Abstract
The dynamics of abundance and biomass of Noctiluca scintillans (Dinoflagellata) and Acartia clausi (Copepoda) in the Bosphorus area of the Black and Marmara Seas during 2005–2009 has been analyzed. Despite the fact that the more abundant Black Sea zooplankton community permanently enters the Marmara Sea, A. clausi and N. scintillans have formed independent populations in the northeast of the Marmara Sea. This may be confirmed on the one hand by the positive correlations between the numbers of A. clausi and N. scintillans individuals in the Marmara Sea near the Bosphorus Strait and near the Prince Islands and, on the other hand, by a lack of correlation between the abundance of these species in the Marmara Sea and in the Bosphorus area of the Black Sea. The abundance of N. scintillans was 6–10 times lower in the Black Sea near the Bosphorus Strait than that in the Marmara Sea. A high correlation (r = 0.84) between the abundance of N. scintillans and the number of dead A. clausi females near the Bosphorus Strait in the Marmara Sea suggests an important role of necrozooplankton in the development of the dinoflagellate.
Introduction
Due to the pronounced vertical stratification of water masses with various hydrological properties that characterize the Marmara Sea, the zooplankton community of the Bosphorus area is formed by organisms with different origins. In the sea, four main groups of mesozooplankton (Copepoda, Cladocera, Sagitta, Oikopleura) and a dinoflagellate Noctiluca scintillans (Macartney) Kofoid et Swezy, 1921 are of Black Sea origin and inhabit predominantly the upper lаyers with a salinity of 18–22‰, while the Mediterranean Sea species (mostly copepods) occupy the deep layers of 38–39‰ (Yuksek et al. Citation2003; Tarkan et al. Citation2005; Isinibilir et al. Citation2011).
Near the Bosphorus Strait in the Marmara Sea, N. scintillans dominated in abundance and biomass among other mesozooplankton species (Yuksek et al. Citation2003; Yılmaz et al. Citation2005). According to Isinibilir et al. (Citation2011), copepods constitute 79–93% of mesozooplankton in this region, with Acartia clausi Giesbrecht, 1889 being the most abundant species (Benli et al. Citation2001). Previously, Svetlichny et al. (Citation2006) and Hubareva et al. (Citation2008) showed that individuals of A. clausi which are sensitive to high salinity and transferred from the Black Sea to the Marmara Sea through the Bosphorus Strait did not survive in the salinity gradient layers and, as a result, deep-water zooplankton of the southern Bosphorus mainly consisted of dead organisms of Black Sea origin. Oğuz and Öztürk (Citation2011) also reported the important role of the salinity gradient in the Bosphorus Strait area as a barrier to species exchange between the two seas. High mortality of copepods in the Marmara Sea near the Bosphorus area (calculated to be 0.69 d−1, see Isinibilir et al. Citation2011) can significantly contribute to the concentration of organic matter in this region. Morever, in the highly polluted areas of the Marmara Sea, the heterotrophic dinoflagellate N. scintillans can develop successfully (Koval Citation1984; Yılmaz et al. Citation2005; Zaika Citation2005; Pavlova & Melnikova Citation2006), consuming A. clausi eggs (Daan Citation1987; Quevedo et al. Citation1999). Thus, determining the mutual effects between A. clausi and N. scintillans populations may contribute to improving our understanding of the functioning of zooplankton communities in the adjacent areas of the Black and Marmara Seas.
The aim of the present study was to analyze the dynamics of N. scintillans population abundance in relation to regional changes in the numbers of live and dead A. clausi in the Bosphorus region of the Black and Marmara Seas during 2005–2009.
Materials and methods
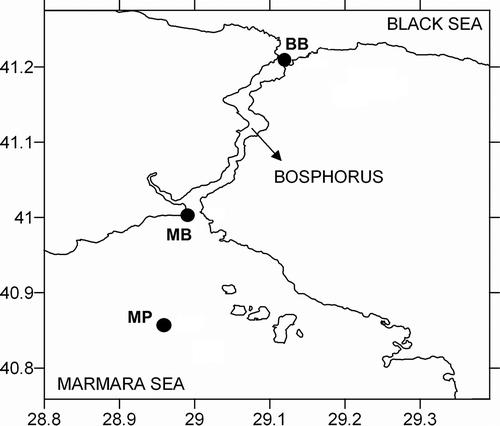
Zooplankton samples were collected seasonally during 2005–2009 with a closing Nansen net (opening diameter: 50 cm, mesh size: 200 μm) by vertical hauls between the bottom and the surface at the permanent stations (MP) located in the Marmara Sea near the Prince Islands (40°51,715 N, 28°57,901 E, depth of 150 m) and the inlet of the Bosphorus Strait (MB, 40°59,32 S, 28°59,33 E, depth of 50 m), and in the Black Sea near the northern inlet of the Bosphorus Strait (BB, 41°13,35 N, 29°08,09 E, depth of 80 m) from the layers of 0–50 and 50–80 m (). Sampling in the Marmara Sea was carried out from the surface layers (0–20 m) formed by the brackish Black Sea water, the intermediate layers (20–50 m) and the deep layers (50–200 m) consisting of the high-saline Mediterranean Sea water. In the comparative analysis, we used also the samples collected by Dr. B. Anninsky from the layer of 0–70 m in the Bosphorus area of the Black Sea on 14 October 2005. The samples were immediately preserved with 4% borax-buffered formaldehyde. In the laboratory, the numbers of N. scintillans and A. clausi developmental stages (being alive or dead before sampling) were determined under a dissecting microscope using a Bogorov chamber. A. clausi individuals with opaque exoskeletons and destructive changes in muscles and internal organs were assumed to be dead before the sampling.
Figure 1. Positions of the sampling stations: Black Sea near the Bosphorus Strait (BB); Marmara Sea near the Bosphorus Strait (MB); Prince Islands (MP).
The total length (Ltot, mm) and length and width of the prosome (lpr and dpr, mm) of Acartia clausi were measured under a light microscope fitted with an eyepiece micrometer. The body weight of adults and copepodits of Acartia clausi (mg) was equal to their body volume (mm3) calculated as Vb = 0.47 Ltot0.21 lpr0.93 dpr1.86, where Ltot is the total length (mm) of body and lpr and dpr are length and width of the prosome (Svetlichny et al. Citation2012).
Statistical evaluation of the data was conducted by one-way analysis of variance (ANOVA). Average values presented in the figures are means ± standard deviation. To evaluate the statistical significance of the difference between the means, Bailey’s td – criteria was used. The correlation analysis was conducted using Spearman’s rank correlation coefficients.
Results and discussion
According to our observations, the biomass of A. clausi was approximately 47 times higher near the Bosphorus Strait in the Black Sea than in the southern Bosphorus region of the Marmara Sea during 2005–2009 (except April and November 2008) () . On the contrary, the percentage of dead organisms increased from 3.9 ± 4% in the Black Sea to 30 ± 20% in the Marmara Sea. Near the Prince Islands (MP), the main part of the A. clausi population was aggregated in the brackish upper layers (0–20 m) where the mean share of dead individuals amounting to 15%. In the deep layers formed with the Mediterranean water, the biomass of this species decreased while the mean percentage of dead organisms increased up to 50%. However, maximum values of dead A. clausi individuals in deep layers in the south Bosphorus area (57 and 80% of total biomass) were found in June and November 2007, respectively ().
Table I. Total biomass (B, mg m−2) and the share of dead Acartia clausi (%) in the Marmara Sea near the Prince Islands and near the Bosphorus Strait, and in the Black Sea near the Bosphorus Strait.
In April and November 2008, the biomass of A. clausi in the southern Bosphorus area (1898.2 and 830.9 mg m−2, respectively) was significantly higher than those near the Prince Islands and near the Bosphorus in the Black Sea, mainly due to late developmental stages (up to 90% of the total population number).
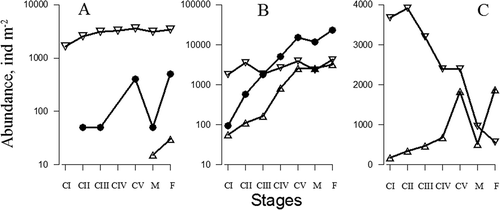
In April 2008, pre-adults and adults of A. clausi were located in the intermediate layer (15–30 m) in the southern Bosphorus area, while the early developmental stages aggregated predominantly in the upper layers (). The percentage of dead organisms in the intermediate layer constituted about 20%. In contrast, early developmental stages of A. clausi were dominant in the northern Bosphorus in April 2008, with the mean percentage of dead individuals being 2.7%. In November 2008, the maximum abundance values for this species (mainly the late developmental stages) also were found below 30 m depth in the southern Bosphorus. The deep location of the A. clausi population in the studied area may be due to the prevalence of the southwestern winds during spring and autumn, which results in a decrease in the Black Sea water inflow into the Marmara Sea and an increase in the thickness of the brackish layer down to the bottom of the southern exit of the Bosphorus Strait (Bogdanova & Shmeleva Citation1967; Oğuz & Öztürk Citation2011). Previously, age and size segregation of copepod populations in the Black Sea in relation to hydrodynamic processes were reported by Arashkevitch et al. (Citation2002).
Figure 2. Age structure of Acartia clausi population in the Marmara Sea (A) near the Prince Islands in the layers 0–20 m (∇), 20–50 m (●), 50–150 m (∆), near the Bosphorus Strait (B) in the layers 0–15 m (∇), 15–30 m (●), 30–50 m (∆) and in the Black Sea (C) near the Bosphorus Strait in the layers 0–50 m (∇) and 50–80 m (∆) in April 2008. CI–CV: the first to fifth copepodite stages; M: males; F: females.
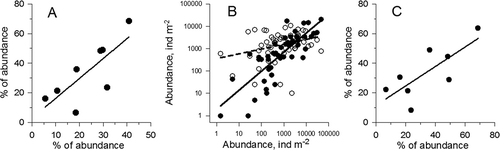
Hubareva et al. (Citation2008) suggested that the population of A. clausi from the Marmara Sea recruited specimens from the Black Sea constantly through the Bosphorus Strait. This may be confirmed by the fact that the percentages of females in the northern Bosphorus area and near the Prince Islands during 2005–2009 were positively (r = 0.77, p < 0.05) correlated (). Nevertheless, we found a high correlation (r = 0.8, p < 0.05) between the total number of A. clausi in the southern Bosphorus region and that near the Prince Islands (), whilst there was a lack of correlation between the total abundance of this species in the southern Bosphorus and that in the northern Bosphorus area (r = 0.29). Moreover, the share of I–IV copepodite stages in the A. clausi population in the Marmara Sea near the Bosphorus Strait was positively correlated with the share of females near the Prince Islands (r = 0.76, p < 0.05) (). Therefore, despite the fact that the biomass of the Black Sea A. clausi population from the northern Bosphorus is higher than that in the southern Bosphorus area, the population dynamics of this species near the Bosphorus Strait are predominantly influenced by the Marmara Sea A. clausi development.
Figure 3. Acartia clausi. Correlations between the share (%) of females near the Prince Islands (MP) and the share of females in the Black Sea (BB) near the Bosphorus Strait (А), between total population abundance (ind. m−2) in the Marmara Sea near the Bosphorus Strait (MB) and total population abundance in the MP (●) and BB (○) (B), between the share of the copepodites I–IV in the MB and the share of females in the MP (C) during 2005–2009.
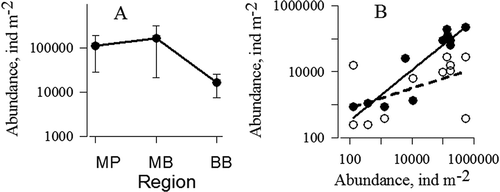
In contrast to A. clausi, the abundance of N. scintillans in the northern Bosphorus was on average 10 and seven times lower than those in the southern Bosphorus and near the Prince Islands, respectively (). The maximum abundance of this species in the northern Bosphorus in winter amounted to 28,000 ind. m−2 while in the open regions of the Black Sea, N. scintillans abundance can reach densities up to 200,000 ind. m−2 (Kovalev Citation1993; Kovalev et al. Citation1996). This may be explained by the aggregation of N. scintillans during the period of mass development in the cold layers of the Black Sea, which do not flow into the Bosphorus Strait (Bityukov Citation1969). In the Marmara Sea, the population of N. scintillans occupies the thin surface layer formed by the Black Sea water (Isinibilir et al. Citation2011) due to the fact that body density of this dinoflagellate is close to the Black Sea water density (Bityukov Citation1969).
Figure 4. A, Regional changes in the numbers of Noctiluca scintillans in the Black Sea near the Bosphorus Strait (BB), in the Marmara Sea near the Bosphorus Strait (MB) and near the Prince Islands (MP). B, Correlations between the numbers of Noctiluca scintillans in the MB and MP (●), and in the MB and BB (○).
During 2005–2009, the maximum abundance of N. scintillans reached 550,000 ind. m2 and was close to the magnitudes reported by Yılmaz et al. (Citation2005). According to our data, the maximum abundance of this species were recorded in five samples from the southern Bosphorus area and in three samples collected near the Prince Islands. We found a positive correlation (r = 0.85, p < 0.01) between the abundance of N. scintillans in these regions of the Marmara Sea (), while there was a lack of correlation between the abundances of this species in the southern and northern Bosphorus areas.
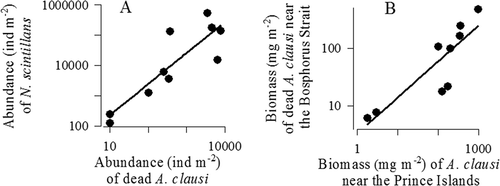
The heterotrophic dinoflagellate N. scintillans is known to develop successfully in disturbed environments at high organic matter concentrations (Koval Citation1984; Pavlova & Melnikova Citation2006). In the Bosphorus Strait, the copepod A. clausi basically contributes to necrozooplankton. Isinibilir et al. (Citation2011) calculated the mean total biomass of dead organisms in the southern Bosphorus as 123 mg m−2 (varying from 10 to 473 mg m−2). According to our refined estimation, the biomass of dead A. clausi during the same studied period amounted to 92.5 mg m−2. A positive correlation (r = 0.84, p < 0.01) between the abundance of N. scintillans and dead A. clausi females in the southern Bosphorus may be considered evidence of an important role for necrozooplankton in the development of N. scintillans (). Based on a positive correlation (r = 0.78, p < 0.05) between the number of dead A. clausi in the southern Bosphorus and the total abundance of this species near the Prince Islands in the Marmara Sea (В), and a lack of correlation between the number of dead A. clausi near the Bosphorus Strait and the total abundance of the specimens in the Black Sea, one can suggest that the zooplankton community in the Bosphorus area is relatively independent of the complex of the Black Sea ecological factors but strongly affected by the environmental conditions of the Marmara Sea.
Figure 5. Correlation between the number of Noctiluca scintillans and the number of dead Acartia clausi females near the Bosphorus Strait in the Marmara Sea (A), and correlation between the biomass of dead Acartia clausi females near the Prince Islands and the total biomass of Acartia clausi females near the Bosphorus Strait (В).
Conclusion
Despite the fact that both studied species are of Black Sea origin and adapted to brackish water, the positive correlations between the abundance of A. clausi and N. scintillans in the Marmara Sea near the Bosphorus Strait and near the Prince Islands, and a lack of correlation between the abundance of these species in the Marmara Sea and in the Bosphorus area of the Black Sea during 2005–2009, indicate that A. clausi and N. scintillans have formed independent populations in the northwestern Marmara Sea. The abundance of N. scintillans was 6–10 times higher near the Bosphorus Strait in the Marmara Sea than in the Black Sea. A high correlation (r = 0.84) between the abundance of N. scintillans and the number of dead A. clausi females near the Bosphorus Strait in the Marmara Sea seems to suggest an important role for necrozooplankton in the development of the dinoflagellate.
Polyphagous N. scintillans inhabiting the brackish, highly productive upper layers, competing with A. clausi for food and consuming copepod eggs, can significantly reduce the abundance of the A. clausi population in the Marmara Sea. The Bosphorus Strait region of the Marmara Sea, heavily affected by anthropogenic stress, seems to be considered a favorable area for the mass development of such opportunistic species as N. scintillans.
Acknowledgements
The present study was partly supported by the Turkish Scientific Technical Research Council (TUBITAK)--National Academy of the Ukraine (NASU) joint projects (104Y208) and (107Y001), and a North Atlantic Treaty Organization (NATO) Linkage Grant (EST NUKR CLG 983036).
References
- Arashkevitch EG, Drits AV, Musaeva EI, Gagarin VI, Sorokin PY. 2002. Mesozooplankton spatial distribution in relation to circulation pattern in the north-eastern part of the Black Sea. In: Zatsepin A, Flint M, editors. Multi-disciplinary investigations of the north-east part of the Black Sea. Moscow: NAUKA. pp. 257–272. (in Russian).
- Benli HA, Tarkan AN, Sever TM. 2001. Comparison of the mesozooplankton composition the southwestern Black Sea, Sea of Marmara and eastern Aegean Sea. Turkish Journal of Marine Sciences 7:163–179.
- Bityukov EP. 1969. Distribution and ecology of Noctiluca miliaris in the Black Sea. Biology of the Sea 17:76–95. (in Russian).
- Bogdanova TK, Shmeleva AA. 1967. Hydrologic conditions of introduction of the Mediterranean species into the Black Sea. In: Vodyanitskiy VA, editor. Water dynamics and issues on hydrochemistry of the Black Sea. Kiev: Naukova Dumka. pp. 156–166. (in Russian).
- Daan R. 1987. Impact of egg predation by Noctiluca miliaris on the summer development of copepod populations in the southern North Sea. Marine Ecology Progress Series 37:9–17. doi:10.3354/meps037009.
- Hubareva E, Svetlichny L, Kideys A, Isinibilir M. 2008. Fate of the Black Sea Acartia clausi and Acartia tonsa (Copepoda) penetrating into the Marmara Sea through the Bosphorus. Estuarine, Coastal and Shelf Science 76:131–140. doi:10.1016/j.ecss.2007.06.009.
- Isinibilir M, Svetlichny L, Hubareva E, Yilmaz IN, Ustun F, Belmonte G, Toklu-Alicli B. 2011. Adaptability and vulnerability of zooplankton species in the adjacent regions of the Black and Marmara Seas. Journal of Marine Systems 84:18–27. doi:10.1016/j.jmarsys.2010.08.002.
- Koval LG. 1984. The zoo- and necrozooplankton of the Black Sea. Kiev: Naukova Dumka. 127 pp. (in Russian).
- Kovalev AV. 1993. Mesozooplankton. In: Kovalev AV, Finenko ZZ, editors. Plankton of the Black Sea. Kiev: Naukova Dumka. pp. 144–165. (in Russian).
- Kovalev AP, Zagorodnyaya YA, Ostrovskaya NA. 1996. The studies of zooplankton of the Black Sea. In: Eremeev VN, editor. The diagnosis of environmental condition of coastal and shelf zones of the Black Sea. Sevastopol: NAS of the Ukraine, MHI. pp. 254–265. (in Russian).
- Oğuz T, Öztürk B. 2011. Mechanisms impeding natural Mediterranization process of Black Sea fauna. Journal of the Black Sea/Mediterranean Environment 17:234–253.
- Pavlova EV, Melnikova EB. 2006. Annual fluctuations of zooplankton quantity indicators near Sevastopol (1998–2003). Marine Ecological Journal 5:63–73. (in Russian).
- Quevedo M, Gonzalez-Quiros R, Anadon R. 1999. Evidence of heavy predation by Noctiluca scintillans on Acartia clausi (Copepoda) eggs off the central Cantabrian coast (NW Spain). Oceanologica Acta 22:127–131. doi:10.1016/S0399-1784(99)80039-5.
- Svetlichny L, Hubareva E, Khanaychenko A. 2012. Calanipeda aquaedulcis and Arctodiaptomus salinus are exceptionally euryhaline osmoconformers: Evidence from mortality, oxygen consumption, and mass density patterns. Marine Ecology Progress Series 470:15–29. doi:10.3354/meps09907.
- Svetlichny LS, Kideys AE, Hubareva ES, Besiktepe S, Isinibilir M. 2006. Development and lipid storage in Calanus euxinus from the Black and Marmara Seas: Variabilities due to habitat conditions. Journal of Marine Systems 59:52–62. doi:10.1016/j.jmarsys.2005.09.003.
- Tarkan AN, Isinibilir M, Tarkan AS. 2005. Seasonal variations of the zooplankton composition and abundance in the Istanbul Strait. Pakistan Journal of Biological Sciences 8:1327–1336. doi:10.3923/pjbs.2005.1327.1336.
- Yılmaz NI, Okus E, Yüksek A. 2005. Evidences for influence of a heterotrophic dinoflagellate (Noctiluca scintillans) on zooplankton community structure in a highly stratified basin. Estuarine, Coastal and Shelf Science 64:475–485. doi:10.1016/j.ecss.2005.03.011.
- Yuksek A, Yilmaz N, Okus E, Uysal Z, Shmeleva AA, Gubanova AD, Altukhov D, Polat-Beken SC. 2003. Spatio-temporal variations in zooplankton communities and influence of environmental factors on them in SW Black Sea and the Sea of Marmara. In: Yilmaz A, editor. Oceanography of the eastern mediterranean and Black Sea: Similarities and differences of two interconnected basins. Ankara, Turkey: TUBITAK Publishers. pp. 774–784.
- Zaika VE. 2005. Ecology of Noctiluca scintillans (Macartney) in the Black Sea. Marine Ecological Journal 4:42–48. (in Russian).
