Abstract
To understand the effects of island and mainland on life history traits (e.g. longevity, age at maturity, body size and body mass) of the smooth newt Lissotriton vulgaris (Linnaeus, 1758), we generated data on age and body measurements for an island (Bozcaada) and a mainland (Çanakkale) population in northwestern Turkey. Age was determinated by skeletochronology. The maximum life span was 4 years in the island population and 5 years in the mainland population. Age at maturity was estimated to be 2–3 years for both sexes and the populations. While mean snout-venth length (SVL) was calculated as 35.56 mm for females and 31.95 mm for males in the mainland population, it was found to be 32.83 mm for females and 31.78 mm for males in the island population. Females from the mainland population were found to be significantly larger and heavier than those of the island population. A significant positive correlation was found between SVL and age for only males in the mainland population. Unexpectedly, age was not correlated with body size for the island individuals. Since there were hardly any demographic studies on any Urodela species comparing island and mainland populations, in this paper we contribute to the literature on this subject.
Introduction
Differently from morphological, physiological and behavioural characteristics, life-history traits, such as longevity, growth rate, age at maturity, fecundity and the timing of reproduction, coevolve to maximise an organism’s fitness (Tomašević et al. Citation2010). Life-history traits of an individual indicate important variation among populations along environmental and altitudinal gradients. Variation in life-history traits among populations of the same species arises from differences in environmental variables that substantially affect the genetically fixed growth pattern (Özdemir et al. Citation2012). In temperate regions, studies comparing age, size or growth along a gradient of latitude, altitude or temperature provide convincing evidence of a relationship between life-history traits and environmental variables (Ficetola et al. Citation2010; Altunışık & Özdemir Citation2013).
Variations in the body measurements (body size and body mass) and age of island populations of amphibian species, as measured against mainland populations, have been shown in some studies (Castellano et al. Citation1999; Wu et al. Citation2006; Li et al. Citation2011). According to Palkovacs’s life-history theory (Citation2003), three main factors (competition, predation pressures and resource availability) may vary between island and mainland environments. Life-history theory mainly focuses on predation pressures and resource availability, which are considered to be common causal mechanisms for variations in the insular body size of vertebrates (Adler & Levins Citation1994; Palkovacs Citation2003, reviewed in Li et al. Citation2011). The theory explains that a fluctuation in body size (increase or decrease) of a vertebrate species on islands, with respect to the mainland, depends on two hypotheses. The first hypothesis is the effects of decreased extrinsic mortality by reduced predation pressures, and the second one is the altered resource availability dominancy (Palkovacs Citation2003).
Accurately assessed individual age provides greater understanding of life-history traits in long-lived animals such as amphibians. The use of the skeletochronology method, which is the most reliable and appropriate for age determination in amphibians, has been the subject of many recent studies (Üzüm & Olgun Citation2009; Liao & Lu Citation2010; Kutrup et al. Citation2011; Özdemir et al. Citation2012). Age structures of some newts are available (e.g. Triturus cristatus: Francillon-Vieillot et al. Citation1990; Triturus carnifex: Cvetković et al. Citation1996; Triturus marmoratus: Jakob et al. Citation2002; Ommatotriton ophryticus: Kutrup et al. Citation2005; Salamandra infraimmaculata: Warburg Citation2007; Triturus karelinii: Üzüm & Olgun Citation2009; Mertensiella caucasica: Üzüm Citation2009; Salamandrella keyserlingii: Hasumi Citation2010).
The few demographic studies concerning Lissotriton vulgaris (Linnaeus, 1758) were conducted in different regions of Europe including Sweden (Hagström Citation1977), Norway (Dolmen Citation1982), England (Verrell & Francillon Citation1986), Yugoslavia (Kalezić et al. Citation1996), Italy (Nobili & Accordi Citation1997), Ireland (Marnell Citation1998), Romania (Cogalniceanu & Miaud Citation2003) and Austria (Maletzky et al. Citation2004). Despite the species being widespread in Turkey, no data are avaible for the Turkish populations. Our study also provides prominent evidence of differences between an island and a mainland population in some life-history traits (longevity, body size and mass) for a Urodela species, Lissotriton vulgaris.
The smooth newt, Lissotriton (= Triturus) vulgaris, is a small-bodied member of the Salamandridae family. It is widely distributed throughout Europe. L. vulgaris ranges from Ireland and Great Britain through west and central Europe and Scandinavia, south to Italy, the Balkans, northern and western Turkey, and east through much of the steppes of Ukraine and Russia (Arntzen et al. Citation2009). Also, an isolated population in the Caucasus is considered to be valid. The smooth newt occurs at elevations from sea level up to 2150 m above sea level (a.s.l.) (Austria) (Arntzen et al. Citation2009). L. vulgaris usually lives in humid areas, agricultural fields and hilly grasslands, and needs water bodies only for reproduction (Bell Citation1977).
The purposes of this study were (i) to investigate life-history traits (e.g. age at maturity, longevity and body size) of an island (Bozcaada) and a mainland population (Çanakkale) of the smooth newt, L. vulgaris, using skeletochronological methods; (ii) to compare our results for Turkey with those reported for other European populations of L. vulgaris.
Materials and methods
Sampling and studied populations
We used a total of 37 (21 ♂♂ and 16 ♀♀) L. vulgaris adults from Çanakkale (12 males, 8 females) and Bozcaada Island (Tenedos) (nine males, eight females) populations collected during the breeding season in 2013 as a part of a research project (The Scientific And Technological Research Council Of Turkey, TÜBİTAK), with the guidelines of the local ethics committee (Çanakkale Onsekiz Mart University approval reference number 2011/09-03) in Turkey. For each individual, we determined sex by examination of external secondary sexual characters (males have a high dorsal crest and prominent cloaca), weighed to the nearest 0.001 g using microbalances, measured snout-vent length (SVL) to the nearest 0.01 mm using digital callipers (Mitutoyo Corp., Kawasaki, Japan) and clipped the longest toe including the first and second phalanges. Toe samples were fixed in 10% formalin, stored in 70% ethanol and successively used in histological analysis.
The first population inhabited Çanakkale (32 m a.s.l., 40° 06' 38.31'' N, 26° 24' 38.32'' E), in northwestern Turkey (). The samples were collected from a water canal near the Terzioğlu campus of Çanakkale University in March 2013. The maximum size of the water canal is 1 km and it is watery all year. L. vulgaris lives sympatrically with Pelophylax ridibundus, Pseudepidalea variabilis, Hyla orientalis, Bufo bufo, Mauremys rivulata, Natrix natrix and Natrix tesellata in the habitat.
The second population was from Bozcaada (Tenedos) Island (3 m a.s.l., 39° 49' 33.86 ''N, 26° 02' 20.24 ''E), located in the northeast of the Aegean Sea, southwest of the Çanakkale Strait (the Dardanelles). Specimens were collected from the seasonal rain puddles and channels in different parts of the island in March and April 2013. L. vulgaris was encountered particularly in the wetland located on the northern part of the island (Çayır Mevkii).
Age determination and statistical analysis
Individual age was investigated using skeletochronological methods (Castanet & Smirina Citation1990). After dissection and preparation, the phalanges were were washed in running tap water for 24 h, decalcified in 5% nitric acid for 2 h and then washed again under running tap water for 12 h. Cross-sections (17 μm) of the diaphyseal part of each phalanx were acquired using a freezing microtome (Thermo Shandon Cryotome SME) and later stained in Ehrlich’s hematoxylin. Sections were submerged in water-based mounting media for ascertainment under a light microscope. Endosteal resorption of the first lines of arrested growth (LAGs) was assessed by comparing the diameters of eroded marrow cavities with the diameters of noneroded marrow cavities in sections from the youngest specimens. The number of LAGs was assessed independently by two observers (A. Altunışık and T.E. Kalaycı). The distance between two adjoining LAGs is a good indicator of individual growth in a given year (Kleinenberg & Smirina Citation1969). Consequently, where we observed an obvious decrease in spacing between two subsequent LAGs, we took it to mark the age when sexual maturity was achieved (Ryser Citation1988).
Because body measurement (SVL and body mass) and age were not normally distributed (Kolmogorov-Smirnov test, p < 0.05), nonparametric tests were utilized to compare variables between sexes (Mann-Whitney U test). Levene tests were used to test for changes in variances, while Pearson’s correlation coefficient was computed to conclude the pattern of relationships among body size, body mass and age. Regression analysis was applied to calculate the correlation equation between age and body measurement, and the regression model was selected according to R2 values. Data analysis was performed using SPSS21® statistical software package.
We quantified sexual size dimorphism (SSD) with the Lovich and Gibbons (Citation1992) index: sexual dimorphism index (SDI) = (size of larger sex/size of smaller sex) ± 1, where the result is arbitrarily defined as positive (minus one) when females are larger and negative (plus one) in the opposite case.
Results
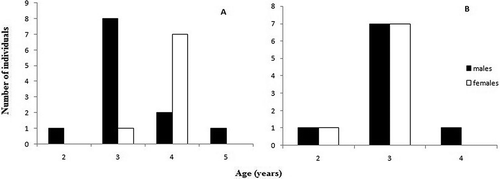
Descriptive statistics of age and body length are given . The age distributions observed in the two populations are separately shown for the two sexes in .
Table I. Descriptive statistics on age (year), snout-venth length (SVL, mm) and body mass (g) of the Lissotriton vulgaris from mainland and island populations.
For the mainland population; age ranged from 2–5 years for males and 3–4 years for females. A cross-section of a 4-year-old female from the mainland population is shown in . Mean SVL was calculated as 35.56 ± 1.40 mm for females and 31.95 ± 1.75 mm for males. According to the results of the Mann-Whitney U test, the age of females was significantly greater than that of males (U = 21, p < 0.05). Age at maturity determined from cross-sections was 2–3 years for both males and females. We found significant sex differences in both SVL (Mann-Whitney U test: U = 4, p < 0.05) and body mass (Mann-Whitney U test: U = 20, p < 0.05) as well. Females of the mainland population were larger than males, and the sexual dimorphism index was found to be 0.11. A significant positive correlation was found between SVL and age for males (Pearson’s correlation r = 0.649, p < 0.05, cubic model best fits the data (y = 30.35 − 2.47 × + 1.425 x2–0.155 x3)) but not for females (r = 0.671, p > 0.05). Also, there was a significant positive correlation between body mass and SVL for only females (r = 0.807, p < 0.05, y = 12.898 − 0.576 x).
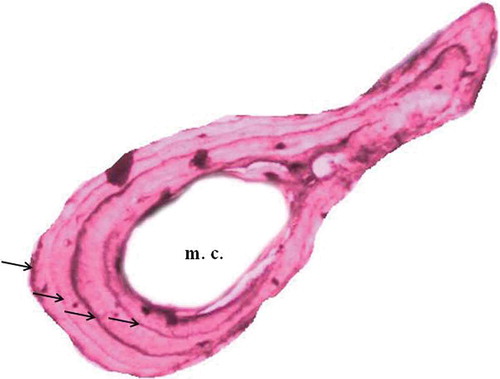
Figure 3. A cross-section (17 μm thick) at the diaphysis level of a phalanx of a male Lissotriton vulgaris individual (m.c. = marrow cavity). The four lines of arrested growth (LAGs) are indicated by black arrows.
For the island population, age ranged from 2–3 years for females and 2–4 years for males. Average SVL was found to be 32.83 ± 1.73 mm for females and 31.78 ± 1.59 mm for males. Mean age, body mass and SVL of specimens from the island population did not differ significantly between the sexes (Mann-Whitney U test, p > 0.05). Age at maturity determined from cross-sections was 2–3 years for both sexes. A significant positive correlation was found between SVL and body mass according to Pearson’s correlation (r = 0.752, p < 0.05), and a cubic model best fits the data (y = 23.558 − 0.067 x2 + 0.001 x3) for females but not for males. Unexpectedly, age was not correlated with body size for either sex (p > 0.05).
Comparison of age, length and body mass between populations
Females from the mainland population were found to be significantly older, larger and heavier than those of the island population (for age: U = 3.65, p < 0.01; for SVL: U = 6, p < 0.01; for body mass: U = 7, p < 0.01; Mann-Whitney U test). In contrast, we did not observe any significant difference in terms of age, body mass or SVL between males of the two populations (Mann-Whitney U test, p > 0.05). Mean SVL and age in the Çanakkale population were slightly higher than in the Bozcaada population (). The sexual size dimorphism index (SDI) was 0.11 for the Çanakkale population and 0.03 for the Bozcaada population.
Discussion
In amphibians, the adult body size depends on a variety of factors, such as tadpole growth rate, time of and size at metamorphosis, juvenile and adult growth rates, age at maturity and longevity. The interaction of environmental factors on each of these processes can strengthen or attenuate the extent of growth in a complex scenario (Özdemir et al. Citation2012).
Body size, also considerable for many organisms, is a prominent feature in Lissotriton vulgaris life history. In previous studies conducted on this newt species, it was reported that large males as compared with small ones have a greater amount of sperm during the breeding season (Verrell et al. Citation1986), and males mate preferentially with larger (Verrell Citation1986) and more fertile females (Verrell & Francillon Citation1986; reviewed in Nobili & Accordi Citation1997). In the present study, the body length of females was found to be larger than that of males in the mainland population. Although females were on average slightly larger than males on the island, there was no statistically significant difference between sexes. As shown in previous studies (Castellano et al. Citation1999; Kutrup et al. Citation2011; Li et al. Citation2011), anurans from island populations were found to be larger than those from mainland populations. However, our results for L. vulgaris are not consistent with this trend; considering both sexes, individuals from the mainland population were on average larger than those from the island population. In most European populations (England: Verrell & Francillon Citation1986; Yugoslavia: Kalezić et al. Citation1996; Austria: Maletzky et al. Citation2004), the mean SVL of L. vulgaris (referred as to Triturus vulgaris) was found to be larger than that of Turkish populations (this study). However, the results found in Italy (Nobili & Accordi Citation1997) and Romania (Cogalniceanu & Miaud Citation2003) were similar to ours in terms of average SVL. When we examine these studies, we can see altitude differences among them (see ). The maximum body length (47 mm) was found in a female smooth newt from 1282 m altitude (Maletzky et al. Citation2004). Since Turkish and Italian populations were at the same altitude, their results resemble each other in terms of body size.
Table II. Age structure of Lissotriton vulgaris populations in Europe assessed by skeletochronology.
Many amphibians exhibit sexual size dimorphism and it is common that females are larger than males in many urodeles (Olgun et al. Citation2001; Maletzky et al. Citation2004; Olgun et al. Citation2005). Analogously, in this study, females of the mainland population were found to be significantly larger than males, while SVL of newts from the island population did not differ significantly between the sexes. Similarly to our results, Maletzky et al. (Citation2004) did not observe any significant differences between sexes of L. vulgaris in terms of body length. In addition, females from the mainland population were found to be significantly heavier than those of the island population (Mann-Whitney U test: p < 0.05). Since the island and the mainland in this study are exposed to different ecological and biological factors, individuals from two populations living in different habitats may show some variations in their life-history traits. Significant differences in terms of age between our island and mainland populations were found in females, but not in males. Similarly, for anurans, it was reported that differences in age between mainland and island populations were significant in the study of Pseudepidalea variabilis, indicating that the former toads were higher in average age than the latter (Castellano et al. Citation1999). Contrary to these results, in the rice frog, Rana limnocharis, no significant differences were found in the mean age of both sexes among the two sites on the mainland and the islands (Li et al. Citation2011).
The maximum lifespan of mainland individuals was assessed as 5 years and 4 years in males and females, respectively. These results are similar to the studies conducted in Italy and England, but different from those in other European countries as can be seen in . The maximum observed longevity was recorded as 11 years in a highland population of L. vulgaris in Austria (Maletzky et al. Citation2004). In general, the age at maturation and lifespan tend to be higher at high altitudes than at low altitudes. Many studies have shown this tendency (Caetano et al. Citation1985; Marunouchi et al. Citation2000; Maletzky et al. Citation2004; Kutrup et al. Citation2005). Confirming this trend, in our study, all individuals from low altitudes (3–32 m a.s.l.) reached maturity at the age of 2 or 3 years. Cold temperatures affect the stage of the amphibian larval and juvenile growth and development rates and, finally, the timing of maturity. Consequently, amphibians inhabiting high altitudes attain sexual maturity later than those at low altitudes. This trend was also shown in the Yugoslavia (Kalezić et al. Citation1996) and Austria (Maletzky et al. Citation2004) populations. Smooth newts from Yugoslavia (990 m a.s.l) and Austria (1282 m a.s.l.) reached sexual maturity at the age of 4 for males and 5 for females.
In our study, we found no significant correlation between age and SVL except in mainland males. Similarly, Verrell and Francillon (Citation1986) and Cvetković et al. (Citation1996) reported that age was significantly correlated with SVL, but only in males of T. vulgaris and T. carnifex, respectively. In a study conducted on T. karelinii (Üzüm & Olgun Citation2009) a significant relationship between age and SVL was reported for both highland and lowland populations.
In conclusion, we provide the first demographic data on L. vulgaris populations in Turkey. Age, body size and body mass were found to be higher in the mainland population than the island population only for females. Since the density of L. vulgaris populations was relatively low in the studied areas, the sample size was limited. For a better understanding of the life-history traits of this newt, the number of individuals should be increased. However there were hardly any demographic studies on any Urodela species comparing island and mainland populations, so in this paper we contribute to the literature on this subject.
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK Project No. 112T063).
References
- Adler G, Levins R. 1994. The island syndrome in rodent populations. The Quarterly Review of Biology 69:473–490. doi:10.1086/418744.
- Altunışık A, Özdemir N. 2013. Body size and age structure of a highland population of Hyla orientalis Bedriaga, 1890, in northern Turkey. Herpetozoa 26:49–55.
- Arntzen JW, Kuzmin S, Beebee T, Papenfuss T, Sparreboom M, Ugurtas IH, Anderson S, Anthony B, Andreone F, Tarkhnishvili D, Ishchenko V, Ananjeva N, Orlov N, Tuniyev B. 2009. Lissotriton vulgaris. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2. Available: http://www.iucnredlist.org. Accessed Feb 2014 6.
- Bell G. 1977. The life of the smooth newt (Triturus vulgaris) after metamorphosis. Ecological Monographs 47:279–299. doi:10.2307/1942518.
- Caetano MH, Castanet J, Francillon H. 1985. Détermination de l’âge de Triturus marmoratus marmoratus (Latreille 1800) du Parc National de Peneda Gerês (Portugal) par squelettochronologie. Amphibia-Reptilia 6:117–132. doi:10.1163/156853885X00010.
- Castanet J, Smirina E. 1990. Introduction to the skeletochronological method in amphibians and reptiles. Annales Des Sciences Naturelles 11:191–196.
- Castellano S, Rosso A, Doglio S, Giacoma C. 1999. Body size and calling variation in the green toad (Bufo viridis). Journal of Zoology 248:83–90. doi:10.1111/j.1469-7998.1999.tb01025.x.
- Cogalniceanu D, Miaud C. 2003. Population age structure and growth in four syntopic amphibian species inhabiting a large river floodplain. Canadian Journal of Zoology 81:1096–1106. doi:10.1139/z03-086.
- Cvetković D, Kalezić ML, Djorović A, Džukić G. 1996. The crested newt (Triturus carnifex) in the Submediterranean: Reproductive biology, body size, and age. Italian Journal of Zoology 63:107–111. doi:10.1080/11250009609356116.
- Dolmen D. 1982. Skeletal growth marks and testis lobulation as criteria for age in Triturus spp. (Amphibia) in central Norway. Acta Zoologica 63:73–80. doi:10.1111/j.1463-6395.1982.tb00761.x.
- Ficetola GF, Scali S, Denoël M, Montinaro G, Vukov TD, Zuffi MAL, Padoa-Schioppa E. 2010. Ecogeographical variation of body size in the newt Triturus carnifex: Comparing the hypotheses using an information-theoretic approach. Global Ecology and Biogeography 19:485–495.
- Francillon-Vieillot H, Arntzen JW, Geraudie J. 1990. Age, growth and longevity of sympatric Triturus cristatus, T. marmoratus and their hybrids (Amphibia, Urodela): A skeletochronological comparison. Journal of Herpetology 24:13–22. doi:10.2307/1564284.
- Hagström T. 1977. Growth studies and ageing methods for adult Triturus vulgaris L. and T. cristatus Laurenti (Urodela, Salamandridae). Zoologica Scripta 6:61–68. doi:10.1111/j.1463-6409.1977.tb00760.x.
- Hasumi M. 2010. Age, body size, and sexual dimorphism in size and shape in Salamandrella keyserlingii (Caudata: Hynobiidae). Evolutionary Biology 37:38–48. doi:10.1007/s11692-010-9080-9.
- Jakob C, Seitz A, Crivelli AJ, Miaud C. 2002. Growth cycle of the marbled newt (Triturus marmoratus) in the Mediterranean region assessed by skeletochronology. Amphibia-Reptilia 23:407–418. doi:10.1163/15685380260462329.
- Kalezić ML, Cvetković D, Djorović A, Džukić G. 1996. Alternative life-history pathways: Paedomorphosis and adult fitness in European newts (Triturus vulgaris and T. alpestris). Journal of Zoological Systematics and Evolutionary Research 34:1–7. doi:10.1111/j.1439-0469.1996.tb00804.x.
- Kleinenberg SE, Smirina EM. 1969. On the method of determination of age in amphibians. Zoologichesky Zhurnal 48:1090–1094. (in Russian).
- Kutrup B, Bulbul U, Yilmaz N. 2005. Age structure in two populations of Triturus vittatus ophryticus at different altitudes. Amphibia-Reptilia 26:49–54. doi:10.1163/1568538053693314.
- Kutrup B, Cakir E, Colak Z, Bulbul U, Karaoglu H. 2011. Age and growth of the green toad, Bufo viridis (Laurenti, 1768) from an island and a mainland population in Giresun, Turkey. Journal of Animal and Veterinary Advances 10:1469–1472. doi:10.3923/javaa.2011.1469.1472.
- Li Y, Xu F, Zhongwei G, Liu X, Jin C, Wang Y, Wang S. 2011. Reduced predator species richness drives the body gigantism of a frog species on the Zhoushan Archipelago in China. Journal of Animal Ecology 80:171–182. doi:10.1111/j.1365-2656.2010.01746.x.
- Liao WB, Lu X. 2010. Age structure and body size of the Chuanxi Tree Frog Hyla annectans chuanxiensis from two different elevations in Sichuan (China). Zoologischer Anzeiger - A Journal of Comparative Zoology 248:255–263. doi:10.1016/j.jcz.2009.10.002.
- Lovich JE, Gibbons JW. 1992. A review of techniques for quantifying sexual size dimorphism. Growth, Development and Aging 56:269–281.
- Maletzky A, Pesta J, Schabetsberger R, Jehle R, Sztatecsny M, Goldschmid A. 2004. Age structure and size of the syntopic populations of Triturus carnifex (Laurenti, 1768), Triturus vulgaris (Linnaeus, 1758) and Triturus alpestris (Laurenti, 1768) in the lake Ameisensee (1,282 m a.s.l.). Herpetozoa 17:75–82.
- Marnell F. 1998. A skeletochronological investigation of the population biology of smooth newts Triturus vulgaris L. at a pond in Dublin, Ireland. Biology and Environment: Proceedings of the Royal Irish Academy 98(B):31–36.
- Marunouchi J, Ueda H, Ochi O. 2000. Variation in age and size among breeding populations at different altitudes in the Japanese newts, Cynops pyrrhogaster. Amphibia-Reptilia 21:381–396. doi:10.1163/156853800507444.
- Nobili G, Accordi F. 1997. Body size, age and fecundity variation in different populations of the smooth newt Triturus vulgaris meridionalis in central Italy. Italian Journal of Zoology 64:313–318. doi:10.1080/11250009709356219.
- Olgun K, Miaud C, Gautier P. 2001. Age, growth, and survivorship in the viviparous salamander Mertensiella luschani from southwestern Turkey. Canadian Journal of Zoology 79:1559–1567. doi:10.1139/cjz-79-9-1559.
- Olgun K, Uzum A, Avci A, Miaud C. 2005. Age, size and growth of the southern crested newt Triturus karelinii (Strauch 1870) in a population from Bozdag (Western Turkey). Amphibia-Reptilia 26:223–230. doi:10.1163/1568538054253465.
- Özdemir N, Altunışık A, Ergül T, Gül S, Tosunoğlu M, Cadeddu G, Giacoma C. 2012. Variation in body size and age structure among three Turkish populations of the treefrog Hyla arborea. Amphibia-Reptilia 33:25–35.
- Palkovacs E. 2003. Explaining adaptive shifts in body size on islands: A life history approach. Oikos 103:37–44. doi:10.1034/j.1600-0706.2003.12502.x.
- Ryser J. 1988. Determination of growth and maturation in the common frog, Rana temporaria, by skeletochronology. Journal of Zoology 216:673–685. doi:10.1111/j.1469-7998.1988.tb02465.x.
- Tomašević K, Ljubisavljević K, Polović L, Džukić G, Kalezić ML. 2010. The body size, age structure and growth pattern of the endemic Balkan mosor rock lizard (Dinarolacerta mosorensis Kolombatović, 1886). Acta Zoologica Academiae Scientiarum Hungaricae 56:55–71.
- Üzüm N. 2009. A skeletochronological study of age, growth and longevity in a population of the Caucasian Salamander, Mertensiella caucasica (Waga 1876) (Caudata: Salamandridae) from Turkey. North-Western Journal of Zoology 5:74–84.
- Üzüm N, Olgun K. 2009. Age, size and growth in two populations of the southern crested newt, Triturus karelinii (Strauch 1870) from different altitudes. Herpetologica 65:373–383. doi:10.1655/08-008.1.
- Verrell PA. 1986. Male discrimination of larger, more fecund females in the smooth newt, Triturus vulgaris. Journal of Herpetology 20:416–422. doi:10.2307/1564504.
- Verrell PA, Francillon H. 1986. Body size, age and reproduction in the smooth newt, Triturus vulgaris. Journal of Zoology 210:89–100. doi:10.1111/j.1469-7998.1986.tb03622.x.
- Verrell PA, Halliday TR, Griffiths ML. 1986. The annual reproductive cycle of the smooth newt (Triturus vulgaris) in England. Journal of Zoology 210:101–119. doi:10.1111/j.1469-7998.1986.tb03623.x.
- Warburg ML. 2007. Longevity in Salamandra infraimmaculata from Israel with a partial review of life expectancy in urodeles. Salamandra 43:21–34.
- Wu Z, Li Y, Murray B. 2006. Insular shifts in body size of rice frogs in the Zhoushan Archipelago, China. Journal of Animal Ecology 75:1071–1080. doi:10.1111/j.1365-2656.2006.01126.x.