found at least once. Intensity was assessed by using the faecal egg count (FEC) as the number of eggs found in 1 g of faeces per individual (Raharivololona & Ganzhorn Citation2010). The FEC was calculated without considering larvae. The average number of parasite eggs per gram was calculated for duplicate collected samples. Nematode eggs can be very difficult to identify to the species level (Setchell et al. Citation2007); thus, we did not attempt to describe taxon diversity in this work. Since we were interested in relative differences of overall infection prevalence between different areas rather than parasite diversity we considered this limitation acceptable.
Statistical analysis
Chi-square was used to detect potential differences in the proportion of infected lemurs between sites and sexes. Fisher’s exact test was performed for two-by-two contingency tables when there was at least one expected value below five. The Mann-Whitney non-parametric test was used to compare the FEC of infected individuals between sites and sexes.
Results
Twenty-one out of 45 red-collared brown lemurs were found to be infected by nematodes at the two sites, while no cestodes or trematodes were detected. At the MAN site, six out of eight males and five out of seven females were infected. The sample size at MAN was unavoidably small, since no more individuals were present in the area during the data collection. At the STL site, two out of 15 males and eight out of 15 females were found positive for nematode presence. This difference in the prevalence of infection between the two sites was significant (χ2 = 6.43, df = 1, n = 45, P < 0.01)().
Figure 2. (a) Prevalence and (b) intensity of nematode infection in males and females of Eulemur collaris at the two study sites. FEC: number of parasite eggs per gram of faecal material. In (b), quartiles and ranges are shown. * p < 0.05; ** p < 0.01.
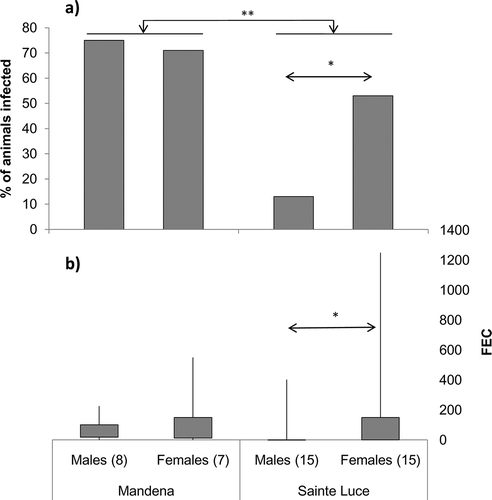
Overall, we did not find a significant difference in the prevalence of infection between males and females (χ2 = 2.67, df = 1, n = 45, P = 0.10). However, separate analyses at each site revealed that while there was no difference between sexes in MAN (Fisher’s exact test: n = 15, P = 0.66) females were significantly more infected than males in STL (χ2 = 5.4, df = 1, n = 30, P = 0.02) ().
The intensity of parasite infection (FEC) for the infected red-collared brown lemurs at the two sites ranged from 25 to 1250 (median: 150, n = 21) eggs per gram. The average intensity of infection was higher in STL (median: 200; min-max: 57–1250, n = 10) than in MAN (median: 75, min-max: 25–550, n = 11). This difference did not reach a significant level (z = –1.90, n1 = 11, n2 = 10, P = 0.06) ().
Overall, average infection intensity at the two sites was similar in males (median: 134, min–max: 25–402, n = 8) and in females (median: 150, min–max: 25–1250, n = 13)(z = –0.73, n1 = 8, n2 = 13, P = 0.47). Separate analyses at each site revealed that there was no difference between males and females both in MAN (z = –0.73, n1 = 6, n2 = 5, P = 0.53) and in STL (z = –1.06, n1 = 2, n2 = 8, P = 0.40)().
Discussion
The results of our study suggest that habitat disturbance had a major effect on parasite prevalence in the two small populations of E. collaris studied in this research. Even though both populations were found to be infected by gastrointestinal parasites, the proportion of infected lemurs increased significantly with forest degradation. The association between intensity of parasite infection and habitat disturbance was less clear. In terms of pathogenic effects, the consequence of this difference is at the moment unknown. The lemurs did not show evident clinical signs, e.g. blood in faeces or diarrhoea, and behavioural data do not suggest striking differences between MAN and STL (Donati et al. Citation2011).
The prevalence of infected lemurs in MAN (60%) is among the highest recorded for Malagasy lemurs (Clough et al. Citation2010; Schwitzer et al. Citation2010; Gabriel, in prep.), while the value in STL (33%) is in line with other studies on lemurs (Dutton et al. Citation2003; Muehlenbein et al. Citation2003; Loudon et al. Citation2006; Junge & Louis Citation2007). Interestingly, a parasite prevalence similar to our figure from MAN was recorded in a different lemur species, M. murinus, in the same area (Raharivololona & Ganzhorn Citation2009). High values of parasite prevalence have been also recorded in two congeneric lemurs, E. flavifrons in the Sahamalaza fragmented forests of the northwest (Schwitzer et al. Citation2010), and E. rufifrons in the western dry forest of Kirindy (Clough et al. Citation2010). The very high prevalence of infection recorded in MAN may be explained by a number of factors. First, although red-collared brown lemurs are mainly arboreal species, in MAN, individuals were frequently observed moving on the ground (Donati et al. Citation2009, Citation2011), which might have resulted in the infection of all the animals examined. In fact, in the degraded habitat of MAN, the forest floor is often used both for travelling between food patches and for searching for fruits dropped from small trees, which are unable to sustain all the group members (Lazdane, pers. observ.). Terrestriality has been repeatedly suggested as a factor increasing the risk of infection (Nunn et al. Citation2000; Loudon et al. Citation2006) and the ability of Eulemur species to use all the forest layers may make them more exposed than strictly arboreal species (Clough et al. Citation2010). Second, MAN is near Fort Dauphin and very close to a large mining area for ilmenite extraction (Vincelette et al. Citation2007). As a consequence, the forest there is regularly visited by people (mainly employers or local villagers), possibly exposing the lemurs to contacts with potential vectors. Parasitological studies on Lemur catta (Linnaeus, 1758) (Mammalia: Lemuridae), E. rufifrons and E. macaco macaco (Linnaeus, 1766) (Mammalia: Lemuridae) indicate that these lemurs are more contaminated by parasites in protected habitats with high encounter rates with humans and domestic animals (Loudon et al. Citation2006; Junge & Louis Citation2007; Miller et al. Citation2007; Junge et al. Citation2008). The exploitation of resources from human trash pits and potential coprophagy of domestic animals’ or humans’ faeces may explain these differences (Loudon et al. Citation2006). Thus, habitat disturbance seems to play a role on the susceptibility to parasite infections in red-collared brown lemurs, although we cannot separate further the effect of forest size, habitat degradation and distance from human settlements, due to the small sample size of our study. These findings match other studies in mainland Africa (Gillespie et al. Citation2005). Interestingly, some of the factors usually positively associated with parasite prevalence, density and group size (Freeland Citation1976; Clough et al. Citation2010), did not seem to have an effect, since both lemur density and average group size were higher in STL (Donati et al. Citation2007, Citation2011) despite the fact that infection prevalence was lower in this area.
Although parasite diversity was not the focus of our study, the presence of only nematodes in these red-collared brown lemurs at two field sites is remarkable. Other lemur populations have been shown to be infected by cestodes in addition to nematodes (Raharivololona & Ganzhorn Citation2009; Clough et al. Citation2010), while the presence of only the latter group was recorded in blue-eyed black lemurs (Schwitzer et al. Citation2010). However, the potential for underestimation is particularly high for some parasite taxa (Clough et al. Citation2010) and their diversity correlates strongly with sampling effort (Nunn & Altizer Citation2006), precluding us from discussing further this result of our short-term assessment until long-term data are available.
Sex differences in terms of vulnerability to parasite infections are still debated for primates, and different studies found opposite trends (Hausfater & Watson Citation1976; Müller-Graf et al. Citation1997; Clough et al. Citation2010). Theoretically, males should be more susceptible to infections, since testosterone depresses the immune response (Wingfield et al. Citation2001), while female oestrogens enhance it (Zuk & McKean Citation1996). However, pregnancy and lactation may expose females to immunosuppressive stress via augmented energetic costs (Nunn & Altizer Citation2006). In Papio cynocephalus (Linnaeus, 1766) (Mammalia: Cercopithecidae), for instance, males have higher egg emission than females during periods of group ranking instability, while egg shedding in adult females fluctuates with their reproductive cycle (Hausfater & Watson Citation1976). In Mandrillus sphinx (Linnaeus, 1758) (Mammalia: Cercopithecidae), nematode egg prevalence increases in pregnant females compared to lactating and cycling females (Setchell et al. Citation2007). Other sex-biased differences may also influence parasite susceptibility, such as body size, social interactions and access to mates or food (Nunn & Altizer Citation2006). Our data showed that in STL parasite prevalence was significantly higher in females than in males, while in MAN the difference was not significant. This is in contrast with our hypothesis which predicted a higher prevalence and infection intensity in males, following the rationale that data collection was performed during the lemur mating season, i.e. when testosterone levels are expected to peak. On the contrary, our results support recent data indicating that high testosterone levels may actually have an immune-enhancing effect in E. rufifrons (Clough et al. Citation2010).
During the second part of our sampling, females were at the beginning of their gestation, which could have been a possible reason for the greater number of animals infected. Several studies indicate that lemur females are under significant physiological stress during the reproductive season due to increased energetic costs, compared to the lean season [Lemur catta: Cavigelli Citation1999; Eulemur rubriventer (Geoffroy, 1850) (Mammalia: Lemuridae): Tecot Citation2013]. The idea is also supported by the observation that in some mammals, immunity seems to decrease during pregnancy to avoid possible harm to the foetus (Cattadori et al. Citation2005). As for the lack of differences between sexes in MAN lemurs, the overall high probability to get infected may have hidden sex-biased differences at that site. Long-term studies controlling the simultaneous effect of social and ecological variables are necessary to clarify whether and when sex-biased factors affect infection prevalence.
In conclusion, the overall high parasite load of E. collaris living in a degraded forest fragment compared to conspecifics living in a geographically close but semi-intact area suggests that habitat disturbance shape the dynamics of parasite infections. Since these seed-disperser lemurs play a key role in the maintenance of forest diversity (Bollen & Donati Citation2006) and high parasite loads have been demonstrated to have detrimental effects on fecundity and survival of wild animals (Tompkins & Begon Citation1999 but see Clough et al. Citation2010), this susceptibility may have cascade consequences for the remnant fragments of this highly threatened habitat.
Acknowledgements
We would like to thank the Commission Tripartite of the Malagasy government for permission to conduct research, as well as the Malagasy Institute for the Conservation of Tropical Environments (MICET) for the logistical assistance. We would also like to thank QIT Madagascar Minerals for their help in the field and the staff of the Oxford Brookes Primate Conservation MSc Program. Thanks are due to Joerg Ganzhorn for helping with the parasite analysis and for his scientific support during this research.
References
- Bollen A, Donati G. 2006. Conservation status of the littoral forest of south-eastern Madagascar: A review. Oryx 40:57–66. doi:10.1017/S0030605306000111.
- Cattadori IM, Boag B, Bjornstad ON, Cornell SJ, Hudson PJ. 2005. Peak shift and epidemiology in a seasonl host-nematode system. Proceedings of the Royal Society B: Biological Sciences 272:1163–1169.
- Cavigelli SA. 1999. Behavioural patterns associated with faecal cortisol levels in free ranging female ring-tailed lemurs, Lemur catta. Animal Behaviour 43:166–179.
- Chapman CA, Speirs ML, Gillespie TR, Holland T, Austad KM. 2006. Life on the edge: Gastrointestinal parasites from the forest edge and interior primate groups. American Journal of Primatology 68:397–409. doi:10.1002/ajp.20233.
- Clough D, Heistermann M, Kappeler PM. 2010. Host intrinsic determinants and potential consequences of parasite infection in free-ranging red-fronted lemurs (Eulemur fulvus rufus). American Journal of Physical Anthropology 142:441–452. doi:10.1002/ajpa.21243.
- Cowlishaw G, Dunbar R. 2000. Primate conservation biology. Chicago: Chicago University Press.
- Donati G, Baldi N, Morelli V, Ganzhorn JU, Borgognini Tarli SM. 2009. Proximate and ultimate determinants of cathemeral activity in brown lemurs. Animal Behaviour 77:317–325. doi:10.1016/j.anbehav.2008.09.033.
- Donati G, Kesch K, Ndremifidy K, Schmidt SL, Ramanamanjato JB, Borgognini SM, Ganzhorn JU. 2011. Better few than hungry: Flexible feeding ecology of collared lemurs Eulemur collaris in littoral forest fragments. PLoS One 6:e19807.doi:10.1371/journal.pone.0019807.
- Donati G, Ravoahangy AM, Ramanamanjato JB, Vincelette M. 2007. Translocation as a conservation measure for an endangered species in the littoral forest of southeastern Madagascar: The case of Eulemur collaris. In: Ganzhorn JU, Goodman SM, Vincelette M, editors. Biodiversity, ecology and conservation of littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort-Dauphin). Washington, DC: Smithsonian Institution. pp. 237–245.
- Dutton CJ, Junge RE, Louis EE. 2003. Biomedical evaluation of free-ranging ring-tailed lemurs (Lemur catta) in Tsimanampetsotsa Strict Nature Reserve, Madagascar. Journal of Zoology and Wildlife Medicine 34:16–24.
- Freeland WJ. 1976. Pathogens and the evolution of primate sociality. Biotropica 8:12–24. doi:10.2307/2387816.
- Gillespie TR. 2006. Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. International Journal of Primatology 27:1129–1143. doi:10.1007/s10764-006-9064-x.
- Gillespie TR, Chapman CA, Greiner EC. 2005. Effects of logging on gastrointestinal parasite infections and infection risk in African primates. Journal of Applied Ecology 42:699–707. doi:10.1111/j.1365-2664.2005.01049.x.
- Gould L, Ziegler TE. 2007. Variation in fecal testosterone levels, inter-male aggression, dominance rank and age during mating and post-mating periods in wild adult male ring-tailed lemurs (Lemur catta). American Journal of Primatology 69:1325–1339. doi:10.1002/ajp.20438.
- Hausfater G, Watson DF. 1976. Social and reproductive correlates of parasite ova emissions by baboons. Nature 262:688–689. doi:10.1038/262688a0.
- Irwin MT, Raharison JL. 2009. A review of the endoparasites of the lemurs of Madagascar. Malagasy Nature 2:66–93.
- Junge RE, Dutton CJ, Knightly F, Williams CV, Rasambainarivo FT, Louis EE. 2008. Comparison of biomedical evaluation for white-fronted brown lemurs (Eulemur fulvus albifrons) from four sites in Madagascar. Journal of Zoo Wildlife and Medicine 39:567–575. doi:10.1638/2007-0137.1.
- Junge RE, Louis EE. 2007. Biomedical evaluation of black lemurs (Eulemur macaco macaco) in Lokobe Reserve, Madagascar. Journal of Zoo Wildlife and Medicine 38:67–76. doi:10.1638/06-006.1.
- Junge RE, Sauther ML. 2006. Overview on the health and disease ecology of wild lemurs: Conservation implications. In: Gould L, Sauther ML, editors. Lemurs: Ecology and adaption. Chicago: University of Chicago press. pp. 423–440.
- Loudon JE, Sauther ML, Fish KD, Hunter-Ishikawa M, Ibrahim Y. 2006. One reserve, three primates: Applying a holistic approach to understand the interconnections among ring-tailed lemurs (Lemur catta), Verreaux’s sifaka (Propithecus verreauxi), and humans (Homo sapiens) at Beza Mahafaly Special Reserve, Madagascar. Ecological and Environmental Anthropology 2:54–74.
- McCallum H. 2008. Landscape structure, disturbance, and disease dynamics. In: Ostfeld RS, Keesing F, Eviner VT, editors. Infectious disease ecology: Effects of ecosystems on disease and of disease on ecosystems. Princeton: Princeton University Press. pp. 100–122.
- Miller DS, Sauther ML, Hunter-Ishikawa M, Fish K, Culbertson H, Cuozzo FP, Campbell TW, Andrews GA, Chavey PS, Nachreiner R, Rumbeiha W, Stacewicz-Sapuntzakis M, Lappin MR. 2007. Biomedical evaluation of free-ranging ring-tailed lemurs (Lemur catta) in three habitats at the Beza Mahafaly Special Reserve, Madagascar. Journal of Zoo Wildlife and Medicine 38:201–216. doi:10.1638/1042-7260(2007)038[0201:BEOFRL]2.0.CO;2.
- Mittermeier RA, Ganzhorn JU, Konstant WR, Glander K, Tattersall I, Groves CP, Rylands AB, Hapke A, Ratsimbazafy J, Mayor MI, Louis EE, Rumpler Y, Schwitzer C, Rasoloarison RM. 2008. Lemur diversity in Madagascar. International Journal of Primatology 29:1607–1656. doi:10.1007/s10764-008-9317-y.
- Muehlenbein MP, Schwartz M, Richard A. 2003. Parasitologic analyses of the sifaka (Propithecus verreauxi verreauxi) at Beza Mahafaly, Madagascar. Journal of Zoological and Wildlife Medicine 34:274–277.
- Mul IF, Paembonan W, Singleton I, Wich SA, van Bolhuis HG. 2007. Intestinal parasites of free-ranging, semicaptive, and captive Pongo abelii in Sumatra, Indonesia. International Journal of Primatology 28:407–420. doi:10.1007/s10764-007-9119-7.
- Müller-Graf CDM, Collins DA, Packer C, Woolhouse MEJ. 1997. Schistosoma mansoni infection in a natural population of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology 115:621–627. doi:10.1017/S0031182097001698.
- Nunn CL, Altizer S. 2006. Infectious diseases in primates: Behavior, ecology and evolution. Oxford: Oxford University Press.
- Nunn CL, Dokey ATW. 2006. Ranging patterns and parasitism in primates. Biology Letters 2:351–354.
- Nunn CL, Gittleman JL, Antonovics J. 2000. Promiscuity and the primate immune system. Science 290:1168–1170. doi:10.1126/science.290.5494.1168.
- Ostner J, Kappeler PM, Heistermann M. 2002. Seasonal variation and social correlates of androgen excretion in male redfronted lemurs (Eulemur fulvus rufus). Behavioral Ecology and Sociobiology 52:485–495. doi:10.1007/s00265-002-0532-9.
- Rabenantoandro J, Randriatafika F, Lowry IIPP. 2007. Floristic and structural characteristics of remnant littoral forest sites in the Tolagnaro area. In: Ganzhorn JU, Goodman SM, Vincelette M, editors. Biodiversity, ecology and conservation of littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort-Dauphin). Washington, DC: Smithsonian Institution. pp. 65–77.
- Raharivololona BM, Ganzhorn JU. 2009. Gastrointestinal parasite infection of the gray mouse lemur (Microcebus murinus) in the littoral forest of Mandena, Madagascar: Effects of forest fragmentation and degradation. Madagascar Conservation and Development 4:103–112. doi:10.4314/mcd.v4i2.48650.
- Raharivololona BM, Ganzhorn JU. 2010. Seasonal variations in gastrointestinal parasites excreted by the gray mouse lemur Microcebus murinus in Madagascar. Endangered Species Research 11:113–122. doi:10.3354/esr00255.
- Rozsa L, Reiczigel J, Majoros G. 2000. Quantifying parasites in samples of hosts. Journal of Parasitolology 86:228–232.
- Schad J, Ganzhorn JU, Sommer S. 2005. Parasite burden and constitution of major histocompatibility complex in the Malagasy mouse lemur, Microcebus murinus. Evolution 59:439–450.
- Schwitzer N, Clough D, Zahner H, Kaumanns W, Kappeler P, Schwitzer C. 2010. Parasite prevalence in blue-eyed black lemurs Eulemur flavifrons in differently degraded forest fragments. Endangered Species Research 12:215–225. doi:10.3354/esr00304.
- Setchell JM, Bedjabaga IB, Goossens B, Reed P, Wickings EJ, Knapp LA. 2007. Parasite prevalence, abundance, and diversity in a semi-free-ranging colony of Mandrillus sphinx. International Journal of Primatology 28:1345–1362. doi:10.1007/s10764-007-9225-6.
- Tecot S. 2013. Variable energetic strategies in disturbed and undisturbed rain forest habitats: Fecal cortisol levels in southeastern Madagascar. In: Masters J, Gamba M, Génin F, Tuttle R, editors. Leaping ahead: Advances in prosimian biology (developments in primatology: Progress and prospects). New York: Springer. pp. 185–195.
- Tompkins DM, Begon M. 1999. Parasites can regulate wildlife populations. Parasitology Today 15:311–313. doi:10.1016/S0169-4758(99)01484-2.
- Vincelette M, Théberge M, Randrihasipara L. 2007. Evaluations of forest cover at regional and local levels in the Tolagnaro region since 1950. In: Ganzhorn JU, Goodman SM, Vincelette M, editors. Biodiversity, ecology and conservation of littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort-Dauphin). Washington, DC: Smithsonian Institution. pp. 49–58.
- Wallis J, Lee DR. 1999. Primate conservation: The prevention of disease transmission. International Journal of Primatology 20:803–826. doi:10.1023/A:1020879700286.
- Wingfield JC, Lynn SE, Soma KK. 2001. Avoiding the ‘costs’ of testosterone: Ecological bases of hormone-behavior interactions. Brain, Behavior and Evolution 57:239–251. doi:10.1159/000047243.
- Wright PC, Arrigo-Nelson SJ, Hogg KL, Bannon B, Morelli TL, Wyatt J, Harivelo AL, Ratelolahy F. 2009. Habitat disturbance and seasonal fluctuations of lemur parasites in the rain forest of Ranomafana National Park, Madagascar. In: Chapman C, Huffman MA, editors. Primates and their parasites. Cambridge: Cambridge University Press. pp. 311–330.
- Zuk M, McKean KA. 1996. Sex differences in parasite infections: Patterns and processes. International Journal for Parasitology 26:1009–1023. doi:10.1016/S0020-7519(96)00086-0.
